Sildenafil citrate (Viagra) is an oral medication for the treatment of erectile dysfunction caused by diverse factors. It selectively inhibits phosphodiesterase type 5, which is specific for cyclic guanosine monophosphate (c-GMP). By inhibiting c-GMP catabolism in cavernosal smooth muscle, it enhances c-GMP activity, which is stimulated by nitric oxide transmission (
1).
Although sildenafil is currently indicated for male erectile dysfunction, progress in understanding sexual function suggests analogous mechanisms for female sexual dysfunction (
2). For women for whom sildenafil is prescribed, reports describe increased vaginal vascular engorgement, enhanced clitoral responsiveness, and improved lubrication (
3). Such reports suggest further study of sildenafil's ability to alleviate female sexual dysfunction induced by antidepressant medications.
Sexual dysfunction is a common and troublesome side effect associated with selective serotonin reuptake inhibitors (SSRIs) and other classes of antidepressants. Its occurrence frequently results in medication switching, discontinuation, or dosage reductions to ineffective levels. Approximately 50 percent of patients of both genders experience some degree of sexual dysfunction while taking SSRIs; the most common complaints among women include decreased libido, difficulty with lubrication, dyspareunia (pain during intercourse), and delayed orgasm or anorgasmia (
4). Such adverse medication effects are overrepresented in women because of their higher rates of major depression and related disorders. Management of antidepressant-induced sexual dysfunction for patients requiring these agents could improve treatment effectiveness by increasing compliance and decreasing relapse or recurrence.
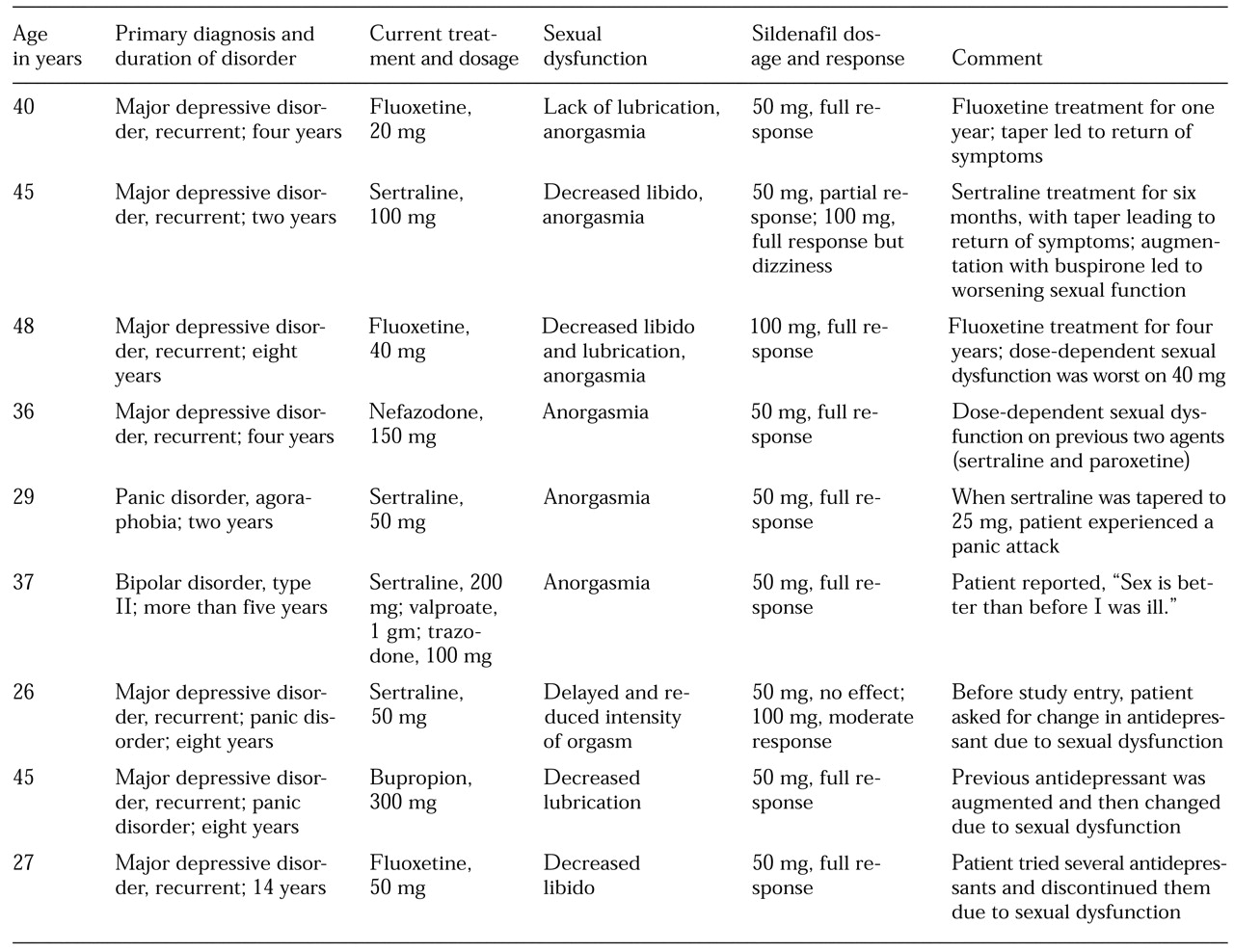
We report on offering sildenafil in open fashion to ten women who developed symptoms of sexual dysfunction as a consequence of treatment with antidepressant agents.
Methods
Patients were recruited from outpatient psychiatry settings at the schools of medicine at the University of New Mexico and Texas Tech University between May 15 and August 15, 1998. Clinicians were asked to identify female patients between the ages of 18 and 60 who were in stable relationships and who developed sexual dysfunction, particularly anorgasmia, with or without other sexual disturbances (loss of libido, lubrication difficulties, or painful intercourse) while being effectively treated with antidepressant medication. Subjects had to be taking the antidepressant at a stable dosage for at least six weeks, with improvement of the presenting condition, and they had to have experienced sexual side effects continuously for more than four weeks.
Exclusion criteria were concurrent unstable medical illness, poor overall physical health, pre-existing sexual dysfunction (other than that associated with an antidepressant), psychiatric disorder not under control, previous or current alcohol or substance abuse or dependence, diabetes mellitus, neurologic disorder, genital anatomical defects, or pregnancy. Previous stroke, myocardial infarction, or use or likely use of any nitrate also explicitly excluded entry into the study.
Ten women patients were identified and gave informed consent. Sexual function was assessed in investigator-conducted interviews. Each patient was given three 50 mg tablets of sildenafil and instructed to take one tablet at least one hour, and not more than two hours, before sexual intercourse. If the medication did not reverse the sexual dysfunction, the patient was instructed to take two tablets (100 mg) in the same time frame before the next sexual encounter. After the initial interview, one of the ten women revealed that her sexual dysfunction of recent onset was not a direct effect of major depression or antidepressant treatment but resulted from a significant relationship problem with her husband. Thus the study sample was reduced to nine women.
Discussion and conclusions
This report adds to findings of the reversal of male and female antidepressant-induced sexual dysfunction by sildenafil (
5). The women in our study developed sexual dysfunction as a side effect of prescribed antidepressant treatment. The adverse effects were heterogeneous and involved the domains of libido, arousal (sensitivity, engorgement, and lubrication), orgasm, and experience (pain, discomfort, or lack of pleasure).
Depression itself often leads to disturbances of sexual function and may obscure the detection of disturbances due to other causes. Antidepressant treatment may resolve a patient's depression but also may produce sexual dysfunction in a similar or other domain. Three women reported that before entering the study their antidepressant dosage was reduced in an effort to reverse the associated sexual dysfunction; however, the reduction led to a return of presenting symptoms. In three other cases, the antidepressant was changed, but sexual dysfunction recurred with the second agent.
Sildenafil consistently improved these iatrogenic adverse effects. An added benefit was that the patients were able to continue the antidepressant regimen that improved their presenting condition and were able to continue its use at a therapeutic dosage, which is indicated for six to nine months after remission. Sildenafil would also be helpful for prolonged treatment in recurrent cases of depression. With the exception of occasional mild transient headache and dizziness, no other significant side effects were observed with brief sildenafil treatment.
A relationship between sexual dysfunction and SSRIs is well established, but such side effects are not exclusive to that class of drug (
6). Other classes of antidepressants also implicated include tricyclics, monoamine oxidase inhibitors, and newer novel agents, although less sexual dysfunction has been noted with bupropion. Sexual dysfunction has been particularly underrecognized and underreported among women for a variety of reasons. Some are embarrassed to report it, some misattribute sexual dysfunction to depression, and in some cases it is masked by other side effects of medication or is inadequately assessed by the treatment provider.
However, the widespread use of SSRIs in primary care and specialty sectors has increased awareness of this problem. Nevertheless, 15 to 45 percent of patients given a prescribed antidepressant fail to complete treatment with the initial agent (
7); approximately two-thirds discontinue treatment due to adverse side effects (
8). From 45 to 60 percent of patients on SSRIs develop some form of sexual dysfunction (
4). Effective management of these side effects may benefit outcome by improving compliance and reducing the morbidity and mortality associated with the disorders for which antidepressants are prescribed but too commonly discontinued.
Sildenafil appears to be an effective intervention for antidepressant-induced sexual dysfunction. This agent offers an additional approach to existing strategies for managing sexual dysfunction, including use of cyproheptadine as a serotonin antagonist, bethanechol as a cholinergic agonist, amantadine as a dopamine agonist, methylphenidate as a stimulant, yohimbine as an alpha-2 antagonist, and ginkgo biloba as an herbal treatment, as well as augmentation or substitution of other agents such as bupropion (
2,
9). With "drug holidays," discontinuation symptoms may emerge (
10). Further studies are needed to determine whether sildenafil can reverse sexual dysfunction associated with pharmacological treatments in which the dysfunction results from other mechanisms, such as dysfunctions associated with neuroleptic-induced hyperprolactinemia.
We recognize the methodological limitations of the study, including open assignment to treatment, lack of a placebo group, small sample size, and lack of a standardized assessment instrument. Thus we caution against overreaching inferences about the sildenafil's effects beyond its primary action in the area of increased vascular function. We do not know whether reversal of antidepressant-induced sexual dysfunction in women would persist with repeated use of sildenafil. In addition, the study did not compare the effectiveness of sildenafil and existing interventions for antidepressant-induced sexual dysfunction. Because improved functioning in one sexual domain is generally associated with improvements in others, improved arousal (engorgement and lubrication) might extend through halo or placebo effects to improved orgasmic functioning. A subset of patients with antidepressant-induced sexual dysfunction may develop tolerance of sexual side effects over time, and sexual dysfunction may abate. In such cases, limited use of sildenafil on an as-needed basis may complement a "watchful waiting" strategy (
2).