Over the past 13 years, five second-generation antipsychotics have been marketed in the United States, beginning with risperidone (1994) and followed by olanzapine (1996), quetiapine (1997), ziprasidone (2001), and aripiprazole (2002). Although these second-generation antipsychotics emerged as alternative treatments for otherwise treatment-resistant patients, they have quickly become first-line agents in treating schizophrenia (
1,
2,
3,
4 ).
Concern about their metabolic side effects and higher cost (
5,
6 ) has renewed questions about the preferred use of second-generation medications. Large clinical trials, including the recent Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE), have further challenged widely held assumptions regarding the clinical advantages of second- over first-generation agents (
7,
8,
9 ).
In this brief report we describe preferences of psychiatrists for second-generation antipsychotics and evaluate their optimism toward second-generation drugs for the management of treatment-resistant schizophrenia. We then seek to identify factors that are related to these clinical opinions and subsequent antipsychotic prescribing practices. Survey information was gathered from September 2003 to January 2004 to assess psychiatrists' management of schizophrenia.
Methods
A nationally representative sample of U.S. psychiatrists was selected as previously described (
10 ) from the American Medical Association's Masterfile of Physicians. Survey information was gathered from September 2003 to January 2004 to assess psychiatrists' management of schizophrenia. Among a final sample of 815 psychiatrists, 473 responded, for a response rate of 58%. The analytic sample was limited to psychiatrists who responded to a set of questions about antipsychotic prescribing preferences (N=431).
Optimism toward individual second-generation antipsychotics was ascertained with a 7-point Likert scale in response to the following vignette: "An adult outpatient with schizophrenia has persistent positive symptoms despite adherence with a three-month course of oral Haldol (haloperidol) at optimal dose. In your view, what is the most likely clinical consequence of switching the patient to each of the following medications?" Separate responses ranged from 1, much worse, to 7, much improved, with 4 indicating no change, for olanzapine, risperidone, quetiapine, ziprasidone, and aripiprazole. On the basis of responses about the five antipsychotics, a mean optimism rating was calculated for each psychiatrist. Because of risks of agranulocytosis, the second-generation antipsychotic clozapine is often considered in its own class and was therefore not included in this analysis, although it received the highest optimism rating of all antipsychotics (data not shown). Psychiatrists were then divided evenly into three groups on the basis of their mean optimism score.
Psychiatrists were subsequently asked, "Which four sources of information most influenced your response to the last question?" Choices included experience prescribing these medications, research on these antipsychotic medications, practice guideline recommendations, recommendations of colleagues, recommendations of drug representatives or advertisements, information in the Physician's Desk Reference, or other. Psychiatrists were also asked to indicate the antipsychotic medication they most often use in schizophrenia.
Data were also collected on physician age, gender, race and ethnicity, board certification, practice setting, and involvement in teaching. Psychiatrists were also asked to estimate how many hours they spent reading peer-reviewed psychiatric journals and how many times they met with a drug representative over the last typical month. Psychiatrists were asked to estimate the total number of patients that they treated in the last typical work month, as well as the total number of patients that they treated with schizophrenia. Psychiatrists were also asked to list approximately how many formal or informal consultations they provided to, or received from, other psychiatrists about patient management in the last typical work month. Psychiatrist familiarity with practice guidelines was rated on a 7-point Likert response scale from 1, not familiar, to 7, very familiar, for four practice guidelines for the management of schizophrenia (American Psychiatric Association guidelines, National Institute of Mental Health's Schizophrenia Patient Outcomes Research Team (PORT) Project, Expert Consensus Guideline Series, and the Texas Medication Algorithm Project). Psychiatrists whose ratings were 1 or 2 for all of the guidelines were considered to be unfamiliar with the guidelines, and those whose ratings were higher were considered to have at least some familiarity.
All study procedures were approved by institutional review boards of the New York State Psychiatric Institute and the American Psychiatric Institute for Research and Education.
Descriptive statistics for psychiatrists' demographic, practice, and influence factors were examined for each optimism group. Multinomial logistic regression was used to examine the effect of psychiatrists' factors on levels of optimism (low, medium, and high) toward second-generation antipsychotics, after controlling for psychiatrists' age, sex, and race, as well as primary treatment setting (inpatient versus outpatient) and average monthly caseload (number of patients treated for schizophrenia). The latter two covariates were selected with the expectation that experience in treating schizophrenia would be related to attitudes toward second-generation antipsychotics. Results are presented as adjusted odds ratios (ORs) with associated 95% confidence intervals (CIs) using low levels of optimism as the reference category. All analyses, including calculated percentages, were weighted to account for survey nonresponse.
Results
A great majority of psychiatrists (97%) reported using second-generation antipsychotics most often in treating schizophrenia. The psychiatrists were most likely to identify risperidone (50%), followed by olanzapine (34%) and quetiapine (7%), as their preferred antipsychotic medication. The newer second-generation antipsychotics, ziprasidone and aripiprazole, were each listed by <5% of psychiatrists. First-generation antipsychotics (haloperidol, thiothixene, perphenazine, and fluphenazine) together accounted for only 3% of preferred prescribing, and <1% identified clozapine as their most common antipsychotic for schizophrenia.
A majority of psychiatrists (88%) felt that switching to any of the second-generation antipsychotics would offer improvement in persistent positive symptoms after a failed first-line trial of haloperidol. A significantly greater proportion of psychiatrists predicted improvement with olanzapine (79%; CI=75%–83%) or risperidone (79%; CI=75%–83%), compared with aripiprazole (64%; CI=59%–68%), quetiapine (60%; CI=56%–65%), or ziprasidone (58%; CI=54%–63%). Almost half of the psychiatrists surveyed (45%) felt that all five of the second-generation antipsychotics would offer improvement over haloperidol.
Psychiatrists were divided into three distinctive groups on the basis of their average optimism rating for the five second-generation antipsychotics (low, medium, and high levels of optimism). The mean±SD optimism ratings of the three groups were as follows: low, 3.8±.6; medium, 4.9±.2; and high, 5.8±.5 (F=748, df=2 and 424, p<.001). All of the 288 psychiatrists with medium or high levels of optimism believed that at least one of the second-generation antipsychotics would offer improvement (100%), as compared with only 62% of the 143 psychiatrists with low levels of optimism (Pearson χ 2 =124.0, df=2, p<.001). Furthermore, 86% of 144 psychiatrists with high levels of optimism believed that all five of the antipsychotics would offer some improvement, compared with 48% of 144 psychiatrists with medium levels of optimism and none of the psychiatrists with low levels of optimism (0 of 143 psychiatrists) (Pearson χ 2 =214.3, df=2, p<.001).
Most psychiatrists (98%) considered their personal experience in prescribing these medications as one of the top four sources of information in determining their clinical predictions regarding the success of a second-generation antipsychotic after a failed trial of haloperidol. Psychiatrists also commonly identified research on these antipsychotics (90%), recommendations of colleagues (81%), and practice guideline recommendations (71%) as influential sources of their opinion. Recommendations of drug representatives or advertisements were acknowledged by 22% of psychiatrists as one of the four most influential factors in shaping their clinical opinion.
Most psychiatrists (89%) reported that they were somewhat familiar with at least one treatment guideline or algorithm for the management of schizophrenia (
1,
2,
3,
4 ). The majority of psychiatrists reported familiarity with the American Psychiatric Association's practice guidelines (82%), followed by the Texas Medication Algorithm Project (57%), the Expert Consensus Guideline Series (50%), and the National Institute of Mental Health's PORT Project (32%).
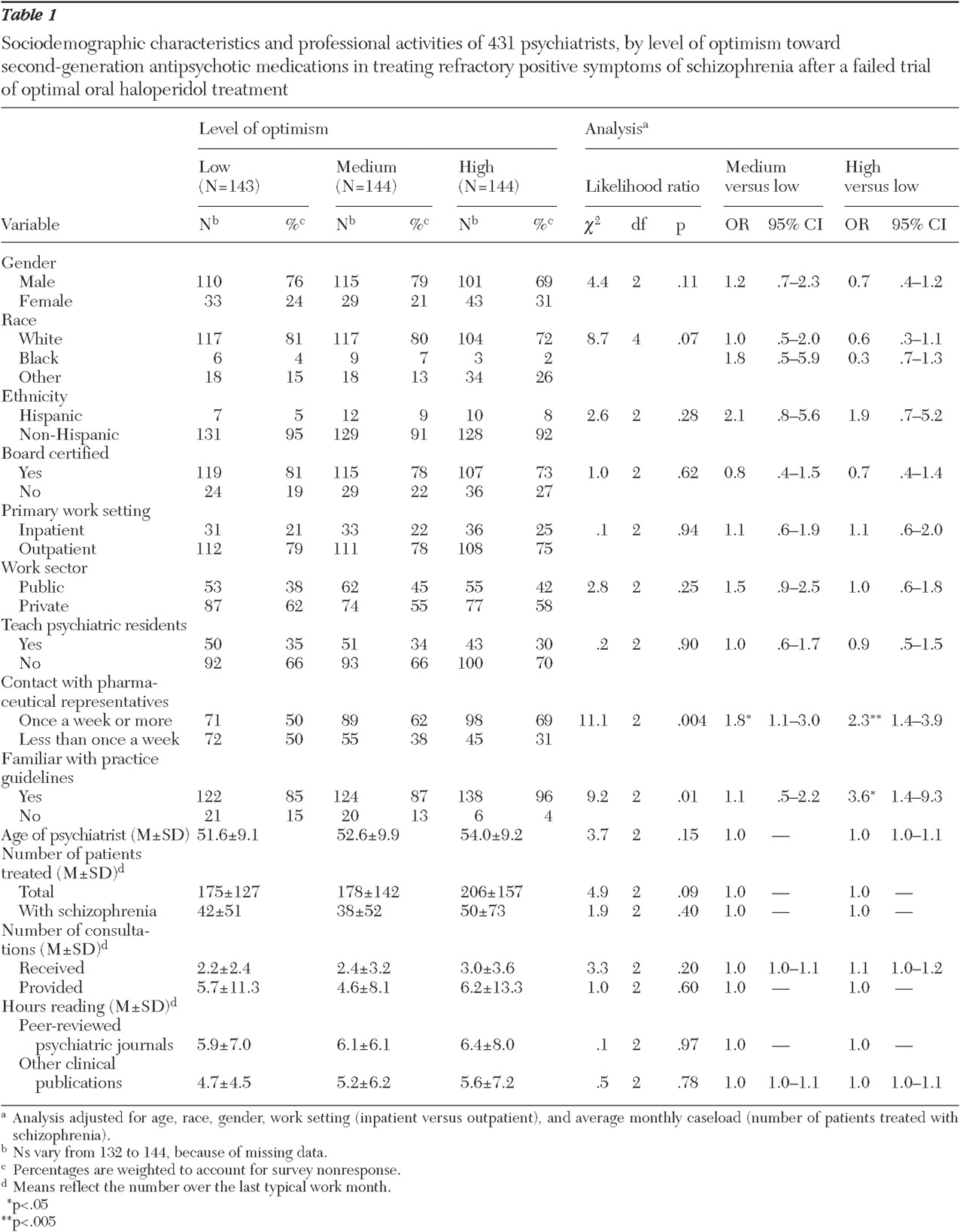
As shown in
Table 1, optimism was not related to any demographic or professional characteristics with two notable exceptions. Psychiatrists who met with a pharmaceutical representative at least once a week were more likely than those who met less frequently to have high levels of optimism toward second-generation antipsychotics for treatment resistance (OR=2.3, CI=1.4–3.9, p=.001). Psychiatrists who reported familiarity with at least one of the practice guidelines for the management of schizophrenia were also more likely to have high levels of optimism (OR=3.6, CI=1.4–9.3, p=.009). Specifically, familiarity with the American Psychiatric Association guidelines (OR=2.1, CI=1.0–4.4, p=.04) and the Texas Medication Algorithm Project (OR=1.8, CI=1.1–2.9, p=.02) were significantly associated with high levels of optimism toward second-generation antipsychotics.
The sources of information psychiatrists considered to influence their opinions toward second-generation antipsychotics did not differ between optimism groups with the exception of practice guidelines. Psychiatrists who cited practice guideline recommendations as one of the top four influential factors in shaping clinical opinion were more likely to be optimistic toward second-generation antipsychotics (OR=1.9, CI=1.1–3.3, p=.02).
There was no significant difference between psychiatrists' choice of a particular second-generation antipsychotic and their overall optimism toward these medications as a group. Among the second-generation antipsychotics, psychiatrists were most likely to use a medication that they believed would offer an improvement in positive symptoms after a failed trial of oral haloperidol (83%).
Discussion
Our study found that among psychiatrists surveyed between September 2003 and January 2004 virtually all used second-generation medications as their first-line treatment for schizophrenia. The findings indicate that psychiatrists are optimistic about the ability of second-generation medications to succeed where first-generation antipsychotics have failed and that psychiatrists report familiarity with the treatment guidelines for schizophrenia and report frequent contact with pharmaceutical representatives, both of which were associated with higher levels of optimism.
Consistent with treatment guidelines (
1,
2,
3,
4 ) almost all psychiatrists reported using second-generation antipsychotics as first-line agents in schizophrenia. Research has suggested that there may be a therapeutic advantage of second-generation over first-generation antipsychotics in treating global psychopathology and cognitive, negative, and mood symptoms (
1 ). However, large clinical trials, including CATIE and the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (
8,
9 ), meta-analyses, and reviews (
11,
12 ) have raised uncertainty about the magnitude of benefits conferred by second-generation antipsychotics.
Several studies have examined sources of information contributing to the adoption of new prescription drugs by general practitioners. Although the pharmaceutical industry, particularly through pharmaceutical representatives, is often the first source of information regarding new medications, the ultimate decision to prescribe a medication has been related to various other information sources, including data from journal articles, other medical literature, the recommendation of colleagues, and prescribing guidelines (
13 ).
Psychiatrists in this survey frequently regarded research and treatment guidelines as contributors to their optimism for second-generation antipsychotics to offer clinical improvement in positive psychotic symptoms after a failed trial of oral haloperidol. However, according to treatment recommendations and recent literature reviews, there is no definitive evidence that patients whose positive symptoms have not responded to a first-generation antipsychotic will respond to any of the second-generation antipsychotics, with the notable exception of clozapine (
2,
11,
12 ).
Although frequent contact with pharmaceutical representatives was associated with higher levels of optimism toward second-generation antipsychotics, only a quarter of psychiatrists cited pharmaceutical representatives or advertisements as influential. Because psychiatrists were relatively unlikely to report that pharmaceutical recommendations play a role in their opinion, it is possible that some psychiatrists are not entirely aware of the influence of pharmaceutical marketing on their assessment of pharmacological options.
Prior studies have suggested that the pharmaceutical industry may have a greater influence on shaping views than is commonly acknowledged by physicians. Consistent with this study, 62% of physicians surveyed by Avorn and colleagues (
14 ) indicated that scientific articles were very important in shaping their opinions and prescribing practices, whereas only 20% cited pharmaceutical representatives as very important. However, a majority of those physicians gave opinions that were favorable toward pharmaceutical companies and unsupported by scientific articles (
14 ). The findings reported in this study are consistent with the view that the pharmaceutical industry influences physician attitudes and practices. Prior studies have estimated that physicians interact with pharmaceutical representatives approximately four times each month (
15 ). The pharmaceutical industry also finances more than one-half of the costs for formal programs of continuing medical education (
16 ) and provides most of the funding for clinical trials (
16 ). Physicians who author clinical practice guidelines also often receive financial support to perform research or serve as consultants for pharmaceutical companies (
17 ). Of course, our findings do not establish that frequent contact with pharmaceutical representatives directly influences prescribing practices.
This study presented here is constrained by several limitations. The study examined optimism concerning the advantage of second-generation antipsychotics over haloperidol in treatment-resistant positive symptoms and did not evaluate physician opinion toward other treatments, such as first-generation antipsychotics, or toward other symptom domains frequently considered in choosing among these medications (such as negative symptoms, cognition, or side effect profiles). It also did not evaluate quality of health care or clinical outcomes of patients with schizophrenia. Prescribing preferences and reported professional activities among psychiatrists were determined through self-reports rather than direct observation, and we have no means of assessing the extent to which social desirability influenced survey responses. Psychiatrists were asked to report on the medication they used most often in treating schizophrenia, which is likely to underestimate the percentage of physicians who use other agents, such as first-generation antipsychotics. The effects were not examined for several psychiatric practice characteristics, such as solo versus group practice, fee-for-service care versus prepaid care, and self-employed versus employed by an organization. Although we adjusted the results for nonresponse related to known psychiatrist characteristics, we cannot exclude the possibility that response patterns are related to unmeasured respondent characteristics that confound group comparisons.
Conclusions
Widespread optimism existed in our small sample of American psychiatrists concerning the effectiveness of newer second-generation antipsychotics in improving the symptom control of patients who have persistent positive symptoms despite optimal treatment with a first-generation antipsychotic medication. This optimism was associated with contact with pharmaceutical representatives and reported familiarity with treatment guidelines in the management of schizophrenia. An important challenge ahead is to assess through prospective practice-based research the factors that drive psychiatrist prescribing practices in treatment-resistant schizophrenia and the influence of pharmaceutical representative contact and treatment guideline adherence on the costs and clinical effectiveness of the routine management of schizophrenia.
Acknowledgments and disclosures
This research was supported by grant NIH-MH-61530 from the National Institutes of Health to Dr. Olfson.
Dr. Marcus has received research support from Ortho-McNeil Janssen Scientific Affairs and has been a consultant for Eli Lilly and Company, Bristol-Myers Squibb,and Ortho-McNeil Janssen Scientific Affairs. Dr. West has received research support from AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, Forest Laboratories, Janssen Pharmaceuticals, Pfizer, Sanofi-Aventis, and Wyeth Pharmaceuticals. Dr. Wilk has received research support from a consortium of industry supporters, including AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, Forest Laboratories, Janssen Pharmaceuticals, Pfizer, and Wyeth Pharmaceuticals. Dr. Olfson has been a consultant for Eli Lilly and Company, Bristol-Myers Squibb, Pfizer, and Ortho-McNeil Janssen Scientific Affairs. Dr. Olfson has also received research or grant support from Eli Lilly and Company and Bristol-Myers Squibb and has been on the speaker's bureau for Janssen Pharmaceuticals. Dr. Arbuckle and Dr. Gameroff report no competing interests.