Schizophrenia is a severe mental illness that may result in long-term disability and heavy disease burden (
1). Although antipsychotics are the standard treatment for schizophrenia, benzodiazepines are often concomitantly prescribed for anxiety, sleep disturbance, side effects of antipsychotics, and rapid tranquilization (
2,
3). However, a systematic review of 31 studies that included more than 2,000 participants demonstrated that the addition of benzodiazepines to antipsychotics did not have a consistently therapeutic effect on schizophrenia symptoms and did not lead to overall improvement (
4). In addition, persons with schizophrenia may be particularly prone to abuse of and dependence on benzodiazepines (
5,
6). Furthermore, because patients with schizophrenia may already be neurocognitively compromised (
7,
8), underlying cognitive impairments may be aggravated by long-term use of benzodiazepines (
9,
10).
Information about long-term use of benzodiazepines by persons with schizophrenia is scarce, and virtually no study has specifically examined clinical factors associated with long-term use and the context in which benzodiazepines are prescribed (
11,
12). Reported prevalence rates of benzodiazepine use among patients with schizophrenia vary widely, from 14% to 41% (
11–
15). Among large-scale observational clinical studies, the CATIE study (Clinical Antipsychotic Trials of Intervention Effectiveness) reported baseline use rates of anxiolytics and hypnotics of 18% and 16%, respectively, among patients with schizophrenia (
11). In the Worldwide Schizophrenia Outpatient Health Outcomes study, rates of anxiolytic-hypnotic use varied from 16% in North Africa and the Middle East to 57% in East Asia, indicating that prescription of benzodiazepines may be highly variable by country (
12,
14). Furthermore, because the rates reported are point prevalence rates, actual prevalence of use of benzodiazepines over an extended period is likely to be underestimated. Indeed, a population-based survey using administrative data indicated a one-year prevalence rate of benzodiazepine use of 41% among patients with schizophrenia (
13).
The wide range of prevalence estimates may result from heterogeneity in the definition of benzodiazepine use, characteristics of the study population, and study duration. Previous clinical drug trials applied strict clinical inclusion criteria, and persons who used multiple prescription drugs were excluded. Therefore, even though these studies involved large samples, the findings may not be representative of people with schizophrenia in general. Use of data from an unselected, population-based sample of patients with schizophrenia may therefore be an important step in avoiding selection bias.
The National Health Insurance Research Database in Taiwan (NHIRD-TW) is a population-based, health insurance claims database that contains complete information on use of health care, including comprehensive information on prescriptions, for 98% of the population of Taiwan. Because every prescription must be submitted and registered, the data are not subject to reporting bias and provide an unusual opportunity to examine the overall pattern of benzodiazepine prescription and factors associated with use of benzodiazepines in the population of persons with schizophrenia in Taiwan. Therefore, this study aimed to describe the pattern of benzodiazepine use among patients with schizophrenia and explore clinical factors associated with use of benzodiazepines. More specifically, because long-term use of benzodiazepines is a clinical concern, factors associated with such use were examined.
Methods
Data source
Data for the study were obtained from the ambulatory claims database of the NHIRD-TW. Taiwan launched a single-payer National Health Insurance program on March 1, 1995. In 2005, a total of 22.72 million individuals, 98% of the population, were enrolled in the program. Prescription records provide details about the medication used, dosage, date of prescription, days of supply dispensed, and up to three diagnoses with
ICD-9-CM codes. The NHIRD-TW has been shown to be useful for the study of several diseases, including schizophrenia (
16). In this study, we used one subset of NHIRD-TW, the Longitudinal Health Insurance Database 2005 (LHID2005), which contains original claims data for one million beneficiaries randomly sampled from the 2005 Registry of Beneficiaries of the NHIRD-TW. Thus the LHID2005 includes about 5% of the total population. The gender and age distributions of the sampled participants are not significantly different from those of the general population (
10).
Study population
From the LHID2005, a total of 4,186 patients with schizophrenia aged 18 and older were identified on the basis of at least one ambulatory claims record with a schizophrenia-spectrum disorder diagnosis (ICD-9-CM 295.x). Descriptive data were not available for patients who were not covered by the National Health Insurance program for more than 90 days (N=33) or who were hospitalized for more than 90 days in 2005 (N=464), and these patients were excluded. The final analyses included 3,690 patients with schizophrenia.
Measures
Demographic and clinical characteristics.
Several demographic and clinical variables were included in the analysis: age, gender, duration of illness, comorbid medical and psychiatric disorders, and concomitant psychotropic medications. Duration of illness was assessed by the number of years between the date that schizophrenia was diagnosed and the first day of the study year, January 1, 2005. For patients first diagnosed as having schizophrenia in 2005, the duration of illness was counted as zero. Comorbid medical conditions were indicated with a modified version of the Charlson Comorbidity Index score (
17,
18), which was the sum of the weighted score of 19 comorbid conditions. Comorbid psychiatric disorders examined were anxiety disorders (
ICD-9-CM codes 300.x, except 300.4, neurotic depression), mood disorders (
ICD-9-CM codes 296.x and 300.4), sleep disorders (
ICD-9-CM codes 307.4x and 780.5x), and substance use disorders (
ICD-9-CM codes 291.x 292.x, 304.x, 305.x, 375.5, 425.5, 535.3x, and 571.0–571.3).
Benzodiazepine use.
Benzodiazepine exposure status was determined by whether benzodiazepines were prescribed (including refills) in 2005. Benzodiazepines included those listed in the Anatomical Therapeutic Chemical (ATC) classification system in the section on benzodiazepine derivatives (N03AE, N05BA, and N05CD) and benzodiazepine-related drugs (N05CF) (
19). Although the benzodiazepine-related drugs, so-called Z-hypnotics, were not classified as benzodiazepines, they had potential for misuse and thus were included as benzodiazepines in our analyses. Defined daily dose (DDD)—that is, “the assumed average maintenance dose per day for a drug used for its main indication in adults” (
19)—was used as a dose standard unit for various benzodiazepines (
20). The cumulated benzodiazepine dose was the sum of the DDDs of all benzodiazepine prescriptions during the study year. In addition, an individual's cumulative period of benzodiazepine exposure was defined as the sum of the number of days each benzodiazepine was prescribed. Periods of overlap of two or more benzodiazepine prescriptions were counted only once. On the basis of cumulative days of benzodiazepine use, study participants were further categorized into short-term users and long-term users. Short-term users had cumulated prescription of less than 180 days, and long-term users had cumulated prescription of 180 days or longer in the one-year period (
21). The types of benzodiazepines used in the study year were summed for each individual.
Antipsychotic use and concomitant medications.
Patients with schizophrenia were further divided into three groups according to the number of antipsychotics prescribed in the study year (ATC code N05A): nonuser, one antipsychotic, and two or more antipsychotics. In addition, concomitant use of several medications was assessed: antidepressants (N06A), anticholinergic agents (N04A), and mood stabilizers, which included lithium (N05AN01), carbamazepine (N03AF01), valproic acid (N03AG01), and lamotrigine (N03AX09).
Statistical analysis
Demographic and clinical characteristics, including age, sex, Charlson Comorbidity Index score, comorbid psychiatric disorders, number of antipsychotics used, and concomitant use of psychotropic agents, were compared between the benzodiazepine use groups with chi square tests and Student's t tests. The pattern of benzodiazepine use was represented by cumulative days of use, cumulative dosage, and average daily dosage (DDD).
Multivariate logistic regression was used to explore clinical characteristics associated with benzodiazepine prescription. The logistic regression model included a binary dependent variable, whether or not benzodiazepine was prescribed, and independent variables, including age categories (18–24, 25–44, 45–64, and 65 years and older); sex; duration of schizophrenia in years (less than one, one to four, and five or more years); Charlson Comorbidity Index score (0, 1, and 2 or higher); presence of an anxiety, mood, sleep, or substance use disorder (yes or no); number of antipsychotics used (0, one, and two or more); and use of anticholinergic agents, antidepressants, and mood stabilizers (yes or no).
A second multivariate logistic regression was used to compare the characteristics of long- and short-term benzodiazepine users. The model used a new binary dependent variable (long-term versus short-term use), and the same independent variables included in the previous logistic regression model.
All analyses were performed by using SAS version, 9.1. The level of statistical significance for all analyses was set at ≤.05.
Results
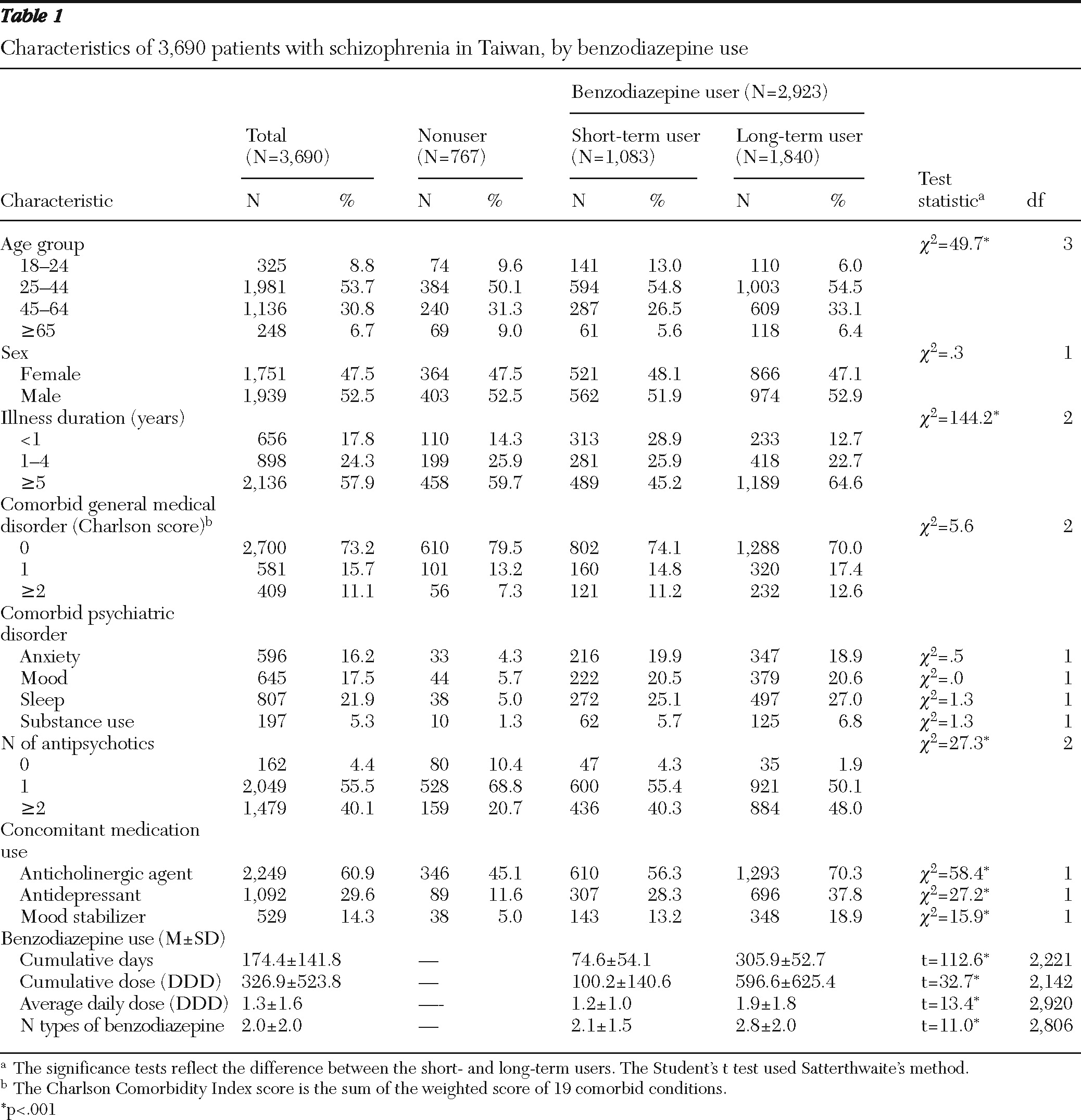
Data for 3,690 patients with schizophrenia were included in the analyses. Their mean±SD age was 42.2±13.6 years. As shown in
Table 1, 52.5% were men, and 57.9% had had a diagnosis of schizophrenia for five or more years. A substantial proportion of the sample had comorbid psychiatric disorders: 16.2% had anxiety disorders, 17.5% had mood disorders, 21.9% had sleep disorders, and 5.3% had substance use disorders. In the sample, 55.5% received prescriptions for one antipsychotic drug, 40.1% had prescriptions for two or more antipsychotics, and 4.4% had no prescriptions for antipsychotics. A variety of other psychotropic agents were prescribed: 14.3% of patients received mood stabilizers, 29.6% received antidepressants, and 60.9% received anticholinergic agents.
During the study year, benzodiazepines were prescribed for 2,923 patients (79.2%), and 1,840 (62.9%) of them were long-term users. Among the 3,690 patients, the mean number of cumulative days of benzodiazepine use was 174.4, the benzodiazepine cumulative dose (DDD) was 326.9, the average daily dose was 1.3 DDD; and the number of types of benzodiazepines used was 2.0. Compared with short-term users, long-term users had prescriptions for more types of benzodiazepine (2.8 compared with 2.1) and had a significantly higher average daily dose (1.9 compared with 1.2 DDD).
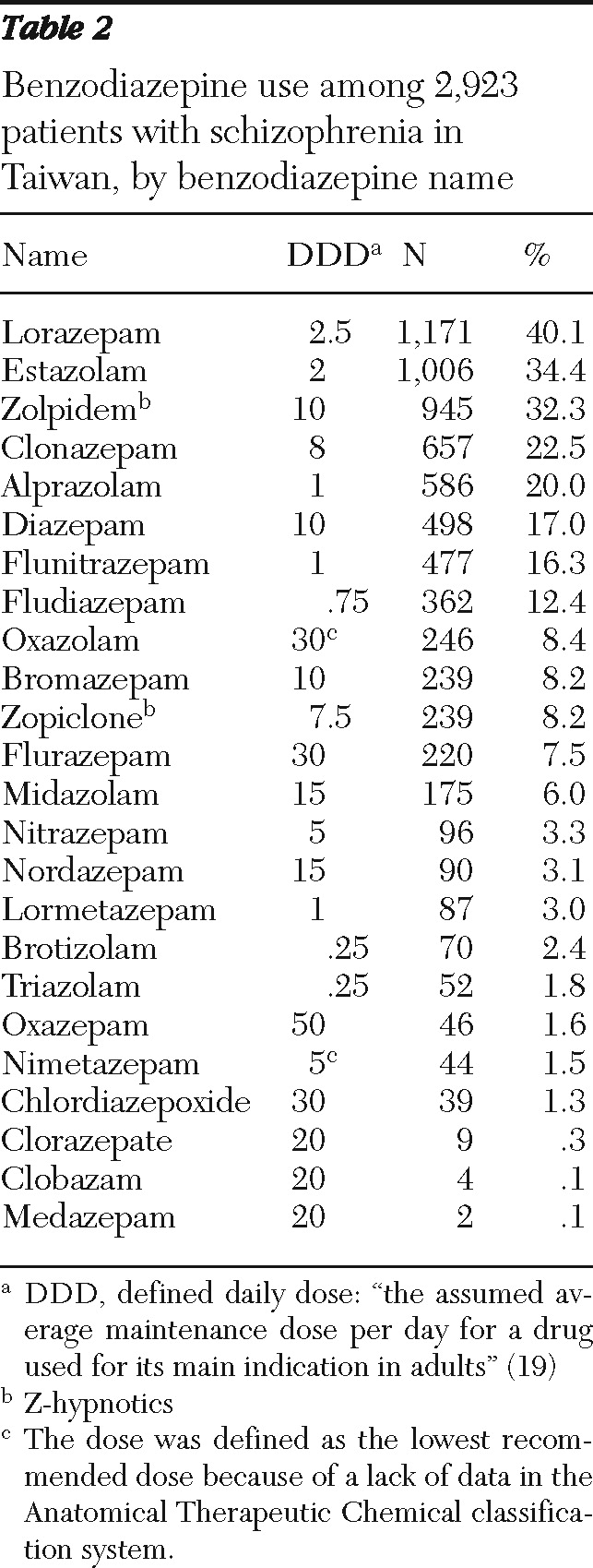
The types of benzodiazepines prescribed are listed in
Table 2. Among the benzodiazepines, the three most frequently prescribed were lorazepam, estazolam, and zolpidem (prescribed to 40.1%, 34.4%, and 32.3%, respectively, of the patients who were prescribed benzodiazepines). Z-hypnotics accounted for a substantial proportion of the cumulative benzodiazepine dose (66.0±222.4; 20.2% of the cumulative dose).
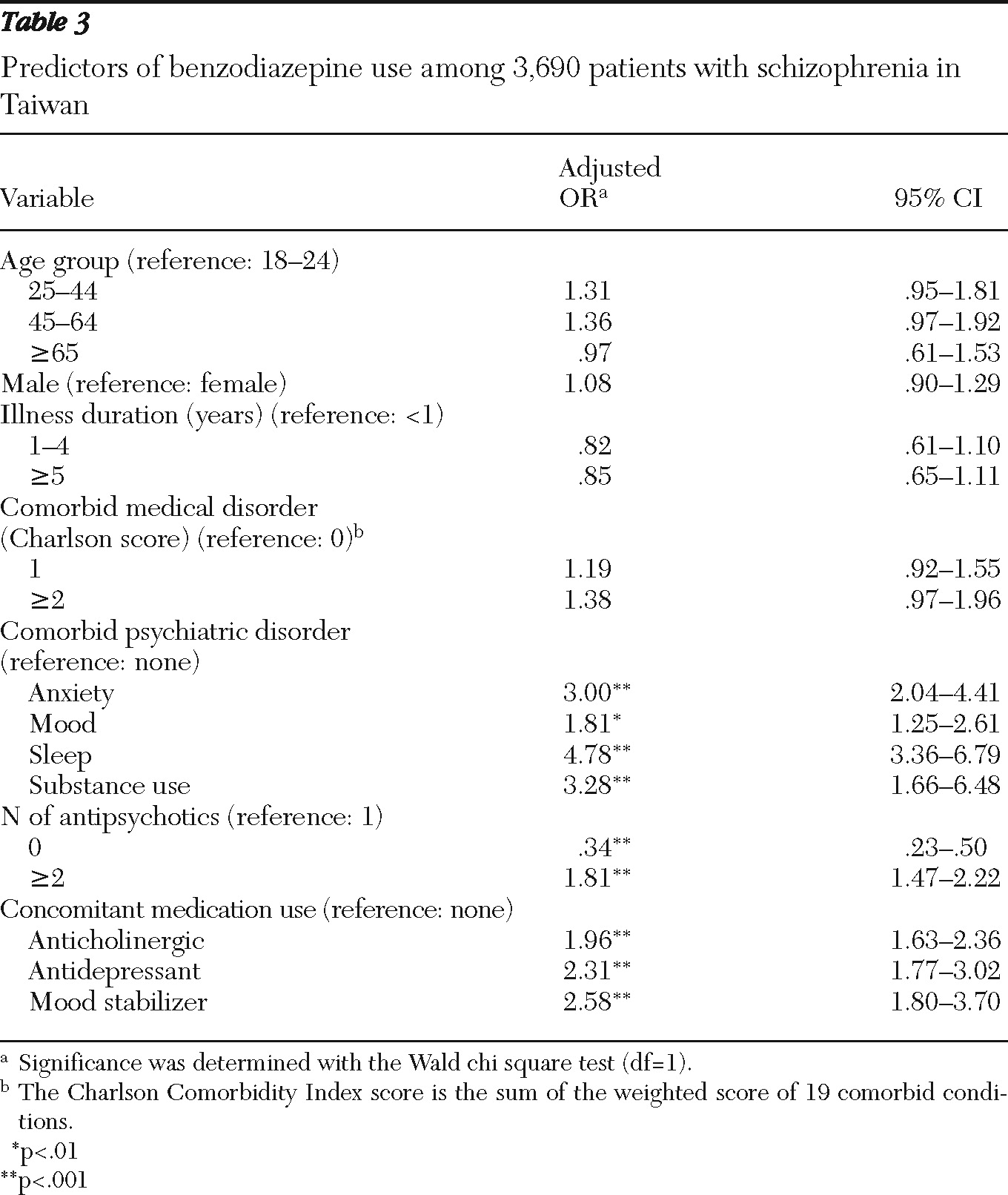
Multivariate logistic regression analyses revealed several factors that were associated with higher odds of benzodiazepine prescription: a comorbid psychiatric disorder, use of two or more antipsychotics, and concomitant use of a mood stabilizer, antidepressant, or anticholinergic agent (
Table 3). Although patients with recent-onset schizophrenia (illness duration of less than one year) were more likely than those with longer illness to be prescribed benzodiazepines, the differences did not reach statistical significance.
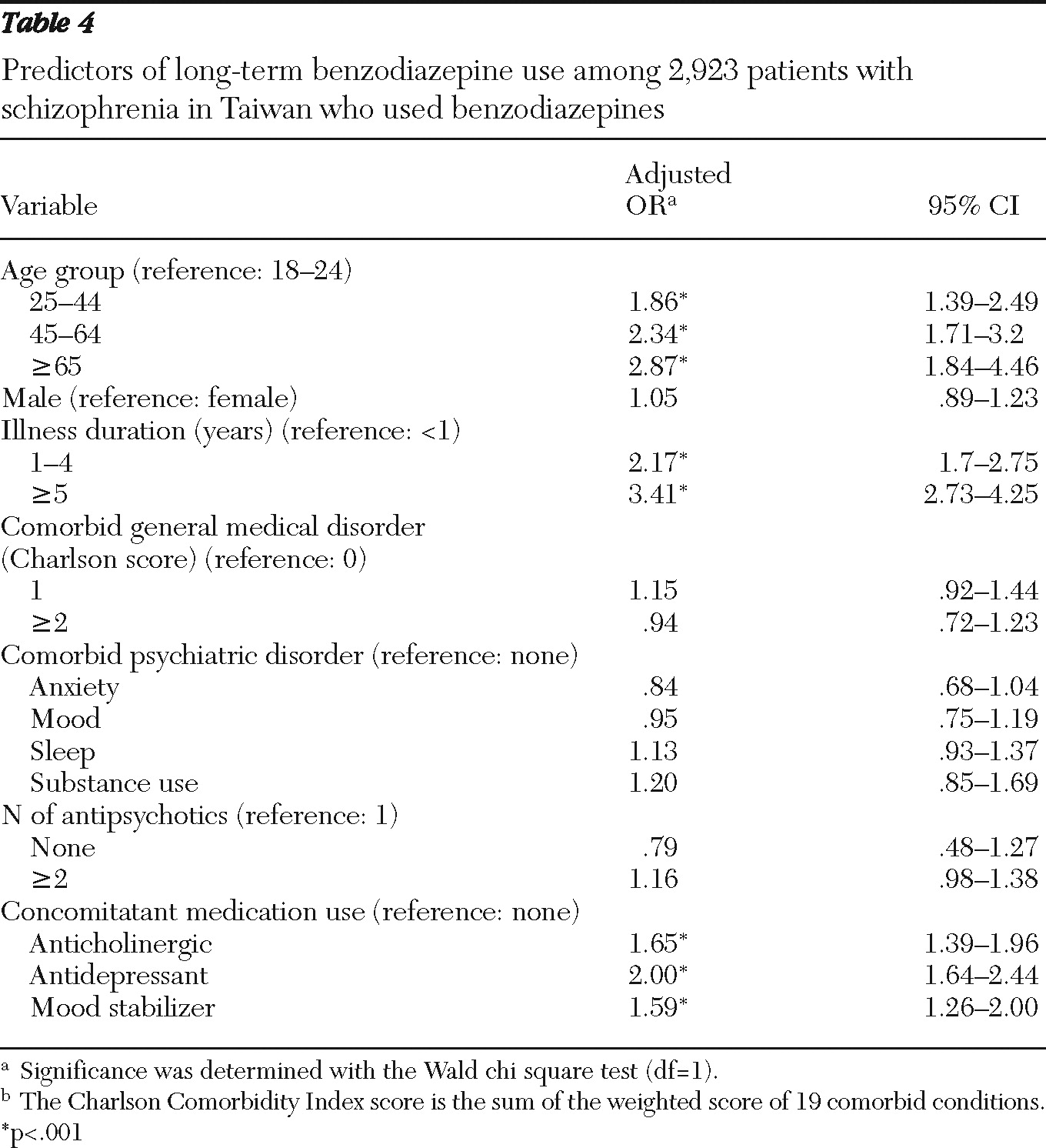
Compared with short-term benzodiazepine users, long-term users were older, had a longer duration of illness, and were more likely to have concomitant use of a mood stabilizer, antidepressant, or anticholinergic agent; however, the groups did not differ significantly in gender, comorbid psychiatric or general medical disorders, or number of antipsychotics used (
Table 4).
Discussion
Analyses of data from a comprehensive nationwide population-based claims database found that 79.2% of patients with schizophrenia in Taiwan received at least one benzodiazepine prescription during the study year. Among these, 62.9% were long-term benzodiazepine users (180 days or longer). Factors associated with prescription of benzodiazepines were comorbid psychiatric disorder (mainly anxiety and sleep disorders), number of antipsychotics used, and concomitant use of other psychotropic agents. Compared with short-term benzodiazepine users, long-term users were older, had a longer duration of illness, and used other psychotropic agents concomitantly.
The overall prevalence rate of 79.2% for benzodiazepine use among patients with schizophrenia in this study is much higher than the rate of 18.6% in the general population in Taiwan (
22). In addition, the proportion of long-term users in the sample is also much higher than the proportion of long-term benzodiazepine users in the general population (10% to 15%) (
22), suggesting that once patients with schizophrenia start using benzodiazepines, they are more likely to stay on them for a longer period than persons in the general population. The results are consistent with previous reports that benzodiazepines are used more frequently by individuals with schizophrenia or other severe mental illnesses (
5,
13,
22,
23). In addition, the higher average daily dose (DDD) among long-term users and the likelihood of receiving more types of benzodiazepines suggest that as the disease progresses, the accumulated dosage also increases.
It is notable that the prevalence rates of benzodiazepine use in this study are substantially higher than the rates reported for patients with schizophrenia in other countries (
13,
15), which likely reflects the fact that this study was an assessment of the cumulative prevalence rate over one year, whereas other cross-sectional studies provided point prevalence rates at the time the studies commenced. The prevalence of benzodiazepine use in the general population in Taiwan is also higher than in other countries (
21,
22,
24). The high prevalence rate might be attributable to the lack of a specific recommendation for duration of benzodiazepine use in treatment guidelines in Taiwan (
25). It also might reflect less public concern about overuse of benzodiazepines in the general population and among patients with schizophrenia. Limiting benzodiazepine prescriptions to two to four weeks is usually highlighted in treatment guidelines based on expert opinion (
26).
Factors shown to be associated with benzodiazepine use in this study are in line with common treatment principles for patients with schizophrenia. The higher rate of benzodiazepine prescription associated with comorbid anxiety, mood, and sleep disorders and with concomitant use of antidepressants or mood stabilizers is consistent with everyday clinical practice of benzodiazepine prescription to treat insomnia and anxiety among patients with schizophrenia (
2,
3,
11). In addition, patients who use anticholinergic agents may have more severe side effects from antipsychotics, and benzodiazepines may be prescribed more frequently in this group to alleviate such adverse effects. Benzodiazepines were more likely to be prescribed among patients using more than one antipsychotic, which may reflect more severe side effects associated with antipsychotic use or more severe clinical symptoms necessitating use of benzodiazepines as an adjuvant treatment for patients who do not respond to antipsychotic monotherapy (
3). This would also explain the finding that patients who were not using antipsychotics were less likely to be prescribed a benzodiazepine; these patients may be clinically stable, with no need for antipsychotics or benzodiazepines. However, it is also possible that people with persistent symptoms had poor treatment compliance and did not attend follow-ups, hence not receiving antipsychotics or benzodiazepines.
Although the difference was not statistically significant, patients with recent-onset schizophrenia (within one year) were more likely than those with a longer duration of illness to receive a benzodiazepine prescription. Patients in the early acute stage of schizophrenia may have more severe symptoms and thus a greater likelihood of receiving a benzodiazepine prescription. Alternately, the greater likelihood may reflect clinicians' reluctance during the initial phase to use a high dosage of antipsychotics; instead they may have resorted to benzodiazepines as an adjunctive medication for nonspecific symptoms. This hypothesis must be further tested by examining relationships between dosages of antipsychotics and benzodiazepines. A positive association would indicate that benzodiazepine use is related to more severe clinical symptoms.
Another finding was that patients who used benzodiazepines plus other psychotropic agents tended to be long-term benzodiazepine users. It was demonstrated that patients with schizophrenia who exhibited symptoms of anxiety or depression at the same time were likely to receive a benzodiazepine prescription; the combined use of multiple psychotropic agents hence might reflect difficulties in treating the more complex and severe clinical symptoms when there are comorbid psychiatric disorders (
11). Combined use is of clinical concern because it exposes patients to side effects of individual medication as well as those caused by complex drug-drug interactions. For long-term benzodiazepine users, side effects related to drug-drug interactions are particularly relevant, given the relatively high dosages and multiple types of benzodiazepines that they were receiving. Research has suggested that the therapeutic effect of benzodiazepines is short-term at best (
3,
4) and that discontinuation of benzodiazepine use during the day can improve cognition without worsening psychotic symptoms (
27). Therefore, clinicians should periodically review the need for benzodiazepines and potential cognitive side effects, especially among patients with complex and severe symptoms.
Long-term benzodiazepine use was associated with longer duration of illness and older age. Schizophrenia is a chronic disease; as the illness progresses, patients are likely to experience fluctuations in clinical symptoms and relapses, which increase the likelihood of receiving a benzodiazepine prescription. Also, as the number of relapses increases, clinicians may be reluctant to discontinue benzodiazepine use, even among patients whose clinical condition has been stabilized. Another possible contributing factor is that some patients may develop benzodiazepine abuse or dependence after prolonged use, particularly patients with schizophrenia (
6). The findings may thus partly reflect the difficulty of discontinuing benzodiazepine use over the long-term course of illness.
The association of long-term benzodiazepine use with older age is consistent with the findings from the general population of Taiwan (
22) and of other countries (
28). A previous study found that the association between age and benzodiazepine use may be mediated by the presence of a comorbid medical illness (
22), a finding not replicated in the study reported here. Patients in this study were relatively young, and the prevalence of medical illness was low; thus the effect of medical illness was small and insignificant.
An important limitation in administrative databases should be considered when interpreting the results of this study. Patients with schizophrenia were identified only by an
ICD-9-CM diagnosis in claims records, which raises a concern about diagnostic accuracy. In general, use of claims data to identify patients with a diagnosis of schizophrenia has been shown to be relatively accurate, given the characteristic clinical manifestations and course of schizophrenia (
29). Specifically, the accuracy of diagnostic coding of schizophrenia in the NHIRD was supported by the following evidence. First, of the 3,690 patients in the sample, 3,378 (91.5%) had two or more claims records with diagnoses of a schizophrenia spectrum disorder, and 3,528 (95.6%) had received an antipsychotic prescription. Given the grave consequences of these clinical decisions, it is likely that both were made only after considerable deliberation. Second, patients who were hospitalized for more than 90 days were excluded from the study, which may have resulted in an underestimation of the prevalence rate of benzodiazepine use; hospitalized patients tend to have more severe clinical symptoms, which would increase the likelihood of their having a benzodiazepine prescription.
The presence of a substance use disorder was strongly associated with benzodiazepine abuse, but treatment of such disorders is not covered by the National Health Insurance plan. Thus the effect of substance abuse on the pattern of benzodiazepine use may not have been accurately estimated. However, patients with schizophrenia in this study who had comorbid substance use disorders were more likely than those without substance use disorders to be benzodiazepine users. Therefore, underreported substance use disorders among study patients may have led to an underestimation of the association between substance use disorders and benzodiazepine use. In addition, because of the study design, no causal attributions can be made about factors associated with patterns of benzodiazepine use. Also, use of claims data does not permit examination of individual physicians' benzodiazepine prescribing preferences; idiosyncratic individual factors may have contributed to prescription behaviors in an unsystematic way.
Despite these limitations, this is one of the first studies that specifically examined use of benzodiazepines by patients with schizophrenia in a national population database. The detailed prescription information and the relatively large sample permitted greater exploration than in previous studies of clinical populations of factors related to benzodiazepine prescription and long-term use. Thus the results more closely reflect the reality of benzodiazepine use by patients with schizophrenia.