Back matter
Glossary of Terms
- Adequate dose
- The dose of a medication at which therapeutic effects occurred when tested in clinical trials in a comparable population of subjects. This dose will differ for each medication and may need to be adjusted in an individual patient to address factors that would influence drug absorption, metabolism, elimination, or other pharmacokinetic properties.
- Adequate response
- A reduction in symptoms as a result of treatment that is associated with clinically significant benefit in functioning and/or quality of life. A reduction in symptoms of 50% or more is sometimes used as a threshold for adequacy of response.
- Agitation
- A state of excessive motor activity, verbal aggression, or physical aggression to oneself or others that is associated with observed or inferred evidence of emotional distress (definition adapted from Cummings et al. 2015).
- Antipsychotic medication
- One of a group of medications used in the treatment of psychosis. Some of the antipsychotic medications are also approved for use in other conditions such as mood disorders or Tourette’s syndrome. The first-generation antipsychotic (FGA) medications, sometimes referred to as “typical” antipsychotic medications, were the initial medications to be discovered. The FGAs include, but are not limited to, chlorpromazine, droperidol, fluphenazine, haloperidol, loxapine, perphenazine, thiothixene, thioridazine, and trifluoperazine. The second-generation antipsychotic (SGA) medications, sometimes referred to as “atypical” antipsychotic medications, include, but are not limited, to aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, iloperidone, olanzapine, paliperidone, quetiapine, risperidone, and ziprasidone. Within each group of antipsychotic medications, there is significant variability in the pharmacological properties, presumed mechanisms, and side effect profiles of specific drugs.
- Assessment
- The process of obtaining information about a patient through any of a variety of methods, including face-to-face interview, review of medical records, physical examination (by the psychiatrist, another physician, or a medically trained clinician), diagnostic testing, or history taking from collateral sources.
- Behavioral and psychological symptoms of dementia
- Signs and symptoms of disturbed perception, thought content, mood, or behavior that occur in the context of dementia (Finkel et al. 1996). Behavioral and psychological symptoms of dementia (BPSD) are distinct from the cognitive impairments of dementia and include agitation and psychosis as well as apathy, depression, anxiety, irritability, disinhibition, sleep disturbances, wandering, and disruptive or socially inappropriate behaviors (Kales et al. 2015). This set of symptoms has also been referred to as noncognitive neuropsychiatric symptoms of dementia (Kales et al. 2014).
- Comprehensive treatment plan
- A plan of treatment that is developed as an outgrowth of the psychiatric evaluation and is modified as clinically indicated. A comprehensive treatment plan can include nonpharmacological and pharmacological interventions. It is individualized to the patient’s clinical presentation, safety-related needs, concomitant medical conditions, personal background, relationships, life circumstances, and strengths and vulnerabilities. There is no prescribed format that a comprehensive treatment plan must follow. The breadth and depth of the initial treatment plan will depend on the amount of time and the extent of information that are available. The fully developed treatment plan will also vary in breadth and depth depending upon factors such as the needs of the patient and the setting in which care is occurring. Additions and modifications to the treatment plan are made as additional information accrues (e.g., from family, staff, medical records, and other collateral sources), and the patient’s responses to clinical interventions are observed.
- Dementia
- A degenerative condition characterized by the development of multiple cognitive deficits that include memory impairment and at least one of the following cognitive disturbances: aphasia, apraxia, agnosia, or a disturbance in executive functioning. The cognitive deficits cannot occur exclusively during the course of a delirium; they must be sufficiently severe to cause impairment in occupational or social functioning, and must represent a decline from a previously higher level of functioning (American Psychiatric Association 2000). The definition of major neurocognitive disorder, as used in the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM–5), is somewhat broader than the term dementia, in that individuals with substantial decline in a single domain can receive the diagnosis of neurocognitive disorder (American Psychiatric Association 2013).
- Nonpharmacological interventions
- Any of a wide variety of interventions other than medications. Nonpharmacological interventions include, but are not limited to, cognitive/emotion-oriented interventions (e.g., reminiscence therapy, validation therapy, simulated presence therapy, cognitive training and rehabilitation), sensory stimulation interventions (e.g., acupuncture, aromatherapy, light therapy, massage and touch therapy, music therapy, Snoezelen multisensory stimulation therapy), individualized behavioral reinforcement strategies, animal-assisted therapy, exercise, environmental modifications (e.g., reducing noise, decreasing clutter, removing access to sharp objects, establishing daily routines, providing orientation, improving lighting, increasing color contrasts), and caregiver support and education (Kales et al. 2015; Brasure et al. 2016). Nonpharmacological interventions do not include restraint or seclusion.
- Quantitative measures
- Clinician- or patient-administered tests or scales that provide a numerical rating of features such as symptom severity, level of functioning, or quality of life and have been shown to be valid and reliable.
- Surrogate decision maker
- The individual who is designated to make decisions on behalf of the patient in circumstances where the patient lacks the capacity to do so. The specific designation of and terminology used to describe a surrogate decision maker will depend on state and federal law.
References
Clinical Questions
Review of Supporting Research Evidence
| Antipsychotic | Symptom domain | Confidence | Effect | SMD (95% CI) |
|---|---|---|---|---|
Aripiprazole | BPSD | Moderate | Small | 0.20 (0.04, 0.35) |
Aripiprazole | Agitation | Low | Small | — |
Aripiprazole | Psychosis | Low | Nonsignificant | 0.14 (− 0.02, 0.29) |
Olanzapine | Overall BPSD | Low | Very small | 0.12 (0.00, 0.25) |
Olanzapine | Agitation | Moderate | Very small | 0.10 (0.07, 0.31) |
Olanzapine | Psychosis | Insufficient | Nonsignificant | 0.05 (− 0.07, 0.17) |
Quetiapine | Overall BPSD | Low | Nonsignificant | 0.13 (− 0.03, 0.28) |
Quetiapine | Agitation | Insufficient | Nonsignificant | 0.06 (− 0.14, 0.25) |
Quetiapine | Psychosis | Insufficient | Nonsignificant | 0.04 (− 0.11, 0.19) |
Risperidone | Overall BPSD | Moderate | Very small | 0.19 (0.00, 0.38) |
Risperidone | Agitation | Moderate | Small | 0.22 (0.09, 0.35) |
Risperidone | Psychosis | Moderate | Small | 0.20 (0.05, 0.36) |
SGAs overall | Overall BPSD | High | Very small | — |
SGAs overall | Agitation | Moderate | Small | — |
SGAs overall | Psychosis | Low | Very small | — |
| Comparison | Symptom domain | Confidence | Effect |
|---|---|---|---|
SGA vs. haloperidol | Overall BPSD | Low | No difference |
SGA vs. haloperidol | Agitation | Low | No difference |
SGA vs. haloperidol | Psychosis | Insufficient | Unable to determine |
Olanzapine or quetiapine vs. risperidone | Overall BPSD | Low | No difference |
Olanzapine or quetiapine vs. risperidone | Agitation | Low | No difference |
Olanzapine or quetiapine vs. risperidone | Psychosis | Insufficient | Unable to determine |
SGA vs. other comparators | Overall BPSD | Insufficient | Unable to determine |
SGA vs. other comparators | Agitation | Insufficient | Unable to determine |
SGA vs. other comparators | Psychosis | Insufficient | Unable to determine |
Lower doses vs. higher doses | Insufficient | Unable to determine | |
Continue on antipsychotic vs. change to placebo | Moderate | Small benefit for continued antipsychotic |
1A. Efficacy and Comparative Effectiveness of Second-Generation Antipsychotics for Overall BPSD
Second-Generation Antipsychotic Versus Placebo
Overview and Quality of Individual Studies
Aripiprazole
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence |
|---|---|---|---|---|---|---|
1A | Nursing home residents with MMSE scores 6–22 and NPI or NPI-NH score > 5 for hallucinations and delusions Interventions: placebo and three fixed doses of aripiprazole (2 mg, 5 mg, 10 mg) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial conducted in long-term care facilities internationally, including the United States and Canada | 487 subjects enrolled; data for 284 were analyzed | 10 weeks | Aripiprazole vs. placebo: total SMD = 0.16 (− 0.05, 0.37); psychosis SMD = 0.24 (0.03, 0.45); agitation SMD = 0.31 (0.10, 0.52) | 1, 2 | |
1A | Non-institutionalized subjects with Alzheimer’s disease and psychosis Interventions: placebo and aripiprazole at doses ranging from 2 to 15 mg/day (average dose: 10 mg/day) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial conducted in the United States, Canada, Western Europe, and Australia/New Zealand | 208 subjects; 83% completed the trial with no difference in dropouts between placebo and aripiprazole | 10 weeks | Aripiprazole vs. placebo: total SMD = 0.06 (− 0.21, 0.34); psychosis SMD = 0.16 (− 0.12, 0.43) | 3 | |
1A | Subjects with Alzheimer’s disease, residing in nursing homes, with psychosis Interventions: placebo, aripiprazole at doses ranging from 0.7 to 15 mg/day (average dose: 8.6 mg/day) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial conducted in long-term care facilities in the United States | 256 subjects enrolled; data for 151 were analyzed | 10 weeks, after 1-week washout | Aripiprazole vs. placebo: total SMD = 0.36 (0.11, 0.61); psychosis SMD = − 0.02 (− 0.27, 0.23); agitation SMD = 0.30 (0.05, 0.55) | 2 |
Olanzapine
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence |
|---|---|---|---|---|---|---|
1A | Subjects with Alzheimer’s dementia, vascular dementia, or mixed dementia, in outpatient or residential settings, with NPI or NPI-NH score > 5 on hallucination and delusion items Interventions: placebo vs. flexibly dosed olanzapine (2.5–10 mg/day; mean dose: 5.2 mg/day) or risperidone (0.5–2 mg/day; mean dose: 1.0 mg/day) Design: double-blind randomized trial Industry-sponsored multicenter trial in the United States | 494 subjects, with 94 receiving placebo, 204 receiving olanzapine, and 196 receiving risperidone | 10 weeks | Olanzapine vs. placebo: total SMD = − 0.02 (− 0.27, 0.23); psychosis SMD = − 0.12 (− 0.36, 0.13); agitation SMD = 0.09 (− 0.16, 0.34) | 2 | |
1A | Subjects with Alzheimer’s disease (MMSE scores 5–26), in long-term care settings, with hallucinations or delusions Interventions: placebo or fixed doses of olanzapine (1, 2.5, 5, or 7.5 mg/day) Design: double-blind randomized trial in Europe, Israel, Lebanon, Australia/New Zealand, and South Africa Industry-sponsored multicenter trial | 652 subjects; 65%–75% of subjects in each study arm completed the trial | 10 weeks | Olanzapine vs. placebo: total SMD = 0.14 (− 0.05, 0.34); psychosis SMD = 0.17 (− 0.02, 0.37); agitation SMD = 0.14 (− 0.05, 0.33) | 2 | |
1A | Subjects with Alzheimer’s disease or probable Alzheimer’s disease (MMSE scores 5–26), ambulatory and residing at home or in assisted living, with moderate or greater levels of psychosis, aggression, or agitation Interventions: placebo vs. masked, flexibly dosed olanzapine (mean dose: 5.5 mg/day), quetiapine (mean dose: 56.5 mg/day), or risperidone (mean dose: 1.0 mg/day) Stable doses of cholinesterase inhibitor were permitted. Design: multicenter, federally funded CATIE-AD trial—Phase 1 | 421 subjects randomly assigned to treatment group, with 142 receiving placebo, 100 receiving olanzapine, 94 receiving quetiapine, and 85 receiving risperidone | Median duration on Phase 1 treatment was 7.1 weeks; clinical outcomes assessed for those continuing to receive antipsychotic at 12 weeks | Olanzapine vs. placebo: total SMD = 0.15 (− 0.11, 0.40); psychosis SMD = 0.07 (− 0.19, 0.33); agitation SMD = 0.28 (0.02, 0.53) | 1 | |
1A | Subjects with possible or probable Alzheimer’s disease, residing in a nursing facility, with NPI-NH score > 2 Interventions: placebo vs. fixed doses of olanzapine (5, 10, or 15 mg/day) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial in the United States | 206 subjects; 66%–80% of subjects in each study arm completed the trial | 6 weeks | Olanzapine vs. placebo: total SMD = 0.30 (− 0.03, 0.63); psychosis SMD = 0.17 (− 0.17, 0.50); agitation SMD = 0.39 (0.05, 0.72) | 5 |
Quetiapine
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence | |
|---|---|---|---|---|---|---|---|
1A | Subjects with Alzheimer’s disease or probable Alzheimer’s disease (MMSE scores 5–26), ambulatory and residing at home or in assisted living, with moderate or greater levels of psychosis, aggression, or agitation Interventions: placebo vs. masked, flexibly dosed olanzapine (mean dose: 5.5 mg/day), quetiapine (mean dose: 56.5 mg/day), or risperidone (mean dose: 1.0 mg/day) Stable doses of cholinesterase inhibitor were permitted. Design: multicenter, federally funded CATIE-AD trial—Phase 1 | 421 subjects randomly assigned to treatment group, with 142 receiving placebo, 100 receiving olanzapine, 94 receiving quetiapine, and 85 receiving risperidone | Median duration on Phase 1 treatment was 7.1 weeks; clinical outcomes assessed for those who continued to take antipsychotic at 12 weeks | Quetiapine vs. placebo: total SMD = 0.15 (− 0.11, 0.42); psychosis SMD = 0.16 (− 0.10, 0.42); agitation SMD = 0.10 (− 0.17, 0.37) | 1 | ||
1A | Subjects with Alzheimer’s disease meeting criteria for DSM-IV (MMSE score > 4), residing in a nursing facility, with psychosis and BPRS score > 23 Interventions: placebo vs. flexibly dosed haloperidol (0.5–12 mg/day; median of the mean daily dose: 1.9 mg) or quetiapine (25–600 mg/day; median of the mean daily dose: 96.9 mg) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial in the United States | 284 subjects; data for 180 were analyzed | 10 weeks | Quetiapine vs. placebo: total SMD = 0.22 (− 0.07, 0.28); psychosis SMD = 0.00 (− 0.29, 0.30); agitation SMD = 0.24 (− 0.05, 0.54) | 4 | ||
1A | Subjects with possible Alzheimer’s disease or vascular dementia, in long-term care facilities, with agitation and PANSS-EC score > 13 Interventions: placebo vs. quetiapine 100 mg vs. quetiapine 200 mg (dose adjusted according to fixed titration) Design: double-blind randomized trial Industry-sponsored multicenter trial in the United States | 333 subjects | 10 weeks | Quetiapine vs. placebo: total SMD = 0.04 (− 0.21, 0.28); psychosis SMD = − 0.03 (− 0.27, 0.21); agitation SMD = − 0.03 (− 0.27, 0.21) | 2 | ||
Risperidone
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
1A | Subjects with DSM-IV diagnosis of dementia of the Alzheimer’s type, vascular dementia, or mixed dementia, residing in nursing homes, with MMSE score < 24 and significant aggressive behavior Interventions: placebo vs. risperidone (flexibly dosed up to 2 mg/day; mean dose: 0.95 mg/day). Design: double-blind randomized trial Industry-sponsored multicenter trial in Australia/New Zealand | 345 subjects | 12 weeks | Risperidone vs. placebo: total SMD = 0.46 (0.23, 0.69); psychosis SMD = 0.36 (0.13, 0.59); agitation SMD = 0.37 (0.14, 0.59) | 3 | |||||||
1A | Subjects with Alzheimer’s dementia, vascular dementia, or mixed dementia, in outpatient or residential settings, with NPI or NPI-NH score > 5 on hallucination and delusion items Interventions: placebo vs. flexibly dosed olanzapine (2.5–10 mg/day; mean dose: 5.2 mg/day) or risperidone (0.5–2 mg/day; mean dose: 1.0 mg/day) Design: double-blind randomized trial Industry-sponsored multicenter trial in the United States | 494 subjects, with 94 receiving placebo, 204 receiving olanzapine, and 196 receiving risperidone | 10 weeks | Risperidone vs. placebo: total SMD = − 0.13 (− 0.38, 0.12); psychosis SMD = − 0.03 (− 0.34, 0.16); agitation SMD = 0.14 (− 0.11, 0.39) | 2 | |||||||
1A | Hospitalized or institutionalized subjects with MMSE score < 24 and BEHAVE-AD score > 7 Interventions: placebo vs. flexibly dosed haloperidol (0.5–4 mg/day; mean dose: 1.2 mg/day) or risperidone (0.5–4 mg/day; mean dose: 1.1 mg/day) Design: randomized trial Industry-sponsored multicenter trial in the United Kingdom and Europe | 344 subjects; 68 of 115 subjects treated with risperidone, 81 of 115 subjects treated with haloperidol, and 74 of 114 subjects receiving placebo completed the trial | 12 weeks | Risperidone vs. placebo: total SMD = 0.12 (− 0.14, 0.38); agitation SMD = 0.31 (0.05, 0.57) | 4 | |||||||
1A | Subjects with DSM-IV diagnosis of Alzheimer’s disease, vascular dementia, or mixed dementia, residing in a nursing home or chronic care facility, with MMSE score < 24 and significant psychotic and behavioral symptoms (BEHAVE-AD score > 7) Interventions: placebo vs. fixed doses of risperidone (0.5 mg/day, 1 mg/day, or 2 mg/day) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial conducted in the United States | 625 subjects; 70% of whom completed the study | 12 weeks | Risperidone vs. placebo: total SMD = 0.32 (0.11, 0.53); psychosis SMD = 0.20 (− 0.01, 0.41); agitation SMD = 0.38 (0.17, 0.60) | 4 | |||||||
1A | Subjects with symptoms meeting criteria for Alzheimer’s dementia (MMSE scores 5–23), residing in nursing homes or long- term care facilities, who were mobile and had psychosis Interventions: placebo vs. flexibly dosed risperidone (0.5–1.5 mg/day; mean dose: 1.03 mg/day) Design: randomized controlled trial Industry-sponsored multicenter trial conducted in the United States | 473 subjects randomly assigned to treatment group, with 238 receiving placebo and 235 receiving risperidone; 354 of the subjects completed the study | 8 weeks, after 1–16 days of placebo run-in/washout | Risperidone vs. placebo: total SMD = −0.01 (− 0.21, 0.18); psychosis SMD = 0.17 (− 0.02, 0.36); agitation SMD = 0.04 (− 0.16, 0.23) | 3 | |||||||
1A | Subjects with Alzheimer’s disease or probable Alzheimer’s disease (MMSE scores 5–26), ambulatory and residing at home or in assisted living, with moderate or greater levels of psychosis, aggression, or agitation Interventions: placebo vs. masked, flexibly dosed olanzapine (mean dose: 5.5 mg/day), quetiapine (mean dose: 56.5 mg/day), or risperidone (mean dose: 1.0 mg/day) Stable doses of cholinesterase inhibitor were permitted. Design: multicenter, federally funded CATIE-AD trial—Phase 1 | 421 subjects randomly assigned to treatment group, with 142 receiving placebo, 100 receiving olanzapine, 94 receiving quetiapine, and 85 receiving risperidone | Median duration on Phase 1 treatment was 7.1 weeks; clinical outcomes assessed for those continuing to receive antipsychotic at 12 weeks | Risperidone vs. placebo: total SMD = 0.40 (0.13, 0.68); psychosis SMD = 0.38 (0.11, 0.66); agitation SMD = 0.10 (− 0.17, 0.37) | 1 | |||||||
Quality of the Body of Research Evidence for Second-Generation Antipsychotics Versus Placebo in Overall BPSD
Second-Generation Antipsychotic Versus Haloperidol
Overview and Quality of Individual Studies
Olanzapine Versus Haloperidol
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
1A | Subjects with DSM-IV dementia, residing in a nursing facility in Italy, who also had probable vascular dementia based on NINDS and AIREN criteria and MMSE score > 13 and were not bedridden Interventions: olanzapine (flexibly titrated between 2.5 mg and 7.5 mg; mean dose: 4.23 mg/day) vs. promazine (mean dose: 54.3 mg/day) vs. haloperidol (mean dose: 1.65 mg/day) Patients were allowed to continue taking nonpsychiatric medications from baseline. Design: Open-label, nonrandomized; groups were divided manually, with matching for age, education levels, and preliminary NPI scores | 346 patients enrolled, with 173 receiving olanzapine, 60 receiving promazine, and 113 receiving haloperidol | 12 months | Olanzapine vs. haloperidol: total SMD = 0.38 (0.17, 0.60) Both treatment groups showed a reduction in NPI scores relative to baseline of about 30%, but there was no significant difference between the groups. | 0 | |||||||
1A | Subjects with DSM-IV dementia, residing in nursing homes or their own homes, who were judged to be in need of treatment for clinically significant agitation (CMAI score > 44) Interventions: haloperidol (1–3 mg/day; mean dose: 1.75 mg) vs. olanzapine (2.5–7.5 mg/day; mean dose: 4.71 mg) Design: double-blind randomized controlled two-arm trial; randomization occurred after 3- to 11-day washout Multicenter study in the Netherlands; funding source not noted | 59 subjects, with 1 excluded for missing data; 3 patients, all of whom were in the olanzapine group, withdrew from the study | 5 weeks total, with titration taking up to 2 weeks and the medication at stable dose for at least 3 weeks | Olanzapine vs. haloperidol: total SMD = − 0.18 (− 0.77, 0.40); agitation SMD = − 0.21 (− 0.73, 0.31) AHRQ does not report SMD for psychosis comparison, but the change in the NPI psychosis item showed no significant difference in the scores for the two treatments. | 3 | |||||||
Quetiapine Versus Haloperidol
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
1A | Inpatients with ICD-10 Alzheimer’s disease and associated behavioral symptoms Interventions: haloperidol (0.5–4 mg/day; mean dose: 1.9 mg/day) vs. quetiapine (25–200 mg/day; mean dose: 125 mg/day); fixed titration schedule with weekly dose increments to final dose Design: randomized controlled open-label trial Trial conducted in Switzerland; two of the three investigators were noted to be supported by an industry-sponsored grant. | 30 subjects enrolled; 4 dropped out, and 4 had missing data; data for 22 were analyzed | 5 weeks, after run-in period of up to 7 days | Quetiapine vs. haloperidol: total SMD = 0.99 (0.10, 1.88); agitation SMD = 0.06 (− 0.78, 0.89) | 2 | |||||||
1A | Subjects with DSM-IV Alzheimer’s disease (MMSE score > 4), residing in nursing facilities, with psychosis and BPRS score > 23 Interventions: placebo vs. flexibly dosed haloperidol (0.5–12 mg/day; mean dose: 1.9 mg/day) or quetiapine (25–600 mg/day; mean dose: 96.9 mg/day) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial in the United States | 284 subjects; data for 180 were analyzed | 10 weeks | Quetiapine vs. haloperidol: total SMD = 0.16 (− 0.16, 0.47); agitation SMD = 0.04 (− 0.26, 0.34) | 4 | |||||||
Risperidone Versus Haloperidol
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence |
|---|---|---|---|---|---|---|
1 | Inpatients or outpatients with DSM-IV diagnosis of dementia of Alzheimer’s type or vascular dementia associated with behavioral symptoms Interventions: flexibly dosed haloperidol (0.5–2 mg/day; mean dose: 0.90 mg/day) vs. risperidone (0.5–2 mg/day; mean dose: 0.85 mg/day) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial conducted in Hong Kong | 58 subjects | 3 months | Haloperidol vs. risperidone: change in BEHAVE-AD dementia (aggressiveness): SMD = 0.057 (− 0.472, 0.585); change in BEHAVE-AD dementia (psychosis): SMD = − 0.383 (− 0.917, 0.15) Scores on the CMAI and BEHAVE-AD were significantly improved by both haloperidol and risperidone, with no significant differences between the two treatments. Patients treated with haloperidol, but not those treated with risperidone, showed an increase in EPS on the SAS. | 3 | |
1A | Hospitalized or institutionalized subjects with MMSE score < 24 and BEHAVE-AD score > 7 Interventions: placebo vs. flexibly dosed haloperidol (0.5–4 mg/day; mean dose: 1.2 mg/day) or risperidone (0.5–4 mg/day; mean dose: 1.1 mg/day) Design: randomized trial Industry-sponsored multicenter trial in the United Kingdom and Europe | 344 subjects; 68 of the 115 risperidone, 81 of the 115 subjects treated with haloperidol, and 74 of the 114 subjects receiving placebo completed the trial | 12 weeks | Risperidone vs. haloperidol: total SMD = − 0.19 (− 0.45, 0.07); agitation SMD = − 0.07 (− 0.19, − 0.33) | 4 | |
1 | Subjects in a nursing facility with a diagnosis of Alzheimer’s disease, vascular dementia, or mixed dementia associated with behavioral disturbance FAST > 3, BEHAVE-AD score > 7, and CMAI score > 2 on at least two items Interventions: flexibly dosed risperidone (0.5–1.5 mg/day; mean dose: 0.80 mg/day) vs. haloperidol (0.5–1.5 mg/day; mean dose: 0.83 mg/day) Design: randomized double-blind crossover trial Industry-sponsored trial at a single center in Korea | 120 | 18 weeks | Compared with treatment with haloperidol, risperidone treatment was associated with greater clinical improvement on total and subscale scores of the Korean version of BEHAVE-AD, total and subscale scores of the Korean version of CMAI, and the CGI-C as well as a lower frequency of EPS. | 4 |
Quality of the Body of Research Evidence for Second-Generation Antipsychotics Versus Haloperidol for Overall BPSD
Olanzapine or Quetiapine Versus Risperidone
Overview and Quality of Individual Studies
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence |
|---|---|---|---|---|---|---|
1A | Subjects with Alzheimer’s dementia, vascular dementia, or mixed dementia, in outpatient or residential settings, with NPI or NPI-NH score > 5 on hallucination and delusion items Interventions: placebo vs. flexibly dosed olanzapine (2.5–10 mg/day; mean dose: 5.2 mg/ day) or risperidone (0.5–2 mg/day; mean dose: 1.0 mg/day) Design: double-blind randomized trial Industry-sponsored multicenter trial in the United States | 494 subjects, with 94 receiving placebo, 204 receiving olanzapine, and 196 receiving risperidone | 10 weeks | Olanzapine vs. risperidone: total SMD = 0.10 (− 0.10, 0.30); psychosis SMD = − 0.03 (− 0.23, 0.17); agitation SMD = − 0.04 (− 0.24, 0.16) | 2 | |
1 | Subjects with DSM-IV diagnosis of dementia in long-term care facilities in the United States Interventions: olanzapine (2.5–10 mg/day; mean dose: 6.65 mg/day) vs. risperidone (0.5–2 mg/day; mean dose: 1.47 mg/day) Design: double-blind parallel study | 39 subjects, with 20 receiving olanzapine and 19 receiving risperidone | 2 weeks | Both risperidone and olanzapine were associated with significant decreases in CGI-C and NPI scores (P < 0.0001) and an improved score on a quality-of-life measure (Quality of Life in Late Stage Dementia) (P < 0.03), however, the drugs did not differ in the magnitude of their effects on these measures. The most common adverse events were drowsiness and falls. At baseline, 42% (16/38) of subjects had extrapyramidal symptoms, and there was no significant change in SAS scores with treatment. | 3 | |
1 | Subjects with a DSM-IV diagnosis of Alzheimer’s disease, vascular dementia, or mixed dementia associated with behavioral symptoms Interventions: promazine 50 mg/day vs. risperidone 1 mg/day vs. olanzapine 5 mg/day; doses could be doubled at 4 weeks if no clinical response Design: double-blind randomized trial conducted in Western Europe; setting of care not specified | 60 enrolled (20 per group); 1 withdrawal in risperidone group | 8 weeks, after 10-day washout | Global improvement was noted in 80% of patients treated with risperidone and olanzapine and in 65% of patients treated with promazine. | 3 | |
1A | Subjects with Alzheimer’s disease or probable Alzheimer’s disease (MMSE scores 5–26), ambulatory and residing at home or in assisted living, with moderate or greater levels of psychosis, aggression, or agitation Stable doses of cholinesterase inhibitor were permitted. Interventions: placebo vs. masked, flexibly dosed olanzapine (mean dose: 5.5 mg/day), quetiapine (mean dose: 56.5 mg/day), or risperidone (mean dose: 1.0 mg/day) Design: multicenter, federally funded CATIE-AD trial—Phase 1 | 421 subjects randomly assigned to treatment group, with 142 receiving placebo, 100 receiving olanzapine, 94 receiving quetiapine, and 85 receiving risperidone | Median duration on Phase 1 treatment was 7.1 weeks; clinical outcomes assessed for those continuing to receive antipsychotic at 12 weeks | Olanzapine vs. risperidone: total SMD = − 0.27 (− 0.56, 0.02); psychosis SMD = − 0.27 (− 0.56, 0.02); agitation SMD = − 0.17 (− 0.12, 0.16) Quetiapine vs. risperidone: total SMD = − 0.24 (− 0.53, 0.06); psychosis SMD = − 0.24 (− 0.54, 0.05); agitation SMD = 0.10 (− 0.20, 0.39) | 1 | |
1A | Outpatients with mild to moderate dementia of the Alzheimer’s, vascular, mixed, or frontotemporal lobe type according to DSM-IV and ICD-10 who had behavioral disturbance and NPI sub-item scores relating to psychosis or agitation/aggression Interventions: flexibly dosed quetiapine (50–400 mg/day; mean dose: 77 mg/day) vs. risperidone (0.5–4 mg/day; mean dose: 0.9 mg/day) Design: single-blind, parallel-group randomized trial Investigator-sponsored multicenter trial in Western Europe | 72 enrolled, with 65 subjects in ITT population (34 patients receiving quetiapine and 31 patients receiving risperidone) | 8 weeks | Quetiapine vs. risperidone: total SMD = − 0.06 (− 0.55, 0.43); agitation SMD = − 0.17 (− 0.66, 0.32) | 3 |
Quality of the Body of Research Evidence for Olanzapine or Quetiapine Versus Risperidone for Overall BPSD
Second-Generation Antipsychotic Versus Other Comparators
Overview and Quality of Individual Studies
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence |
|---|---|---|---|---|---|---|
1 | Subjects with a diagnosis of Alzheimer’s disease, residing in nursing care facilities in England, with clinically significant agitation Interventions: placebo vs. rivastigmine (3–6 mg BID by week 12 and > 8 mg daily by week 26) vs. quetiapine (25–50 mg BID by week 12 and 50 mg BID by week 26) Design: double-blind randomized controlled trial Funded by general donations to the principal investigator’s research program and profits from prior industry-sponsored trials | 93 subjects; 80 started treatment (25 receiving rivastigmine, 26 receiving quetiapine, and 29 receiving placebo), and 71 tolerated the maximum protocol dose (22 rivastigmine, 23 quetiapine, 26 placebo); 56 had a baseline score of > 10 on the SIB, and 46 of these subjects were included in the analysis at 6-week follow up (14 receiving rivastigmine, 14 receiving quetiapine, and 18 receiving placebo). | 26 weeks total; primary outcome was agitation at 6 weeks | Rivastigmine vs. quetiapine: change in CMAI dementia (agitation) SMD = − 0.051 (− 0.601, 0.499) When treated with either rivastigmine or quetiapine as compared with placebo, subjects failed to show an improvement in agitation. Relative to placebo, quetiapine, but not rivastigmine, was associated with greater cognitive decline as measured by the SIB score. | 4 | |
1 | Inpatients with Alzheimer’s dementia who had been admitted for behavioral symptoms, including psychosis and agitation Interventions: risperidone 1 mg/day vs. escitalopram 10 mg/day Design: double-blind randomized trial Trial conducted in Israel | 40 subjects | 6 weeks | Degree of improvement as measured by the NPI was comparable in those treated with risperidone as compared with those treated with escitalopram. Premature discontinuation occurred in 45% of risperidone-treated subjects and 25% of escitalopram-treated subjects, primarily because of adverse events. Serious adverse effects, including severe extrapyramidal side effects and acute illness requiring hospitalization, occurred in 6 risperidone-treated patients. | 5 | |
1 | Subjects with dementia with Lewy body (DLB) or Alzheimer’s disease (AD) who were hospitalized for behavioral disturbance Interventions: risperidone (started at 0.5 mg/day for 3 days, then increased to two capsules/day for 2 weeks, with two additional dosage increases up to four capsules/day allowed) vs. citalopram (started at 10 mg/day for 3 days, then increased to two capsules/day for 2 weeks, with two additional dosage increases up to four capsules/day allowed) Design: double-blind randomized controlled trial Trial conducted at Western Psychiatric Institute and Clinic in Pittsburgh, PA | 31 patients with DLB and 66 patients with AD; of the 408 patients who were prescreened, 111 signed consent and were screened, and 103 were randomly assigned to treatment group | Up to 12 weeks | Efficacy of citalopram or risperidone was comparable for subjects overall, but AD patients showed improved scores on the NPI and CGI-C, whereas DLB patients showed a worsening on both measures. Discontinuation rates were similar for DLB patients who were treated with citalopram (71%) or risperidone (65%). However, premature discontinuation rates were higher in participants with DLB (68%) than in those with AD (50%), and treated DLB subjects who had been randomly assiged to receive risperidone had more side effects. | 4 | |
1 | Subjects age 65 years or older with Alzheimer’s disease, residing in nursing homes or equivalent institutions, with symptoms of psychosis and/or agitation Interventions: XR vs. IR quetiapine; doses were 50 mg/day XR and 25 mg/day IR. Treatment was escalated to 100 mg/day by day 4 (for both XR and IR). At day 8, a period of flexible dosing (50–300 mg/day) began when dose adjustment was made at the investigator’s discretion Design: double-blind, double-dummy, parallel-group randomized controlled trial Trial conducted at 14 sites in Australia, Belgium, Canada, Norway, and South Africa | Of the 109 patients screened, 100 were randomly assigned to receive quetiapine XR (n = 68) or quetiapine IR (n = 32); 90 patients completed the study (1 patient receiving quetiapine XR withdrew because of an adverse event). | 6 weeks; enrollment and screening were conducted between May 2002 and February 2003 | Relative to baseline, both the IR and the XR formulations of quetiapine were associated with improvements in NPI frequency x severity total score and the NPI disruption score, as well as improvements in the CMAI score. Global ratings using the CGI–Severity of Illness and CGI–Improvement scores also showed benefit from both formulations. | 3 | |
1 | Subjects with a diagnosis of dementia and associated neuropsychiatric symptoms who were being treated on an inpatient or outpatient basis at a university hospital in Sweden Interventions: galantamine (target dose: 24 mg) vs. risperidone (target dose: 1.5 mg) Design: open-label randomized trial Trial conducted at a single center in Sweden | 100 subjects (50 in each group); 91 completed the study | 12 weeks | Treatments with galantamine and with risperidone were associated with decreases in agitation. However, improvement was more pronounced with risperidone than with galantamine (mean difference in total CMAI score: 3.7 points at 3 weeks [P = 0.03] and 4.3 points at 12 weeks [P = 0.01]). NPI domains of irritation and agitation also showed greater benefit with risperidone (F(1,97) = 5.2, P = 0.02). However, galantamine treatment was associated with an improvement in MMSE scores, with an increase of 2.8 points compared with baseline (95% CI: 1.96, 3.52). No severe treatment-related side effects were reported with either treatment. | 0 | |
1A | Subjects with severe probable Alzheimer’s disease, residing in nursing home setting, with MMSE score < 6 and CMAI score> 3 for at least 6 weeks Interventions: fixed titration with rivastigmine 3–6 mg/day vs. risperidone 0.5 mg/day Exclusion criteria included prior exposure to cholinesterase inhibitor or antipsychotic (> 20 mg thioridazine equivalents per day). Design: double-blind randomized controlled trial Trial conducted in the United Kingdom | 27 subjects | 6 weeks | Rivastigmine vs. risperidone: change in CMAI (agitation) SMD = 1.31 (0.47–2.15) | 3 | |
1 | Mowla and Pani 2010 | Subjects with mild to moderate DSM-IV Alzheimer’s disease and behavioral disturbance Interventions: flexibly dosed topiramate (average dose: 44 mg/day) or risperidone (average dose: 1.9 mg/day) Design: randomized controlled trial Multisite trial; Bushehr University of Medical Sciences, Iran | 48 subjects, with 25 receiving topiramate and 23 receiving risperidone; 41 total subjects completed the trial | 8 weeks | Topiramate vs. risperidone: change in NPI (total) SMD = 0.23 (− 0.38, 0.85); change in CMAI (agitation) SMD = 0.06 (− 0.56, 0.67) Both topiramate and risperidone were associated with significant improvements in all outcome measures. | 5 |
1 | Subjects with dementia admitted to hospital for moderate to severe agitation or psychosis but no significant depressive symptoms or recent depressive episodes and no unstable physical illness Interventions: flexibly dosed citalopram (average dose: 29.4 mg/day) or risperidone (average dose: 1.25 mg/day) Subjects could continue taking cholinesterase inhibitors or memantine if they had been taking them for at least 12 weeks at a stable dose; lorazepam at up to 2 mg/day was also permitted for extreme agitation or aggression. Design: Double-blind randomized trial Trial conducted in Canada; funding by U.S. Public Health Service and Sandra A. Rotman Program in Neuropsychiatry, Toronto, Ontario, Canada | 408 subjects were screened; 103 were randomly assigned (citalopram, n = 53; risperidone, n = 50); 45 completed treatment (citalopram, n = 25; risperidone, n = 20) | 12-week trial conducted between February 2000 to June 2005 | No significant differences were seen between citalopram and risperidone in outcomes or time to dropout. On the NRS, there were significant decreases in psychosis scores for both medications (32.3% and 35.2% decreases for citalopram and risperidone, respectively). The decrease in agitation scores was significant for citalopram (12.5%) but not for risperidone (8.2%). | 5 | |
1 | Subjects with DSM-IV Alzheimer’s dementia admitted to hospital for unmanageable behavioral symptoms Interventions: flexibly dosed risperidone (average dose: 1.1 mg/day), yokukansan (average dose: 7 mg/day), or fluvoxamine (average dose: 83 mg/day) Subjects could continue taking donepezil and could receive anticholinergic medications for EPS and zopiclone or brotizolam for insomnia. Design: rater-blinded randomized trial Trial conducted at a psychiatric hospital in Japan | 90 subjects screened; 82 enrolled and data for 76 analyzed (risperidone, n = 25; yokukansan, n = 26; fluvoxamine, n = 25) | 8 weeks, preceded by 1-week washout, with data collected between January 2009 and August 2010 | All three drugs significantly reduced NPI-NH total scores from 26.20 (SD, 15.77) to 17.72 (SD, 11.49), with no significant differences among groups. Single-item scores were significantly reduced for delusions, agitation, disinhibition, aberrant motor behavior, and nighttime behavior disturbances, again with no significant group differences. MMSE scores and FIM scores showed no significant change during the study. Constipation was the most common adverse event in all groups, with a significant increase in frequency with risperidone. EPS and muscle rigidity were also significantly increased in frequency with risperidone (19.2% of that treatment group). | 2 |
Quality of the Body of Research Evidence for Second-Generation Antipsychotics Versus Other Medications for Overall BPSD
Discontinuation Studies
Overview and Quality of Individual Studies
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence |
|---|---|---|---|---|---|---|
1 | Nursing home residents with probable or possible Alzheimer’s disease (by NINCDS/ADRDA criteria) who had no severe behavioral disturbances and had been taking neuroleptics for longer than 3 months Interventions: prescriptions written, in a twice-daily regimen, allocating the closest dose to participant’s preexisting prescription from the doses encapsulated (risperidone 0.5 mg, chlorpromazine 12.5 mg, thioridazine 12.5 mg, trifluoperazine 0.5 mg, haloperidol 0.25 mg) After randomization, study medication replaced existing medication on the day of commencement. Design: double-blind, placebo-controlled randomized discontinuation study Multicenter trial in the United Kingdom | 100 subjects enrolled, with 82 completing 1-month assessment (36 receiving placebo, 46 receiving active treatment) | 3 months | Subjects with higher baseline NPI scores (> 14) were significantly more likely to develop marked behavioral problems when antipsychotic medication was discontinued (χ2 = 6.8, P = 0.009). Similar proportions of antipsychotic- and placebo-treated subjects withdrew from the study prematurely, overall and because of worsening behavioral symptoms. | 3 | |
1 | Subjects with dementia, residing in nursing facilities, who had been receiving antipsychotic medication for at least 3 months for behavioral or psychiatric disturbance Interventions: continuation of antipsychotic (thioridazine, chlorpromazine, haloperidol, trifluoperazine, or risperidone) or change to receiving placebo Design: Blinded, placebo-controlled, parallel-group randomized discontinuation trial Multicenter trial (Dementia Antipsychotic Withdrawal Trial) in the United Kingdom | 165 subjects randomly assigned to treatment group (83 to antipsychotic treatment group; 82 to placebo group); 128 initiated intervention (64 in each condition); 13 were lost to follow-up in each study arm. 51 subjects per condition completed the study. | 12 months | Continuation treatment and placebo groups had no significant difference in the estimated mean change between baseline and 6 months in SIB scores (estimated mean difference in deterioration favoring placebo: − 0.4 [95% CI: − 6.4, 5.5]) or NPI scores (estimated mean difference in deterioration favoring continued treatment: − 2.4 [95% CI: − 8.2, 3.5]). There continued to be no difference between continuation treatment and placebo groups at 12 months, although some evidence suggested that subjects with initial NPI scores ≥ 15 showed reduced neuropsychiatric symptoms with continuing treatment. Subjects who continued to receive antipsychotic treatment had a lower cumulative probability of survival at 12 months, with 70% (95% CI: 58%, 80%) survival in the continued treatment group versus 77% (95% CI: 64%, 85%) in the placebo group for subjects receiving at least one dose of drug or placebo. Differences between groups were more pronounced at longer periods of follow-up (24-month survival: 46% vs. 71%; 36-month survival: 30% vs. 59%) with an HR of 0.58 (95% CI: 0.35, 0.95). | 5 | |
1 | Outpatients with Alzheimer’s disease with psychosis or agitation who had responded to 20 weeks of open-label haloperidol (0.5–5 mg/day) as defined by a minimum of a 50% reduction in three target symptoms, and improvement in CGI-C score for psychosis/agitation Interventions: randomization to placebo vs. continuation of haloperidol Design: double-blind randomized trial in the United States | 44 patients at trial entry; of the 22 responders to haloperidol, 21 entered the randomized portion of the trial, and 20 had at least one follow-up visit. | 6 months | Open-label haloperidol was associated with a significant decrease in symptoms but a significant increase in EPS. 4 of 10 patients who continued to take haloperidol relapsed as compared with 8 of 10 patients receiving placebo, but this difference was not statistically significant. (Relapse criteria required 50% worsening in target symptoms and CGI-C scores.) Time to relapse was shorter for placebo than for haloperidol (P = 0.04). | 3 | |
1 | Patients with Alzheimer’s disease and psychosis or agitation-aggression, 50–95 years of age, who were recruited from memory clinics (including Alzheimer’s research centers), geriatric psychiatry clinics, and clinics at Veterans Affairs medical centers; through physician referrals and advertising; or from assisted-living facilities (outpatient or residents) or nursing homes Interventions: 16-week open-label risperidone phase, then randomization to one of three regimens: continued risperidone therapy for 32 weeks (group 1), risperidone therapy for 16 weeks followed by placebo for 16 weeks (group 2), or placebo for 32 weeks (group 3) Design: double-blind randomized controlled trial | 180 patients received open-label risperidone (mean dose, 0.97 mg/day). Criteria for response to treatment were met in 112 patients; 110 of these subjects underwent randomization. | 32 weeks in randomized phase (after the 16-week open-label phase) | Outcome measures: time to relapse of psychosis or agitation, adverse events, mortality. 16-week relapse rate was higher in patients receiving placebo than in those treated with risperidone (60% [24 of 40 patients in group 3] vs. 33% [23 of 70 in groups 1 and 2]; P = 0.004; HR with placebo = 1.94; 95% CI: 1.09, 3.45; P = 0.02). 32-week relapse rate was higher in group 2 than in group 1 (48% [13 of 27 patients in group 2] vs. 15% [2 of 13 in group 1]; P = 0.02; HR = 4.88; 95% CI: 1.08, 21.98; P = 0.02). | 5 | |
1 | Subjects with dementia, residing in nursing homes, who were taking haloperidol, risperidone, or olanzapine for nonpsychotic symptoms for at least 3 months Interventions: continuation of treatment with antipsychotic medication or change to placebo Design: double-blind, placebo-controlled randomized trial Multicenter trial in Norway | 55 subjects; 27 of the subjects had antipsychotic medication discontinued, and 28 had antipsychotic medication continued. | 4 weeks | In subjects who had antipsychotic discontinued, the proportion of individuals who continued not to take an antipsychotic medication was 85%, 46%, and 33% at 1, 2, and 5 months after drug discontinuation. In a subset of 30 patients, antipsychotic discontinuation was associated with reduced sleep efficiency and greater activity levels as measured by actigraphy. | 4 | |
1 | Subjects with dementia, residing in nursing facilities, who had been receiving antipsychotic medication (risperidone, olanzapine, haloperidol, thioridazine, or loxapine) for more than 6 months, and had behavioral symptoms that were currently stable Interventions: continue treatment or change to placebo Design: randomized trial of antipsychotic discontinuation Multicenter trial in Canada; not industry sponsored | 34 subjects; 10 of the 16 subjects receiving placebo and 6 of the 16 subjects receiving active treatment withdrew from the trial before completion. | 6 months | About one-quarter of subjects in each group showed a worsening of behavioral symptoms. More subjects in the placebo group withdrew from the study for worsening behavior, but this difference was not statistically significant. Data suggested that subjects taking a higher baseline dose of antipsychotic were more likely to have a worsening of behavior upon discontinuation of antipsychotic medication. | 3 |
Quality of the Body of Research Evidence From Discontinuation Studies in Terms of Overall BPSD
1B. Efficacy and Comparative Effectiveness of Second- Generation Antipsychotics for Treatment of Agitation
Second-Generation Antipsychotic Versus Placebo
Overview and Quality of Individual Studies
Aripiprazole
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
1A | Nursing home residents with MMSE scores 6–22 and NPI or NPI-NH score > 5 for hallucinations and delusions Interventions: placebo and three fixed doses of aripiprazole (2 mg, 5 mg, 10 mg) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial conducted in long-term care facilities internationally, including the United States and Canada | 487 subjects enrolled; data for 284 analyzed | 10 weeks | Aripiprazole vs. placebo: total SMD = 0.16 (− 0.05, 0.37); psychosis SMD = 0.24 (0.03, 0.45); agitation SMD = 0.31 (0.10, 0.52) | 1, 2 | ||||||
1A | Nursing home residents with Alzheimer’s disease with psychosis Interventions: placebo, aripiprazole at 0.7–15 mg/day (average dose: 8.6 mg/day) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial conducted in long-term care facilities in the United States | 256 subjects enrolled; data for 151 analyzed | 10 weeks, after 1-week washout | Aripiprazole vs. placebo: total SMD = 0.36 (0.11, 0.61); psychosis SMD = − 0.02 (− 0.27, 0.23); agitation SMD = 0.30 (0.05, 0.55) | 2 | ||||||
Olanzapine
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
1A | Subjects with Alzheimer’s dementia, vascular dementia, or mixed dementia, in outpatient or residential settings, with NPI or NPI-NH score > 5 on hallucination and delusion items Interventions: placebo vs. flexibly dosed olanzapine (2.5–10 mg/day; mean dose: 5.2 mg/day) or risperidone (0.5–2 mg/day; mean dose: 1.0 mg/day) Design: double-blind randomized trial Industry-sponsored multicenter trial in the United States | 494 subjects, with 94 receiving placebo, 204 receiving olanzapine, and 196 receiving risperidone | 10 weeks | Olanzapine vs. placebo: total SMD = − 0.02 (− 0.27, 0.23); psychosis SMD = − 0.12 (− 0.36, 0.13); agitation SMD = 0.09 (− 0.16, 0.34) | 2 | |||||||
1A | Subjects with Alzheimer’s disease (MMSE scores 5–26), in long-term care settings, with hallucinations or delusions Interventions: placebo or fixed-dose olanzapine (1, 2.5, 5, or 7.5 mg/day) Design: double-blind randomized trial Industry-sponsored multicenter trial in Europe, Israel, Lebanon, Australia/New Zealand, and South Africa | 652 subjects; 65%–75% of the subjects in each study arm completed the trial | 10 weeks | Olanzapine vs. placebo: total SMD = 0.14 (− 0.05, 0.34); psychosis SMD = 0.17 (− 0.02, 0.37); agitation SMD = 0.14 (− 0.05, 0.33) | 2 | |||||||
1A | Subjects with Alzheimer’s disease or probable Alzheimer’s disease (MMSE scores 5–26), ambulatory and residing at home or in assisted living, with moderate or greater levels of psychosis, aggression, or agitation Interventions: placebo vs. masked, flexibly dosed olanzapine (mean dose: 5.5 mg/day), quetiapine (mean dose: 56.5 mg/day), or risperidone (mean dose: 1.0 mg/day) Stable doses of cholinesterase inhibitor were permitted. Design: multicenter, federally funded CATIE-AD trial—Phase 1 | 421 subjects randomly assigned to treatment group, with 142 receiving placebo, 100 receiving olanzapine, 94 receiving quetiapine, and 85 receiving risperidone | Median duration on Phase 1 treatment was 7.1 weeks; clinical outcomes assessed for those continuing to take antipsychotic at 12 weeks | Olanzapine vs. placebo: total SMD = 0.15 (− 0.11, 0.40); psychosis SMD = 0.07 (− 0.19, 0.33); agitation SMD = 0.28 (0.02, 0.53) | 1 | |||||||
1A | Subjects with possible or probable Alzheimer’s disease, residing in a nursing facility, with NPI-NH score > 2 Interventions: placebo vs. fixed doses of olanzapine (5, 10, or 15 mg/day) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial in the United States | 206 subjects; 66%–80% of the subjects in each study arm completed the trial | 6 weeks | Olanzapine vs. placebo: total SMD = 0.30 (− 0.03, 0.53); psychosis SMD = 0.17 (− 0.17, 0.50); agitation SMD = 0.39 (0.05, 0.72) | 5 | |||||||
Quetiapine
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
1A | Subjects with a diagnosis of Alzheimer’s disease, residing in nursing care facilities, with clinically significant agitation Interventions: placebo vs. rivastigmine (3–6 mg BID by week 12 and > 8 mg/day by week 26) vs. quetiapine (25–50 mg BID by week 12 and 50 mg BID by week 26) Design: double-blind randomized controlled trial Trial conducted in England. Funded by general donations to the PIs’ research program and profits from prior industry-sponsored trials. | 93 subjects; 80 started treatment (25 receiving rivastigmine, 26 quetiapine, 29 placebo), and 71 tolerated the maximum protocol dose (22 receiving rivastigmine, 23 quetiapine, 26 placebo); 56 had a baseline SIB score > 10, and 46 of these subjects were included in the analysis at 6-week follow-up (14 receiving rivastigmine, 14 quetiapine, 18 placebo). | 26 weeks total; primary outcome was agitation at 6 weeks | Placebo vs. quetiapine: dementia (agitation) change in CMAI SMD = 0.276 (− 0.25, 0.603) Rivastigmine vs. quetiapine: dementia (agitation) change in CMAI SMD = − 0.051 (− 0.601, 0.499) When treated with either rivastigmine or quetiapine as compared with placebo, subjects failed to show an improvement in agitation. Relative to placebo, quetiapine, but not rivastigmine, was associated with greater cognitive decline as measured by the SIB score. | 4 | ||||||
1A | Subjects with diagnosis of Alzheimer’s disease (MMSE score < 24) associated with behavioral symptoms (NPI score > 6 on any item) Interventions: placebo vs. flexibly dosed quetiapine (50–300 mg/day; median dose: 200 mg/day) Design: double-blind randomized trial Industry-sponsored trial conducted in Israel | 40 enrolled; 27 completed treatment | 6 weeks | Placebo vs. quetiapine: dementia (agitation) change in NPI SMD = − 0.48 (−1.11, 0.15) Significant reductions occurred in NPI total scores in both groups (79% for placebo and 68.5% for quetiapine). At 6 weeks the CGI-C score had decreased significantly in the quetiapine group (P = 0.009) but not the placebo group (P = 0.48). MMSE, AIMS, and SAS scores and adverse events did not show significant differences between quetiapine treatment and placebo. | 3 | ||||||
1A | Subjects with Alzheimer’s disease or probable Alzheimer’s disease (MMSE scores 5–26), ambulatory and residing at home or in assisted living, with moderate or greater levels of psychosis, aggression, or agitation Interventions: placebo vs. masked flexibly dosed olanzapine (mean dose: 5.5 mg/day), quetiapine (mean dose: 56.5 mg/day), or risperidone (mean dose: 1.0 mg/day) Stable doses of cholinesterase inhibitor were permitted. Design: multicenter, federally funded CATIE-AD trial—Phase 1 | 421 subjects randomly assigned to treatment group, with 142 receiving placebo, 100 receiving olanzapine, 94 receiving quetiapine, and 85 receiving risperidone | Median duration on Phase 1 treatment was 7.1 weeks; clinical outcomes assessed for those continuing to receive antipsychotic at 12 weeks | Quetiapine vs. placebo: total SMD = 0.15 (− 0.11, 0.40); psychosis SMD = 0.16 (− 0.10, 0.42); agitation SMD = 0.20 (− 0.06, 0.46) | 1 | ||||||
1A | Subjects with DSM-IV Alzheimer’s disease (MMSE score > 4), residing in a nursing facility, with psychosis and BPRS score > 23 Interventions: placebo vs. flexibly dosed haloperidol (0.5–12 mg/day; mean dose: 1.9 mg/day) or quetiapine (25–600 mg/day; mean dose: 96.9 mg/day) Design: randomized controlled, double-blind trial Industry-sponsored multicenter trial in the United States | 284 subjects; data for 180 analyzed | 10 weeks | Quetiapine vs. placebo: total SMD = 0.01 (−0.29, 0.30); psychosis SMD = 0.00 (−0.29, 0.30); agitation SMD = 0.24 (−0.05, 0.54) | 4 | ||||||
1A | Subjects with possible Alzheimer’s disease or vascular dementia, in long-term care facility, with agitation and PANSS-EC score > 13 Interventions: placebo vs. quetiapine 100 mg vs. quetiapine 200 mg (adjusted according to fixed titration) Design: double-blind, randomized trial Industry-sponsored multicenter trial in the United States | 333 subjects | 10 weeks | Quetiapine vs. placebo: total SMD = 0.04 (− 0.21, 0.28); psychosis SMD = − 0.03 (− 0.27, 0.21); agitation SMD = − 0.03 (− 0.27, 0.21) | 2 | ||||||
Risperidone
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1A | Subjects with DSM-IV diagnosis of dementia of the Alzheimer’s type, vascular dementia, or mixed dementia, residing in nursing homes, with an MMSE score < 24 and significant aggressive behavior Interventions: placebo vs. flexibly dosed risperidone (up to 2 mg/day; mean dose: 0.95 mg/day) Design: double-blind randomized trial Industry-sponsored multicenter trial in Australia/New Zealand | 345 subjects | 12 weeks | Risperidone vs. placebo total SMD = 0.46 (0.23, 0.69); psychosis SMD = 0.36 (0.13, 0.59); agitation SMD = 0.37 (0.14, 0.59) | 3 | |||||||||||
1A | Subjects with Alzheimer’s dementia, vascular dementia, or mixed dementia, in outpatient or residential settings, with NPI or NPI-NH score > 5 on hallucination and delusion items Interventions: placebo vs. flexibly dosed olanzapine (2.5–10 mg/day; mean dose: 5.2 mg/day) or risperidone (0.5–2 mg/day; mean dose: 1.0 mg/day) Design: double-blind randomized trial Industry-sponsored multicenter trial in the United States | 494 subjects, with 94 receiving placebo, 204 receiving olanzapine, and 196 receiving risperidone | 10 weeks | Risperidone vs. placebo: total SMD = − 0.13 (− 0.38, 0.12); psychosis SMD = − 0.03 (− 0.34, 0.16); agitation SMD = 0.14 ( −0.11, 0.39) | 2 | |||||||||||
1A | Hospitalized or institutionalized subjects with MMSE score < 24 and BEHAVE-AD score > 7 Interventions: placebo vs. flexibly dosed haloperidol (0.5–4 mg/day; mean dose: 1.2 mg/day) or risperidone (0.5–4 mg/day; mean dose: 1.1 mg/day) Design: randomized trial Industry-sponsored multicenter trial in the United Kingdom and Europe | 344 subjects; 68 of the 115 subjects receiving risperidone, 81 of the 115 subjects receiving haloperidol, and 74 of the 114 subjects receiving placebo completed the trial | 12 weeks | Risperidone vs. placebo: total SMD = 0.12 (− 0.14, 0.38); agitation SMD = 0.31 (0.05, 0.57) | 4 | |||||||||||
1A | Subjects with DSM-IV diagnosis of dementia of the Alzheimer’s type, vascular dementia, or mixed dementia, residing in a nursing home or chronic care facility, with MMSE score < 24 and significant psychotic and behavioral symptoms (BEHAVE-AD score > 7) Interventions: placebo vs. fixed doses of risperidone at 0.5, 1, or 2 mg/day Design: double-blind randomized controlled trial Industry-sponsored multicenter trial in the United States | 625 subjects; 70% of the subjects completed the study | 12 weeks | Risperidone vs. placebo: total SMD = 0.32 (0.11, 0.53); psychosis SMD = 0.20 (− 0.01, 0.41); agitation SMD = 0.38 (0.17, 0.60) | 4 | |||||||||||
1A | Subjects who were mobile and had symptoms that met criteria for Alzheimer’s dementia (MMSE scores 5–23), residing in nursing homes or long-term care, with psychosis Interventions: placebo vs. flexibly dosed risperidone (0.5-1.5 mg/day; mean dose: 1.03 mg/day) Design: randomized controlled trial Industry-sponsored multicenter trial in the United States | 473 subjects randomly assigned to treatment group, with 238 receiving placebo and 235 receiving risperidone; 354 completed the study | 8 weeks, after 1–16 days of placebo run-in/washout | Risperidone vs. placebo: total SMD = −0.01 (−0.21, 0.18); psychosis SMD = 0.17 (− 0.02, 0.36); agitation SMD = 0.04 (− 0.16, 0.23) | 3 | |||||||||||
1A | Subjects with Alzheimer’s disease or probable Alzheimer’s disease (MMSE scores 5–26), ambulatory and residing at home or in assisted living, with moderate or greater levels of psychosis, aggression, or agitation Interventions: placebo vs. masked, flexibly dosed olanzapine (mean dose: 5.5 mg/day), quetiapine (mean dose: 56.5 mg/day), or risperidone (mean dose: 1.0 mg/day) Stable doses of cholinesterase inhibitor were permitted. Design: multicenter, federally funded CATIE-AD trial—Phase 1 | 421 subjects randomly assigned to treatment group, with 142 receiving placebo, 100 receiving olanzapine, 94 receiving quetiapine, and 85 receiving risperidone | Median duration on Phase 1 treatment was 7.1 weeks; clinical outcomes assessed for those continuing to take antipsychotic at 12 weeks | Risperidone vs. placebo: total SMD = 0.40 (0.13, 0.68); psychosis SMD = 0.38 (0.11, 0.66); agitation SMD = 0.10 ( −0.17, 0.37) | 1 | |||||||||||
Quality of the Body of Research Evidence for Second-Generation Antipsychotics Versus Placebo in Agitation
Second-Generation Antipsychotic Versus Haloperidol
Overview and Quality of Individual Studies
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
1 | Inpatients or outpatients with a DSM-IV diagnosis of dementia of Alzheimer’s type or vascular dementia associated with behavioral symptoms Interventions: flexibly dosed haloperidol (0.5–2 mg/day; mean dose: 0.90 mg/day) vs. risperidone (0.5–2 mg/day; mean dose: 0.85 mg/day) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial in Hong Kong | 58 subjects | 3 months | Haloperidol vs. risperidone: change in BEHAVE-AD dementia (aggressiveness) SMD = 0.057 (− 0.472, 0.585); change in BEHAVE-AD dementia (psychosis) SMD = − 0.383 (− 0.917, 0.15) Scores on the CMAI and BEHAVE-AD were significantly improved by both haloperidol and risperidone, with no significant differences between the two treatments. Haloperidol-treated patients, but not risperidone-treated patients, showed an increase in EPS on the SAS. | 3 | ||||||
1A | Hospitalized or institutionalized subjects with MMSE score < 24 and a BEHAVE-AD score > 7 Interventions: placebo vs. flexibly dosed haloperidol (0.5–4 mg/day; mean dose: 1.2 mg/day) or risperidone (0.5–4 mg/day; mean dose: 1.1 mg/day) Design: randomized trial Industry-sponsored multicenter trial in the United Kingdom and Europe | 344 subjects | 12 weeks | Risperidone vs. haloperidol: total SMD = − 0.19 (− 0.45, 0.07); agitation SMD = − 0.07 (− 0.19, 0.33) | 4 | ||||||
1A | Inpatients with ICD-10 Alzheimer’s disease and associated behavioral symptoms Interventions: haloperidol (0.5–4 mg/day; mean dose: 1.9 mg/day) vs. quetiapine (25–200 mg/day; mean dose: 125 mg/day); fixed titration schedule with weekly dose increments to final dose Design: open-label randomized controlled trial Trial conducted in Switzerland; two of the three investigators were noted to be supported by an industry-sponsored grant. | 30 subjects enrolled; 4 dropped out, and 4 had missing data; data for 22 analyzed | 5 weeks, after run-in period of up to 7 days | Quetiapine vs. haloperidol: total SMD = 0.99 (0.10, 1.88); agitation SMD = 0.06 (− 0.78, 0.89) | 2 | ||||||
1 | Subjects residing in a nursing facility with a diagnosis of Alzheimer’s disease, vascular dementia, or mixed dementia associated with behavioral disturbance (FAST score > 3, BEHAVE-D score > 7, CMAI score > 2 on at least two items) Interventions: flexibly dosed risperidone (0.5–1.5 mg/day; mean dose: 0.80 mg/day) vs. haloperidol (0.5-1.5 mg/day; mean dose: 0.83 mg/day) Design: double-blind, crossover, randomized trial Industry-sponsored trial at a single center in Korea | 120 subjects | 18 weeks | Compared with haloperidol treatment, risperidone treatment was associated with greater clinical improvement on total and subscale scores of the Korean version of BEHAVE-AD, on total and subscale scores of the Korean version of CMAI, and on the CGI-C, as well as a lower frequency of EPS. | 4 | ||||||
1A | Subjects with DSM-IV Alzheimer’s disease (MMSE score > 4), residing in a nursing facility, with psychosis and BPRS score > 23 Interventions: placebo vs. flexibly dosed haloperidol (0.5–12 mg/day; mean dose: 1.9 mg/day) or quetiapine (25–600 mg/day; mean dose: 96.9 mg/day) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial in the United States | 284 subjects; data for 180 analyzed | 10 weeks | Quetiapine vs. haloperidol: total SMD = 0.16 (− 0.16, 0.47); agitation SMD = 0.04 (− 0.26, 0.34) | 4 | ||||||
1A | Subjects with DSM-IV diagnosis of dementia, living in nursing homes or their own homes, who were judged to be in need of treatment for clinically significant agitation (CMAI score > 44) Interventions: haloperidol (1–3 mg/day; mean dose: 1.75 mg) vs. olanzapine (2.5–7.5 mg/day; mean dose: 4.71 mg) Design: double-blind, two-arm randomized controlled study Randomization took place after a 3- to 11-day washout. Multicenter trial conducted in the Netherlands; funding source not noted | 59 subjects; 1 excluded for missing data; 3 patients, all of whom were in the olanzapine group, withdrew from the study | 5 weeks total; titration for up to 2 weeks, and at least 3 weeks at stable dose | Olanzapine vs. haloperidol: total SMD = − 0.18 (− 0.77, 0.41); agitation SMD = − 0.21 (− 0.73, 0.31) AHRQ does not report SMD for psychosis comparison, but the change in the NPI psychosis item showed no significant difference in the scores for the two treatments. | 3 | ||||||
Quality of the Body of Research Evidence for Second-Generation Antipsychotics Versus Haloperidol in Agitation
Olanzapine or Quetiapine Versus Risperidone
Overview and Quality of Individual Studies
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence | ||||
|---|---|---|---|---|---|---|---|---|---|---|
1A | Subjects with Alzheimer’s dementia, vascular dementia, or mixed dementia and NPI or NPI-NH score > 5 on hallucination and delusion items Interventions: placebo vs. flexibly dosed olanzapine (2.5 –10 mg/day; mean dose: 5.2 mg/day) or risperidone (0.5–2 mg/day; mean dose: 1.0 mg/day) Design: double-blind randomized trial Industry-sponsored multicenter trial in the United States | 494 subjects, with 94 receiving placebo, 204 receiving olanzapine, and 196 receiving risperidone | 10 weeks | Olanzapine vs. risperidone: total SMD = 0.10 (− 0.10, 0.30); psychosis SMD = − 0.03 (− 0.23, 0.17); agitation SMD = − 0.04 (− 0.24, 0.16) | 2 | |||||
1A | Subjects with Alzheimer’s disease or probable Alzheimer’s disease (MMSE scores 5–26), ambulatory and residing at home or in assisted living facilities, with moderate or greater levels of psychosis, aggression, or agitation Interventions: placebo vs. masked, flexibly dosed olanzapine (mean dose: 5.5 mg/day), quetiapine (mean dose: 56.5 mg/day), or risperidone (mean dose: 1.0 mg/day) Stable doses of cholinesterase inhibitor were permitted. Design: multicenter, federally funded CATIE-AD trial—Phase 1 | 421 subjects randomly assigned to treatment group, with 142 receiving placebo, 100 receiving olanzapine, 94 receiving quetiapine, and 85 receiving risperidone | Median duration on Phase 1 treatment was 7.1 weeks; clinical outcomes assessed for those continuing to take antipsychotic at 12 weeks | Olanzapine vs. risperidone: total SMD = − 0.27 (− 0.56, 0.02); psychosis SMD = − 0.27 (− 0.56, 0.02); agitation SMD = 0.17 (− 0.12, 0.16) Quetiapine vs. risperidone: total SMD = − 0.24 (− 0.53, 0.06); psychosis SMD = − 0.24 (− 0.54, 0.05); agitation SMD = 0.10 (− 0.20, 0.39) | 1 | |||||
1A | Outpatients with mild to moderate dementia of the Alzheimer’s, vascular, mixed, or frontotemporal lobe type according to DSM-IV and ICD-10 who had behavioral disturbance and NPI subitem scores relating to psychosis or agitation/aggression Interventions: flexibly dosed quetiapine (50–400 mg/day; mean dose: 77 mg/day) vs. risperidone (0.5–4 mg/day; mean dose: 0.9 mg/day) Design: single-blind, parallel-group randomized trial Investigator-sponsored multi-center trial in Western Europe | 72 subjects, with 65 subjects in the ITT population; 34 receiving quetiapine and 31 receiving risperidone | 8 weeks | Quetiapine vs. risperidone: total SMD = −0.06 (− 0.55, 0.43); agitation SMD = −0.17 (− 0.66, 0.32) | 3 | |||||
Quality of the Body of Research Evidence for Olanzapine or Quetiapine Versus Risperidone in Agitation
1C. Efficacy and Comparative Effectiveness of Second- Generation Antipsychotics for Treatment of Psychosis
Second-Generation Antipsychotic Versus Placebo
Overview and Quality of Individual Studies
Aripiprazole
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1A | Nursing home residents with MMSE scores 6–22 and NPI or NPI-NH score >5 for hallucinations and delusions Interventions: placebo vs. three fixed doses of aripiprazole (2 mg, 5 mg, 10 mg) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial conducted in long-term care facilities internationally, including the United States and Canada | 487 subjects enrolled; data for 284 analyzed | 10 weeks | Aripiprazole vs. placebo: total SMD = 0.16 (− 0.05, 0.37); psychosis SMD = 0.24 (0.03, 0.45); agitation SMD = 0.31 (0.10, 0.52) | 1, 2 | |||||||||
1A | Non-institutionalized subjects with Alzheimer’s disease and psychosis Interventions: placebo vs. aripiprazole (2–15 mg/day) Design: double-blind randomized controlled trials Industry-sponsored multicenter trial conducted in the United States, Canada, Western Europe, and Australia/New Zealand | 208 subjects | 10 weeks | Aripiprazole vs. placebo: total SMD = 0.06 (− 0.21, 0.34); psychosis SMD = 0.16 (− 0.12, 0.43) | 3 | |||||||||
1A | Nursing home residents with Alzheimer’s disease and psychosis Interventions: placebo vs. aripiprazole (0.7–15 mg/day; average dose: 8.6 mg/day) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial conducted in long-term care facilities in the United States | 256 subjects enrolled; data for 151 analyzed | 10 weeks, after 1-week washout | Aripiprazole vs. placebo: total SMD = 0.36 (0.11, 0.61); psychosis SMD = − 0.02 (−0.27, 0.23); agitation SMD = 0.30 (0.05, 0.55) | 2 | |||||||||
Olanzapine
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1A | Subjects with Alzheimer’s dementia, vascular dementia, or mixed dementia, in outpatient or residential settings, with NPI or NPI-NH > 5 on hallucination and delusion items Interventions: placebo vs. flexibly dosed olanzapine (2.5–10 mg/day; mean dose: 5.2 mg/day) or risperidone (0.5–2 mg/day; mean dose: 1.0 mg/day) Design: double-blind randomized trial Industry-sponsored multicenter trial in the United States | 494 subjects, with 94 receiving placebo, 204 receiving olanzapine, and 196 receiving risperidone | 10 weeks | Olanzapine vs. placebo: total SMD = − 0.02 (− 0.27, 0.23); psychosis SMD = − 0.12 (− 0.36, 0.13); agitation SMD = 0.09 (− 0.16, 0.34) | 2 | ||||||||
1A | Subjects with Alzheimer’s disease (MMSE scores 5–26), in long-term care settings, with hallucinations or delusions Interventions: placebo vs. fixed-dose olanzapine (1, 2.5, 5, or 7.5 mg/day) Design: double-blind randomized trial Industry–sponsored multicenter trial in Europe, Israel, Lebanon, Australia/New Zealand, and South Africa | 652 subjects; 65%–75% of the subjects in each study arm completed the trial | 10 weeks | Olanzapine vs. placebo: total SMD = 0.14 (− 0.05, 0.34); psychosis SMD = 0.17 (− 0.02, 0.37); agitation SMD = 0.14 (− 0.05, 0.33) | 2 | ||||||||
1A | Subjects with Alzheimer’s disease or probable Alzheimer’s disease (MMSE scores 5–26), ambulatory and residing at home or in assisted living facilities, with moderate or greater levels of psychosis, aggression, or agitation Interventions: placebo vs. masked, flexibly dosed olanzapine (mean dose: 5.5 mg/day), quetiapine (mean dose: 56.5 mg/day), or risperidone (mean dose: 1.0 mg/day) Stable doses of cholinesterase inhibitor were permitted. Design: multicenter, federally funded CATIE-AD trial—Phase 1 | 421 subjects randomly assigned to treatment group, with 142 receiving placebo, 100 receiving olanzapine, 94 receiving quetiapine, and 85 receiving risperidone | Median duration on Phase 1 treatment was 7.1 weeks; clinical outcomes assessed for those continuing to take antipsychotic at 12 weeks | Olanzapine vs. placebo: total SMD = 0.15 (− 0.11, 0.40); psychosis SMD = 0.07 (− 0.19, 0.33); agitation SMD = 0.28 (0.02, 0.53) | 1 | ||||||||
1A | Subjects with possible or probable Alzheimer’s disease, resided in a nursing facility, with NPI-NH score > 2 Interventions: placebo vs. fixed doses of olanzapine (5, 10, or 15 mg/day) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial in the United States | 206 subjects; 66%–80% of individuals in each study arm completed the trial | 6 weeks | Olanzapine vs. placebo: total SMD = 0.30 (− 0.03, 0.53); psychosis SMD = 0.17 (− 0.17, 0.50); agitation SMD = 0.39 (0.05, 0.72) | 5 | ||||||||
Quetiapine
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1A | Subjects with Alzheimer’s disease or probable Alzheimer’s disease (MMSE scores 5–26), ambulatory and residing at home or in assisted living facilities, with moderate or greater levels of psychosis, aggression, or agitation Interventions: placebo vs. masked, flexibly dosed olanzapine (mean dose: 5.5 mg/day), quetiapine (mean dose: 56.5 mg/day), or risperidone (mean dose: 1.0 mg/day) Stable doses of cholinesterase inhibitor were permitted. Design: multicenter, federally funded CATIE-AD trial—Phase 1 | 421 subjects randomly assigned to treatment group, with 142 receiving placebo, 100 receiving olanzapine, 94 receiving quetiapine, and 85 receiving risperidone | Median duration on Phase 1 treatment was 7.1 weeks; clinical outcomes assessed for those continuing to take antipsychotic at 12 weeks | Quetiapine vs. placebo: total SMD = 0.15 (− 0.11, 0.40); psychosis SMD = 0.16 (− 0.10, 0.42); agitation SMD = 0.10 (− 0.17, 0.37) | 1 | ||||||||||
1A | Subjects with DSM-IV Alzheimer’s disease (MMSE score > 4), residing in a nursing facility, with psychosis and BPRS score > 23 Interventions: placebo vs. flexibly dosed haloperidol (0.5–12 mg/day; mean dose: 1.9 mg/day) or quetiapine (25–600 mg/day; mean dose: 96.9 mg/day) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial in the United States | 284 subjects; data for 180 analyzed | 10 weeks | Quetiapine vs. placebo: total SMD = 0.01 (− 0.29, 0.30); psychosis SMD = 0.00 (− 0.29, 0.30); agitation SMD = 0.25 (− 0.05, 0.54) | 4 | ||||||||||
1A | Subjects with possible Alzheimer’s disease or vascular dementia, in long-term care facility, with agitation and PANSS-EC score > 13 Interventions: placebo vs. quetiapine 100 mg vs. quetiapine 200 mg (dose adjusted according to fixed titration) Design: double-blind randomized trial Industry-sponsored multicenter trial in the United States | 333 subjects | 10 weeks | Quetiapine vs. placebo: total SMD = 0.04 (− 0.21, 0.28); psychosis SMD = − 0.03 (− 0.27, 0.21); agitation SMD = − 0.03 (− 0.27, 0.21) | 2 | ||||||||||
Risperidone
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
1A | Subjects with a DSM-IV diagnosis of dementia of the Alzheimer’s type, vascular dementia, or mixed dementia, residing in nursing homes, with MMSE score < 24 and significant aggressive behavior Interventions: placebo vs. flexibly dosed risperidone (up to 2 mg/day; mean dose: 0.95 mg/day) Design: double-blind randomized trial Industry-sponsored multicenter trial in Australia/ New Zealand | 345 subjects | 12 weeks | Risperidone vs. placebo: total SMD = 0.46 (0.23, 0.69); psychosis SMD = 0.36 (0.13, 0.59); agitation SMD = 0.37 (0.14, 0.59) | 3 | |||||||
1A | Subjects with Alzheimer’s dementia, vascular dementia, or mixed dementia, in outpatient or residential settings, with NPI or NPI-NH score > 5 on hallucination and delusion items Interventions: placebo vs. flexibly dosed olanzapine (2.5–10 mg/day; mean dose: 5.2 mg/day) or risperidone (0.5–2 mg/day; mean dose: 1.0 mg/day) Design: double-blind randomized trial Industry-sponsored multicenter trial in the United States | 494 subjects, with 94 receiving placebo, 204 receiving olanzapine, and 196 receiving risperidone | 10 weeks | Risperidone vs. placebo: total SMD = − 0.13 (− 0.38, 0.12); psychosis SMD = − 0.03 (− 0.34, 0.16); agitation SMD = 0.14 (− 0.11, 0.39) | 2 | |||||||
1A | Hospitalized or institutionalized individuals with MMSE score < 24 and BEHAVE-AD score > 7 Interventions: placebo vs. flexibly dosed haloperidol (0.5–4 mg/day; mean dose: 1.2 mg/day) or risperidone (0.5–4 mg/day; mean dose: 1.1 mg/day) Design: randomized trial Industry-sponsored multicenter trial in the United Kingdom and Europe | 344 subjects; 68 of the 115 subjects receiving risperidone, 81 of the 115 subjects receiving haloperidol, and 74 of the 114 subjects receiving placebo completed the trial | 12 weeks | Risperidone vs. placebo: total SMD = 0.12 (− 0.14, 0.38); agitation SMD = 0.31 (0.05, 0.57) | 4 | |||||||
1A | Subjects with DSM-IV diagnosis of Alzheimer’s disease, vascular dementia, or mixed dementia, residing in a nursing home or chronic care facility, with MMSE score <24 and significant psychotic and behavioral symptoms (BEHAVE-AD score > 7) Interventions: placebo vs. fixed doses of risperidone (0.5, 1, or 2 mg/day) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial conducted in the United States | 625 subjects; 70% of the subjects completed the study | 12 weeks | Risperidone vs. placebo: total SMD = 0.32 (0.11, 0.53); psychosis SMD = 0.20 (− 0.01, 0.41); agitation SMD = 0.38 (0.17, 0.60) | 4 | |||||||
1A | Subjects with presentation that met criteria for Alzheimer’s dementia (MMSE scores 5–23), residing in nursing homes or a long-term care setting, who were mobile and had psychosis Interventions: placebo vs. flexibly dosed risperidone (0.5-1.5 mg/day; mean dose: 1.03 mg/day) Design: randomized controlled trial Industry-sponsored multicenter trial conducted in the United States | 473 subjects randomly assigned to treatment group, with 238 receiving placebo and 235 receiving risperidone; 354 subjects completed the study | 8 weeks, after 1–16 days of placebo run-in/washout | Risperidone vs. placebo: total SMD = − 0.01 (−0.21, 0.18); psychosis SMD = 0.17 (−0.02, 0.36); agitation SMD = 0.04 (− 0.16, 0.23) | 3 | |||||||
1A | Subjects with Alzheimer’s disease or probable Alzheimer’s disease (MMSE scores 5–26), ambulatory and residing at home or in assisted living facilities, with moderate or greater levels of psychosis, aggression, or agitation Interventions: placebo vs. masked flexibly dosed olanzapine (mean dose: 5.5 mg/day), quetiapine (mean dose: 56.5 mg/day), or risperidone (mean dose: 1.0 mg/day) Stable doses of cholinesterase inhibitor were permitted. Design: multicenter, federally funded CATIE-AD trial—Phase 1 | 421 subjects randomly assigned to treatment group, with 142 receiving placebo, 100 receiving olanzapine, 94 receiving quetiapine, and 85 receiving risperidone | Median duration on Phase 1 treatment was 7.1 weeks; clinical outcomes assessed for those who continued to take antipsychotic at 12 weeks | Risperidone vs. placebo: total SMD = 0.40 (0.13, 0.68); psychosis SMD = 0.38 (0.11, 0.66); agitation SMD = 0.10 (− 0.17, 0.37) | 1 | |||||||
Quality of the Body of Research Evidence for Second-Generation Antipsychotics Versus Placebo in Psychotic Symptoms
Olanzapine or Quetiapine Versus Risperidone
Overview and Quality of Individual Studies
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1A | Subjects with Alzheimer’s dementia, vascular dementia, or mixed dementia, in outpatient or residential settings, with NPI or NPI-NH score > 5 on hallucination and delusion items Interventions: placebo vs. flexibly dosed olanzapine (2.5–10 mg/day; mean dose: 5.2 mg/day) or risperidone (0.5–2 mg/day; mean dose: 1.0 mg/day) Design: double-blind, randomized trial Industry-sponsored multicenter trial in the United States | 494 subjects, 94 receiving placebo, 204 receiving olanzapine, and 196 receiving risperidone | 10 weeks | Olanzapine vs. risperidone: total SMD = 0.10 (− 0.10, 0.30); psychosis SMD = − 0.03 (− 0.23, 0.17); agitation SMD = − 0.04 (− 0.24, 0.16) | 2 | |||||||||||||
1A | Subjects with Alzheimer’s disease or probable Alzheimer’s disease (MMSE scores 5–26), ambulatory and residing at home or in assisted living facilities, with moderate or greater levels of psychosis, aggression, or agitation Interventions: placebo vs. masked, flexibly dosed olanzapine (mean dose: 5.5 mg/day), quetiapine (mean dose: 56.5 mg/day), or risperidone (mean dose: 1.0 mg/day) Stable doses of cholinesterase inhibitor were permitted. Design: multicenter, federally funded CATIE-AD trial—Phase 1 | 421 subjects randomly assigned, with 142 receiving placebo, 100 receiving olanzapine, 94 receiving quetiapine, and 85 receiving risperidone | Median duration on Phase 1 treatment was 7.1 weeks; clinical outcomes assessed for those continuing to take antipsychotic at 12 weeks | Olanzapine vs. risperidone: total SMD = − 0.27 (− 0.56, 0.02); psychosis SMD = − 0.27 (− 0.56, 0.02); agitation SMD = − 0.17 (− 0.12, 0.16) Quetiapine vs. risperidone: total SMD = −0.24 (− 0.53, 0.06); psychosis SMD = − 0.24 (− 0.54, 0.05); agitation SMD = 0.10 (− 0.20, 0.39) | 1 | |||||||||||||
Quality of the Body of Research Evidence for Olanzapine or Quetiapine Versus Risperidone in Psychotic Symptoms
2. Appropriate Dosage and Duration of Antipsychotic Treatment in Individuals With Alzheimer’s Disease and Other Dementia Syndromes
Overview and Quality of Individual Studies
| Study type | Study | How subjects were recruited and what intervention(s) were performeda | Sample sizeb | How long subjects were followed | Outcome measures and main results | Rating of quality of evidence | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1A | Nursing home residents with MMSE scores 6–22 and NPI or NPI-NH score > 5 for hallucinations and delusions Interventions: placebo vs. three fixed doses of aripiprazole (2 mg, 5 mg, 10 mg) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial conducted in long-term care facilities internationally, including the United States and Canada | 487 subjects enrolled; data for 284 analyzed | 10 weeks | Beginning at week 6 and continuing to study end point at week 10, subjects who received 10 mg aripiprazole daily had a statistically significant degree of improvement in NPI-NH Psychosis subscale scores as well as significant improvements in CMAI scores and scores on the NPI irritability, agitation/aggression, and anxiety items. A greater proportion of subjects who received aripiprazole 10 mg daily showed response to treatment (defined as a > 50% decrease in NPI-NH Psychosis subscale scores from baseline) compared with subjects receiving placebo. Aripiprazole 5 mg/day differed from placebo in response rate and NPI subscores at early time points but not at 10 weeks, although CMAI scores remained improved. Response to aripiprazole 2 mg/day did not differ from response to placebo at any time point. | 1, 2 | |||||||||||||
1A | Subjects with Alzheimer’s disease (MMSE scores 5–26), residing in long-term care settings, with hallucinations or delusions Interventions: placebo vs. fixed-dose olanzapine (1, 2.5, 5, or 7.5 mg/day) Design: double-blind randomized trial Industry-sponsored multicenter trial in Europe, Israel, Lebanon, Australia/New Zealand, and South Africa | 652 subjects; 65%–75% of the subjects in each study arm completed the trial | 10 weeks | No significant treatment effects, based on NPI-NH Psychosis Total and CGI-C scores, were seen at the 10-week end point for any of the doses of olanzapine. Repeated-measures analysis of the Psychosis Total scores showed significant within-group improvement from baseline in all five treatment groups. Nevertheless, a secondary comparison pooling across all visits showed a significant main effect of treatment with either 2.5 mg/day or 7.5 mg/day of olanzapine as compared with placebo. | 2 | |||||||||||||
1A | Subjects with DSM-IV diagnosis of Alzheimer’s disease, vascular dementia, or mixed dementia, residing in nursing homes or chronic care facilities; with MMSE score < 24 and significant psychotic and behavioral symptoms (BEHAVE-AD score > 7) Interventions: placebo vs. fixed doses of risperidone (0.5, 1, or 2 mg/day) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial conducted in the United States | 625 subjects; 70% of the subjects completed the study | 12 weeks | Subjects who received either 1 mg/day or 2 mg/day of risperidone showed significant improvement relative to placebo on BEHAVE-AD Total scores and Psychosis and Aggressiveness subscale scores. These doses of risperidone remained superior to placebo on measures of aggressiveness after the study authors controlled for the effect of psychosis. | 4 | |||||||||||||
1A | Subjects with possible or probable Alzheimer’s disease, residing in nursing facilities, with NPI-NH score > 2 Interventions: placebo vs. fixed doses of olanzapine (5, 10, or 15 mg/day) Design: double-blind randomized controlled trial Industry-sponsored multicenter trial conducted in the United States | 206 subjects; 66%–80% of individuals completed the trial in each study arm | 6 weeks | On the basis of the sum of the Agitation/Aggression, Hallucinations, and Delusions items of the NPI-NH, individuals receiving 5 mg/day or 10 mg/day of olanzapine had significant improvement relative to placebo, whereas those receiving 15 mg/day did not. A similar pattern of findings occurred in terms of the proportion of individuals who showed a response to treatment (as defined by at least a 50% reduction in score from baseline to end point) and in responses to the Psychosis and Agitation items. | 5 | |||||||||||||
1A | Subjects with possible Alzheimer’s disease or vascular dementia, in long-term care facilities, with agitation and PANSS-EC score > 13 Interventions: placebo vs. quetiapine 100 mg vs. quetiapine 200 mg (dose adjusted according to fixed titration) Design: double-blind, randomized trial Industry-sponsored multicenter trial in the United States | 333 subjects | 10 weeks | There was a greater reduction from baseline to end point in mean PANSS-EC score with quetiapine 200 mg/day compared with placebo, but this difference in reduction was not significant using LOCF analysis. However, CGI-C scores were significantly improved with 200 mg/day quetiapine. At 100 mg/day, treatment with quetiapine did not differ from placebo. In terms of response (as defined by at least a 40% reduction on the PANSS-EC from baseline to end point), there were no differences among the treatment arms. | 2 | |||||||||||||
Quality of the Body of Research Evidence for Dose-Related Effects of Second-Generation Antipsychotics
3. Effects of Specific Patient Characteristics on Effectiveness and Harms of Antipsychotic Medications in Individuals With Dementia
4. Potential Adverse Effects and/or Complications Involved With Prescribing Second-Generation Antipsychotics to Patients
| Adverse effect | Strength of evidence (from the 2011 AHRQ reviewa) | Summary of studies since the 2011 AHRQ review | Overall strength of evidence |
|---|---|---|---|
Mortality | High for SGAs relative to placebo Moderate for FGAs relative to SGAs | Moderate for FGAs relative to SGAs Moderate for haloperidol relative to risperidone and for risperidone relative to quetiapine | High for SGAs relative to placebo High for FGAs relative to SGAs Moderate for haloperidol relative to risperidone and for risperidone relative to quetiapine |
Stroke | Low | Low | Low |
Myocardial infarction and other cardiovascular events | Low | Insufficient | Low |
Pulmonary-related adverse effects | Insufficient | Low | Low |
Cognitive changes | Low | Insufficient | Low |
Sedation/fatigue | Moderate | N/A | Moderate |
Extrapyramidal side effects (excluding tardive dyskinesia) | Moderate | Low | Moderate |
Tardive dyskinesia | Insufficient | N/A | Insufficient |
Falls and hip fractures | Insufficient | Low | Low |
Development of diabetes | Low | Insufficient | Low |
Weight gain | Moderate for elderly and those with dementia High for all uses and ages | N/A | Moderate |
Urinary symptoms | Low | N/A | Low |
Mortality
Overview and Quality of Individual Studies
| Adverse effect | Drug | Number of studies | Drug (adverse events/ sample size) | Placebo (adverse events/sample size) | OR | (95% CI) | NNH |
|---|---|---|---|---|---|---|---|
Death | Aripiprazole | 3 | 8/340 | 3/253 | 2.37 | (0.55, 14.18) | NC |
Death | Olanzapine | 2 | 2/278 | 4/232 | 0.48 | (0.04, 3.62) | NC |
Death | Quetiapine | 2 | 5/185 | 7/241 | 0.91 | (0.22, 3.41) | NC |
Death | Risperidone | 5 | 39/1,561 | 17/916 | 1.19 | (0.63, 2.31) | NC |
| Study type | Study | Subjects/Method/Design/Location | N | Duration | Outcomes/Results | Rating of quality of evidence |
|---|---|---|---|---|---|---|
3 | Older adults with dementia residing in one of nine nursing homes Design: prospective cohort study Location: Hong Kong | 599 subjects | July 2009– December 2010; 18 months of follow-up | The 18-month rate for all-cause mortality in individuals exposed to an antipsychotic medication was 24.1%, while the rate in individuals not exposed to an antipsychotic was 27.5% (P = 0.38). The exposed group also had a lower median rate of all-cause hospitalizations (56 [0–111] per 1,000 person-months vs. 111 [0–222] per 1,000 person-months), median (interquartile range), P < 0.001. | 0 | |
3 | Community- dwelling individuals with mild to moderate Alzheimer’s dementia who were recruited from one of 16 memory centers Design: prospective cohort study Location: France | 534 total subjects; 102 of the subjects were new users of an antipsychotic agent during the follow-up period | 3.5-year follow-up period | 113 deaths occurred during the study. Use of either an FGA or an SGA was not an independent predictive factor of all-cause mortality after adjustment for dementia severity in multivariate analyses using a Cox proportional hazards model (HR = 1.12; 95% CI: 0.59, 2.12). However, there was a suggestion of an increased risk of all-cause mortality with antipsychotic treatment in unadjusted and sociodemographically adjusted models. Common use of tiapride in this study may affect generalizability to U.S. patient populations. | 0 | |
3 | Subjects over 65 years of age who were living in the community and given a new prescription for risperidone, olanzapine, quetiapine, haloperidol, aripiprazole, or ziprasidone. Individuals with a prior diagnosis of schizophrenia, bipolar disorder, or cancer were not included. About one-third of individuals had a diagnosis of dementia, although the proportion of individuals with dementia was greater in those beginning treatment with risperidone, haloperidol, quetiapine, or ziprasidone than in those beginning treatment with olanzapine or aripiprazole. Data were obtained from U.S. Medicare or Medicaid claims databases. Design: retrospective cohort study Location: United States | 136,393 subjects, with 36.2% of the subjects receiving risperidone, 32.5% receiving olanzapine, 19.2% receiving quetiapine, 9.6% receiving haloperidol, 1.4% receiving aripiprazole, and 1.1% receiving ziprasidone | January 1, 2001– December 31, 2005 | Using Cox proportional hazards models to control for dose and propensity score, the study authors found that 180-day mortality risk was increased for haloperidol (HR = 1.18; 95% CI: 1.06, 1.33) and decreased for quetiapine (HR = 0.81; 95% CI: 0.73, 0.89) and olanzapine (HR = 0.82; 95% CI: 0.74, 0.90) relative to risperidone. A similar pattern of findings was observed for specific causes of mortality (e.g., circulatory, cerebrovascular, respiratory). Overall noncancer mortality rate for the sample was 13.6 per 100 person-years (4,216 noncancer deaths, with an additional 180 cancer-related deaths). Unadjusted mortality rates ranged from 31.4 (95% CI: 29.1, 33.7) per 100 person-years for haloperidol to 5.8 (95% CI: 3.5, 8.1) per 100 person-years for aripiprazole. However, haloperidol was given at a higher average dose than were the other agents, and risperidone, olanzapine, and haloperidol each showed a dose–response relationship to mortality risk. (Sample sizes were insufficient to perform such calculation for other agents except quetiapine, which showed no dose-response relationship.) Inclusion of individuals who did not have a diagnosis of dementia limits generalizability. | 0 | |
3A | Subjects over 66 years of age with a diagnosis of dementia who were living in the community or in long-term care and who were identified through Ontario Health Insurance Plan or Discharge Abstract Databases as a new user of antipsychotic medication Design: population-based, retrospective cohort study Location: Canada | 27,259 pairs of individuals matched on the basis of propensity scores | April 1, 1997–March 31, 2002 | In both community-dwelling and long-term care–dwelling individuals, initiating use of an SGA was associated with a significant increase in the risk of death within 30 days as compared with nonuse (adjusted HR = 1.31 [95% CI: 1.02, 1.70] for community-dwelling individuals and 1.55 [95% CI: 1.15, 2.07] for individuals living in long-term care facilities) in multivariate analyses. Corresponding values for absolute risk difference were 0.2% and 1.2%, respectively. Mortality risk remained elevated at 180 days after treatment initiation. Use of an FGA medication was associated with a higher risk of mortality at 30 days than use of an SGA (adjusted HR = 1.55 [95% CI: 1.19, 2.02] for community-dwelling individuals and 1.26 [95% CI: 1.04, 1.53] for individuals living in long-term care facilities). As with initiation of an SGA, this increase in risk was still present at 180 days after initiation of treatment. | 0 | |
3 | Individuals who were residing in a specific city in Finland on January 1, 2000 and were at least 65 years of age Data were obtained from the Finnish National Prescription Register with information on diagnoses obtained from the Special Reimbursement Register. Design: population-based retrospective cohort study Location: Leppävirta, Finland | 2,224 subjects; 332 of the subjects used an antipsychotic medication during the study period. | Follow-up from 2000 to 2008 | Using time-dependent Cox proportional hazard models to assess all-cause mortality, the study authors found that the unadjusted HR for risk of death associated with antipsychotic use was 2.71 (95% CI: 2.3, 3.2). After adjustment for baseline age, sex, antidepressant use, and diagnostic confounders, the HR was 2.07 (95% CI: 1.73, 2.47). Adjusted HR was the highest among antipsychotic users with baseline respiratory disease (HR = 2.21; 95% CI: 1.30, 3.76). Inclusion of individuals who did not have a diagnosis of dementia may limit generalizability. | 0 | |
3 | Subjects age 65 years or older who were taking or had initiated treatment with an antipsychotic, sodium valproate, or carbamazepine Subjects identified through a database of prescriptions written for veterans or war widows. Design: retrospective population-based cohort Location: Australia Funding: Australian Department of Veterans Affairs | 16,634 subjects initiated treatment during the study period, and 9,831 individuals continued treatment with one of the study medications. | 2003–2004 | Using mortality rates, Kaplan-Meier survival analysis, and adjusted Cox proportional hazards analysis, the study authors found that those initiating treatment with haloperidol, chlorpromazine, or risperidone had an increased relative risk of mortality compared with olanzapine (2.26 [95% CI: 2.08, 2.47]; 1.39 [95% CI: 1.15, 1.67], and 1.28 [95% CI: 1.07, 1.40], respectively). For those receiving continued treatment, relative risks of mortality compared with olanzapine were increased for haloperidol (1.38 [95% CI: 1.23, 1.54]) and risperidone (1.24 [95%: 1.10, 1.46]). | 0 | |
3A | Nursing home residents age 65 years or older who had initiated treatment with psychotropics after admission Study design: retrospective population-based cohort Location: British Columbia | 10,900 subjects; 1,942 began treatment with an SGA, 1,902 began treatment with an FGA, 2,169 began treatment with an antidepressant, and 4,887 began treatment with a benzodiazepine. | 1996–2006 | Using proportional hazards models with propensity-score adjustments, the study authors found that users of FGAs had an increased risk of death (RR = 1.47; 95% CI: 1.14, 1.91 for first generation), as compared with users of SGAs. Users of benzodiazepines also had a higher risk of death (RR = 1.28; 95% CI: 1.04, 1.58) compared with users of SGAs. Using subgroup-adjusted propensity scores, individuals who began treatment with an FGA (as compared with users of an SGA) had an increased risk of mortality (RR = 1.37; [95% CI: 0.96, 1.95] for individuals with dementia and 1.61 [95% CI: 1.10, 2.36] for individuals without dementia). Among individuals with no history of antipsychotic treatment, the corresponding RR was 1.33 (95% CI: 0.99, 1.77) as compared with users of an SGA. Inclusion of individuals who did not have a diagnosis of dementia may limit generalizability. | 0 | |
3 | Nursing home residents with dementia age 65 years or older who were eligible for Medicaid and were new users of antipsychotic drugs (haloperidol, aripiprazole, olanzapine, quetiapine, risperidone, ziprasidone) Data were obtained from linked data from Medicaid, Medicare, MDS, National Death Index, and a national assessment of nursing home quality with propensity score adjustment used to control for potential confounders. Design: observational-retrospective cohort Location: United States | 75,445 subjects | 2001–2005 | Compared with users of risperidone, users of haloperidol had an increased 180-day risk of all-cause and cause-specific mortality (HR = 2.07; 95% CI: 1.89, 2.26), and users of quetiapine had a decreased risk (HR = 0.81; 95% CI: 0.75, 0.88). There was a dose-response relationship noted for all drugs except quetiapine, and the risk of mortality was increased with higher doses of medication. | 0 | |
3A | Subjects age 65 years or older who had a diagnosis of dementia and began outpatient treatment with an FGA or an SGA (risperidone, olanzapine, quetiapine, aripiprazole, ziprasidone, clozapine) Data were from Department of Veterans Affairs national database. Design: observational retrospective cohort Location: United States | 10,615 subjects | 2001–2005 | Mortality rates at 12 months did not differ for individuals treated with an SGA as compared with an FGA. Individuals treated with an antipsychotic had a higher rate of mortality at 12 months (22.6%–29.1%) as compared with those treated with non-antipsychotic medications (14.6%). | 0 | |
3 | Subjects age 65 years or older who had a diagnosis of dementia and began outpatient treatment with an antipsychotic (risperidone, olanzapine, quetiapine, or haloperidol) or valproic acid or its derivatives (as a non-antipsychotic comparison) Data were from Department of Veterans Affairs national database; data analyzed using multivariate models and propensity adjustments; covariate-adjusted intent-to-treat analyses; analyses were controlled for site of care and medication dosage Design: observational retrospective cohort Location: data obtained from the United States | 33,604 subjects | Fiscal years 1999–2008; compared 180-day mortality rates | In covariate-adjusted intent-to-treat analyses, haloperidol was associated with the highest mortality rates (relative risk = 1.54; 95% CI: 1.38, 1.73) followed by risperidone (reference), olanzapine (relative risk = 0.99; 95% CI: 0.89, 1.10), valproic acid and its derivatives (relative risk = 0.91; 95% CI: 0.78, 1.06), and quetiapine (relative risk = 0.73; 95% CI: 0.67, 0.80). Mortality risk with haloperidol was highest in the first 30 days but decreased significantly and sharply thereafter. Among the other agents, mortality risk differences were most significant in the first 120 days and declined in the subsequent 60 days during follow-up. | 0 | |
3 | Outpatients with dementia age 65 years or older who were prescribed antidementia drugs and psychotropic medications Subjects were identified through the Norwegian Prescription Database Study. Design: population-based cohort study Location: Norway | 26,940 subjects | 2004–2010 | Using Cox survival analyses, with adjustment for age, gender, mean daily defined dose, and severe medical conditions, the study authors found that antipsychotic use, as compared with use of other psychotropic agents, was associated with an approximately twofold increase in mortality at all studied time points after first dispensation (HR at 30 days = 2.1 [95% CI: 1.6, 2.9] to HR at 730–2,400 days = 1.7 [95% CI: 1.6, 1.9]). Haloperidol was associated with higher mortality risk (HR at 30 days = 1.7 [95% CI: 1.0, 3.0] to HR at 730–2,400 days = 1.4 [95% CI: 1.0, 1.9]) as compared with risperidone. | 0 | |
3A | Subjects with dementia over 65 years of age who were newly prescribed quetiapine, olanzapine, risperidone, clozapine, or an FGA Subjects were identified through the Systematic Assessment of Geriatric Drug Use via Epidemiology database (Medicare- or Medicaid-certified nursing facilities in five states in the United States). Design: observational-retrospective cohort Location: United States | 9,729 subjects | 1998–2000 | Rates of all-cause mortality were greater in individuals using FGAs as compared with those using SGAs (HR = 1.26; 95% CI: 1.13, 1.42). | 0 | |
3 | Outpatients with a diagnosis of probable Alzheimer’s disease (of mild to moderate severity) who had at least one follow-up evaluation Design: observational cohort study Location: United States Funding: National Institute on Aging, National Institute of Mental Health | 957 subjects; 241 (25%) of the subjects were exposed to antipsychotics at some time during follow-up (138 to an FGA, 95 to an SGA, and 8 to both) | Mean follow-up time, 4.3 years (SD = 2.7); range, 0.78–18.0 years | Death was more frequent in individuals taking an FGA than in individuals taking an SGA (69% vs. 34%, respectively). Nursing home admission was also more frequent in individuals taking an FGA than in individuals taking an SGA (63% vs. 23%, respectively). However, after adjusting for psychiatric symptoms using Cox proportional hazard models that adjusted for different combinations of age, gender, education level, dementia severity, hypertension, diabetes mellitus, heart disease, extrapyramidal signs, depression, psychosis, aggression, agitation, and dementia medication use, the study authors found that the associations between antipsychotic use and mortality or nursing home admission were no longer significant. Psychosis was strongly associated with nursing home admission and time to death. Neither FGAs nor SGAs were associated with time to death. | 0 | |
3 | Subjects age 65 years or older with a diagnosis of dementia Subjects were identified through a Veterans Health Administration database. Design: observational-retrospective case-control study Location: United States | 90,786 subjects; 46,008 of the subjects had received a new prescription for an antipsychotic (haloperidol, olanzapine, quetiapine, or risperidone), valproic acid and its derivatives, or an antidepressant | October 1, 1998– September 30, 2009 | In comparisons with respective matched nonusers of psychotropic medication, the increased mortality risk over 180 days of follow-up was 3.8% (95% CI: 1.0%, 6.6%; P < 0.01), with an NNH of 26 (95% CI: 15, 99), in individuals receiving haloperidol; 3.7% (95% CI: 2.2%, 5.3%; P < 0.01), with an NNH of 27 (95% CI: 19, 46), in individuals receiving risperidone; 2.5% (95% CI: 0.3%, 4.7%; P=0.02), with an NNH of 40 (95% CI: 21, 312), in individuals receiving olanzapine; and 2.0% (95% CI: 0.7%, 3.3%; P < 0.01), with an NNH of 50 (95% CI: 30, 150), in individuals receiving quetiapine. In comparisons with antidepressant users, mortality risk ranged from 12.3% (95% CI: 8.6%, 16.0%; P < 0.01), with an NNH of 8 (95% CI: 6, 12), for haloperidol users to 3.2% (95% CI: 1.6%, 4.9%; P < 0.01), with an NNH of 31 (95% CI: 21, 62), for quetiapine users. As a group, SGAs (olanzapine, quetiapine, and risperidone) showed a dose-response increase in mortality risk, with 3.5% greater mortality (95% CI: 0.5%, 6.5%; P = .02) in the high-dose subgroup relative to the low-dose group. In direct comparisons with quetiapine, dose-adjusted mortality risk was increased with both risperidone (1.7%; 95% CI: 0.6%, 2.8%; P = 0.003) and olanzapine (1.5%; 95% CI: 0.02%, 3.0%; P = 0.047). | 0 | |
3 | Musicco et al. 2011 | Subjects with dementia age 60 years or older who were newly prescribed an antidementia drug (donepezil, rivastigmine, or galantamine) Subjects were identified via the Italian Health Information System. Design: observational-retrospective cohort Location: Milan, Italy | All 4,369 residents of Milan (Italy) age 60 years or older who were newly prescribed an antidementia drug. All new users of antipsychotic drugs in this cohort were categorized according to whether antipsychotic was conventional (n = 156) or second-generation (n = 806), for a total of 962 subjects in this cohort taking antipsychotic drugs | January 2002–June 2008 | Mortality was increased two- and fivefold in users of SGAs and conventional antipsychotics, respectively, as compared with nonusers of antipsychotic medication. | 0 |
3 | Outpatients with dementia over 65 years of age who were seen at an Alzheimer Evaluation Unit Design: observational-retrospective cohort Location: Italy | 696 individuals; 375 of the subjects were treated with an SGA (quetiapine, risperidone, or olanzapine) | January 2007–December 2009 | Relative risk of death in patients treated with SGAs was 2.354 (95% CI: 1.704, 3.279) as compared with subjects not treated with antipsychotic medication. Quetiapine was most commonly prescribed, and an association was seen between higher doses of this drug and higher mortality rates. | 0 | |
3 | Subjects age 65 years or older who had dementia with behavioral and psychological symptoms and who were new users of SGAs and were seen at a Dementia Evaluation Unit Design: prospective cohort study Location: Italy | 1,618 subjects | Subjects enrolled between September 2006 and March 2010, with an average follow-up of 309 days | At least one adverse event was noted in 9.3% of the 1,618 new users of SGAs. Adverse effects included drug therapeutic failure (3.0%), extrapyramidal symptoms (0.5%), and stroke (0.2%). Death occurred in 5.1%, and the crude all-cause mortality rate was 6.0 per 100 person-years (95% CI: 4.8, 7.4). Mortality rates were higher in patients aged >85 years (9.0 per 100 person-years; 95% CI: 6.4, 12.7) and among male patients (7.5 per 100 person-years; 95% CI: 5.3, 10.6). In the multivariate analysis, only age was associated with all-cause mortality (HR = 1.1 [95% CI: 1.0, 1.1] and 1.4 [95% CI: 0.9, 2.2], respectively), whereas hallucinations (HR = 0.4; 95% CI: 0.2, 0.6) and dosage changes (HR = 0.4; 95% CI: 0.2, 0.78) were associated with a significantly lower risk of all-cause mortality. | 0 | |
3A | Subjects over 66 years of age with a diagnosis of dementia Subjects were identified via Ontario, Canada, administrative health care data. Design: observational-retrospective cohort Location: Ontario, Canada Funding: Canadian Institutes of Health Research | 20,682 community-dwelling and 20,559 nursing home–dwelling subjects | April 1, 1997 and March 31, 2004 | Likelihood of experiencing a serious adverse event (e.g., life-threatening, causing significant disability or death) was significantly greater in individuals treated with an FGA (3.8-fold increase; 95% CI: 3.31, 4.39) or SGA (3.2-fold increase; 95% CI: 2.77, 3.68) as compared with individuals who were not treated with an antipsychotic medication. | 0 | |
3 | Older adults with dementia who were newly prescribed oral SGA therapy; median age = 84 years Design: observational-retrospective cohort Location: Ontario, Canada | 21,526 subjects (13,760 women, 7,766 men) | April 1, 2007, and March 1, 2010 | 1,889 subjects (8.8%) had a serious event, which was defined as a hospital admission or death within 30 days of treatment initiation (1,044 women, 7.6%; 845 men, 10.9%). Of these, 363 women (2.6%) and 355 men (4.6%) died. Men were more likely than women to be hospitalized or die during the 30-day follow-up period (adjusted OR = 1.47, 95% CI: 1.33, 1.62) and consistently more likely to experience a serious event in each stratum. A gradient of risk according to drug dose was found for the development of a serious event in women and men. | 0 | |
3A | Subjects over 65 years of age with a diagnosis of dementia Subjects were veterans identified through an administrative Veterans Health Administration National Patient Care Database. Design: observational-retrospective cohort Location: United States | 18,127 subjects (predominantly male) Subjects treated with antipsychotic (haloperidol [n = 2,217], olanzapine [n = 3,384], quetiapine [n = 4,277], or risperidone [n = 8,249]) were compared with those not taking an antipsychotic. | October 1999–September 2005 | During the initial 30 days of use, there was greater mortality in individuals exposed to haloperidol (5.4%), olanzapine (2.7%), or risperidone (2.8%), but not quetiapine (1.7%) as compared with individuals not taking an antipsychotic (1.7%), with unadjusted hazard ratios of 1.4, 1.6, 1.4, and 1.4, respectively. After the initial 30-day period, there was no difference in mortality among any of the antipsychotic-treated groups or when compared with individuals who did not receive treatment with an antipsychotic. | 0 | |
3 | Subjects with probable Alzheimer’s disease Design: prospective cohort Location: United States | 641 subjects | Mean follow-up time after the baseline visit to censoring or death: 3.0 (± 1.94) years | Using multivariable Cox proportional hazard regression analysis, the study authors found that time-dependent changes in antipsychotic drug use, development of psychotic symptoms, antidementia drug use, and observed MMSE change were not predictive of time to death. Overall disease severity at baseline, medical comorbidities, and education also did not influence time to death. Baseline covariates significantly associated with increased survival were younger age (P = 0.0016), female sex (P = 0.0001), and a slower rate of initial cognitive decline from symptom onset to cohort entry (P < 0.0001). Median survival time following the onset of symptoms was 11.3 years (95% CI: 10.4, 11.8). | 0 | |
3A | Subjects over 65 years of age who were being treated with an antipsychotic (risperidone, quetiapine, olanzapine, clozapine, or an FGA) Subjects were identified via a British Columbia Ministry of Health Pharmanet database. Design: observational-retrospective cohort Location: British Columbia, Canada Funding: government funded | 37,241 subjects were identified as meeting inclusion criteria; 12,882 of the identified subjects initiated treatment with an FGA, and 24,359 initiated treatment with an SGA. | January 1, 1996 to December 31, 2004 | Risk of death with FGAs was at least as high in terms of all-cause mortality as (and perhaps greater than) risk of death with SGAs (14.1% vs. 9.6%, mortality ratio 1.47 [95% CI: 1.39, 1.56]). Using multivariable and propensity score–adjusted modeling, the study authors found that the adjusted hazard ratio for mortality within 180 days of FGA initiation relative to SGA initiation was 1.27 (95% CI: 1.18, 1.37) for noncancer mortality, 1.23 (95% CI: 1.10, 1.36) for cardiovascular mortality, and 1.71 (95% CI: 1.35, 2.17) for respiratory mortality other than that due to pneumonia. Overall cardiovascular mortality and out-of-hospital cardiovascular mortality were each greater for doses of FGAs greater than the median prescribed dose. Hazard ratios for cardiovascular death with FGA as compared with SGA agents were also greatest in the initial days to weeks after treatment initiation. When data for individuals with dementia were analyzed separately, there was no difference in hazard ratios for overall cardiovascular mortality or for out-of-hospital cardiovascular mortality with FGAs as compared with SGAs (1.12 [95% CI: 0.80, 1.56] and 1.00 [95% CI: 1.22, 1.82], respectively). Inclusion of individuals who did not have a diagnosis of dementia may limit generalizability. | 0 | |
3 | Subjects who had stayed for at least 1 day in a long-term care facility Subjects identified based on data from a Medicare Current Beneficiary Survey. Design: retrospective cohort Location: United States Funding: government funded | 2,363 subjects; 742 of the subjects were treated with an antipsychotic during the first 6 months of a study year (194 were treated with an FGA and 456 with an SGA). | 1999–2002 | Using multiple Cox proportional hazards models and controlling for covariates, the study authors found that the adjusted hazard ratio for mortality with antipsychotic use relative to no antipsychotic use was 0.83 (95% CI: 0.69, 1.00) and was 0.89 (95% CI: 0.67, 1.19) for FGAs and 0.77 (95% CI: 0.62, 0.96) for SGAs analyzed separately. When the analysis was limited to individuals with a diagnosis of dementia, the adjusted hazard ratio was 0.77 (95% CI: 0.60, 0.98). | 0 | |
3 | Subjects with vascular dementia Subjects were identified via anonymized versions of electronic health records from two National Health Service Foundation Trusts. Design: observational-retrospective cohort study Location: United Kingdom | 1,531 subjects; 337 of the subjects were exposed to quetiapine, risperidone, or olanzapine | 2007–2010 | No significant increases in mortality were noted in subjects exposed to SGAs (HR = 1.05; 95% CI: 0.87, 1.26), risperidone (HR = 0.85; 95% CI: 0.59, 1.24), or quetiapine (HR = 1.14; 95% CI: 0.93, 1.39; P = 0.20) compared with untreated patients. Too few patients were exposed to olanzapine alone to provide reliable results. | 0 | |
3 | Subjects 65 years or older who initiated treatment with antipsychotic medication Subjects identified through a drug insurance benefits database in Pennsylvania Design: retrospective cohort study Location: United States Funding: government funded | 22,890 subjects; 9,142 initiated treatment with an FGA, and 13, 748 initiated treatment with an SGA. | 1994–2003 | Within 180 days of antipsychotic initiation, mortality occurred in 17.9% of individuals treated with an FGA and in 14.6% of those treated with an SGA. Using Cox proportional hazards models, the study authors found that the adjusted hazard ratio for mortality with FGA as compared with SGA treatment was 1.37 (95% CI: 1.27, 1.49), with the highest hazard ratio in the initial 40 days of treatment (1.56 [95% CI: 1.37, 1.78]). For the subgroup of subjects with dementia, the adjusted hazard ratio for mortality with FGA as compared with SGA treatment was 1.29 (95% CI: 1.15, 1.45). | 0 |
Quality of the Body of Research Evidence for Harm Related to Mortality
Cerebrovascular Accidents
Overview and Quality of Individual Studies
| Adverse effect | Drug | Number of studies | Drug (adverse events/ sample size) | Placebo (adverse events/sample size) | OR | (95% CI) | NNH |
|---|---|---|---|---|---|---|---|
Stroke | Aripiprazole | 3 | 2/340 | 2/253 | 0.70 | (0.05, 10.48) | NC |
Stroke | Olanzapine | 2 | 6/278 | 4/232 | 1.46 | (0.33, 7.44) | NC |
Stroke | Quetiapine | 2 | 3/185 | 6/241 | 0.65 | (0.10, 3.08) | NC |
Stroke | Risperidone | 4 | 24/1,099 | 8/753 | 3.12 | (1.32, 8.21) | 53 |
| Study type | Study | Subjects/Method/Design/Location | N | Duration | Outcomes/Results | Rating of quality of evidence | ||
|---|---|---|---|---|---|---|---|---|
3A | Subjects over 65 years of age with a diagnosis of dementia Subjects identified via Veterans Administration and Medicare databases Design: longitudinal cohort study Location: United States | 14,029 subjects | 2002–2003, followed for 18 months | In comparisons with individuals who did not receive an antipsychotic, the risk of a cerebrovascular event (defined as an inpatient admission with a primary or principal diagnosis of cerebrovascular event by ICD-10-CM codes) was comparable for individuals treated with an FGA (HR = 1.29; 95% CI: 0.48, 3.47) or an SGA (HR = 1.20; 95% CI: 0.83, 1.74). | 0 | |||
3 | Subjects age 65 years or older who were diagnosed with Alzheimer’s disease, vascular dementia, or mixed dementia, with behavioral/psychological symptoms and had an initial visit during the study period Design: retrospective cohort study Location: Hong Kong | 1,089 subjects; 654 of the subjects had been treated with an FGA, 72 with an SGA, and 363 with no antipsychotic | January 1, 2000– June 30, 2007 | Risk of cerebrovascular adverse events (calculated by Cox regression analysis) did not differ among those treated with an FGA (adjusted HR = 0.964; 95% CI: 0.584, 1.591) or SGA (adjusted HR = 1.036 (95% CI: 0.350, 3.066) as compared with those not treated with an antipsychotic. Incidence rates for cerebrovascular adverse events were 44.6/1,000, 32.7/1,000, and 49.6/1,000 person years, respectively. | 0 | |||
3 | Community-dwelling elderly subjects, age 50 years or older Risperidone, olanzapine, or quetiapine was initiated anytime during study period. Data were obtained from IMS LifeLink Health Plan claims database. Design: observational-retrospective cohort. Authors used propensity-score adjustments. Location: United States | 12,145 subjects; 5,083 of the subjects were treated with risperidone, 4,377 with olanzapine, and 2,685 with quetiapine | Recruitment occurred from July 1, 2000–June 30, 2008. Patients were followed until hospitalization or an emergency room visit for a cerebrovascular event, or until the end of the study period, whichever occurred earlier. | 2,458 total cerebrovascular events were identified in the study cohort: 1,081 of 5,083 (21%) risperidone users, 816 of 4,377 (19%) olanzapine users, and 561 of 2,685 (21%) quetiapine users. As compared with use of olanzapine, there was a decreased risk of cerebrovascular adverse events associated with use of quetiapine (HR = 0.88; 95% CI: 0.78, 0.99) but not risperidone (HR = 1.05: 95% CI: 0.95, 1.16) as derived from Cox proportional hazard analysis, which adjusted for multiple propensity scores and other medication exposures. | 0 | |||
3 | Subjects with evidence of treatment for dementia who were aged 60 or older and first prescribed an antipsychotic Subjects were identified via a Medicaid database. Design: retrospective cohort Location: United States Funding: Ortho McNeil Janssen | 18,987 subjects, with 1,260 of the subjects starting haloperidol, 4,137 risperidone, 2,928 olanzapine, and 710 on quetiapine | 1999–2002 | Using logistic regression, the study authors found that in comparison to risperidone, the odds ratios for an acute admission for a cerebrovascular event in the initial 90 days of treatment were 1.91 (95% CI: 1.02, 3.60) for haloperidol, 1.05 (95% CI: 0.63, 1.73) for olanzapine, and 0.66 (95% CI: 0.23, 1.87) for quetiapine. | 0 | |||
3 | Subjects with dementia age 65 or older who were first prescribed an antipsychotic during the observation period Design: retrospective population-based cohort Location: Ontario, Canada Funding: Canadian Institute of Health Research | 32,710 subjects; 14,865 of the subjects were started on an FGA, and 17,845 on an SGA | April 1, 1997–March 31, 2002 | Crude incidence rates of hospitalization for ischemic stroke were comparable for FGAs and SGAs (1.5% and 1.6%, respectively). Cox proportional hazards model, including covariates for sociodemographic factors, comorbid conditions, and concomitant medication use, also showed no differences between FGAs and SGAs (adjusted hazard ratio = 1.01 [95% CI: 0.81, 1.26]). | 0 | |||
3 | Subjects over 66 years of age who were first prescribed an antipsychotic during the observation period Subjects identified from approximately 1.4 million potential subjects in administrative health care databases in Ontario, Canada. Design: retrospective population-based cohort Location: Ontario, Canada Funding: no pharmaceutical funding received | 11,400 subjects; 1,015 of the subjects were first prescribed an FGA, 6,964 risperidone, and 3,421 olanzapine | April 1, 1997–March 31, 2002 | In comparisons with treatment with an FGA, covariate adjusted relative risk estimates for stroke were 1.1 (95% CI: 0.5, 2.3) for olanzapine and 1.4 (95% CI: 0.7, 2.8) for risperidone, suggesting no statistically significant increase in the risk of stroke. Inclusion of individuals who did not have a diagnosis of dementia limits generalizability. | 0 | |||
3 | Subjects age 65 years or older with an incident diagnosis of Alzheimer’s or vascular dementia, compared with a group of dementia-free patients. Subjects were identified from the United Kingdom–based General Practice Research Database. Design: nested case-control follow-up study Location: United Kingdom Funding source: unconditional pharmaceutical company grant | 18,729 subjects; 6,443 case subjects had Alzheimer’s dementia, 2,302 had vascular dementia, and 9,984 had no dementia diagnosis | 1998 and 2008 | During the follow-up, there were 281 case subjects with incident ischemic stroke, 139 with hemorrhagic stroke, and 379 with a transient ischemic attack. The incidence rates of ischemic stroke for patients with Alzheimer’s dementia, vascular dementia, or no dementia were 4.7/1,000 person-years (95% CI: 3.8, 5.9), 12.8/1,000 person-years (95% CI: 9.8, 16.8), and 5.1/1,000 person-years (95% CI: 4.3, 5.9), respectively. In comparison with dementia-free patients, the odds ratio of developing a transient ischemic attack when treated with SGAs was increased for patients with Alzheimer’s dementia (OR = 4.5; 95% CI: 2.1, 9.2) but not those with vascular dementia. | 0 | |||
3 | Community-dwelling patients age 50 years or older who were prescribed at least one antipsychotic medication during the study period without having received an antipsychotic prescription for at least the preceding year Subjects were identified through Dutch community pharmacies and hospital discharge records. Design: nested case-control study Location: Netherlands Funding: no external funding | 26,157 individuals (mean age = 76 ± 9.7 years) met inclusion criteria; 518 of these individuals had a hospital admission for a cerebrovascular event and were matched by sex and age to four randomly selected individuals from the cohort | 1986–2003 | Current exposure and recent exposure to antipsychotics were associated with an increased risk of a cerebrovascular event compared with non-users (OR = 1.7; 95% CI: 1.4, 2.2). A strong temporal relationship was found; the OR for a history of use less than a week is 9.9 (5.7–17.2). Risk decreases in time and is comparable to that for non-users after 3 months of use (OR = 1.0; 95% CI: 0.7, 1.3). Inclusion of individuals who did not have a diagnosis of dementia limits generalizability. | 0 | |||
3 | Subjects age 65 years or older with a diagnosis of dementia who were prescribed an FGA or SGA Subjects were identified via electronic primary care records in the General Practice Research Database. Design: observational–case control Location: United Kingdom Funding: Foundation | 26,885 subjects; 3,149 of the subjects were eligible for the study and were matched to 15,613 control subjects | January 1, 1995– June 22, 2007 | After adjustment for confounding variables, the OR of a CVA associated with use of only FGAs versus no antipsychotic use in individuals with dementia age 65 or older was 1.16 (95% CI: 1.07, 1.27), and the OR for use of only SGAs versus no antipsychotics was 0.62 (95% CI: 0.53, 0.72). In the comparison of FGAs and SGAs, the OR was 1.83 (95% CI: 1.57, 2.14). FGAs appear to be associated with a higher risk of CVA, although the risk disappears with medication discontinuation. | 0 | |||
3 | Subjects 65 years or older, residing in nursing facilities, with a diagnosis of dementia. Subjects were identified via Medicare data and the Systematic Assessment of Geriatric drug use via Epidemiology database. Design: retrospective, case-control Location: United States Funding: National Institutes of Health | 4,788 subjects; 1,130 of the subjects had been hospitalized for a stroke or transient ischemic attack; 3,658 control case subjects from the same facility had been hospitalized for septicemia or a urinary tract infection | June 30, 1998–December 27, 1999 | Using conditional logistic regression, the study authors found that users of antipsychotic medication did not have a significant difference in the odds ratio of being hospitalized for a cerebrovascular event as compared with non-users of antipsychotic medication. Odds ratio of a cerebrovascular event was 0.87 (95% CI: 0.67, 1.12) for risperidone, 1.32 (95% CI: 0.83, 2.11) for olanzapine, 1.57 (95% CI: 0.65, 3.82) for other SGAs, and 1.24 (95% CI: 0.95, 1.63) for FGAs. | 0 | |||
3 | Subjects age 65 years or older who either had dementia with at least one inpatient service claim or at least two ambulatory care claims or were randomly chosen from the population as a sex-, age-, and index year–matched comparison subject All subjects were identified using the Taiwanese Longitudinal Health Insurance Database for 2005. Design: case-control Location: Taiwan | 2,243 subjects with dementia; 1,450 of the subjects were treated with antipsychotic; 6,714 matched comparison subjects | 5 years of follow-up | Using Cox proportional-hazard regression, the study authors found that dementia patients had a twofold greater risk of developing stroke within 5 years of diagnosis compared with matched nonsubjects, after adjustment for other risk factors (95% CI: 2.58, 3.08; P < 0.001). Antipsychotic usage among patients with dementia increases risk of stroke 1.17-fold compared with patients without antipsychotic treatment (95% CI: 1.01, 1.40; P < 0.05). | 0 | |||
3 | Subjects age 65 or older who had an inpatient admission for a cerebrovascular-related event Subjects were identified via a national database of outpatient prescriptions with record linkage. Design: retrospective population-based cohort Location: Lombardy, Italy Funding: not specified | 1,645,978 subjects; 36,075 of the subjects were exposed to an antipsychotic, with 9,265 exposed to an SGA | 2001 | Using logistic regression analysis, the study authors found that the odds ratio of a CVA in subjects receiving an antipsychotic was 1.24 (95% CI: 1.16, 1.32) relative to those not receiving an antipsychotic. Adjusted odds ratios were 1.42 (95% CI: 1.24, 1.69) for those receiving an SGA as compared with an FGA and 1.57 (95% CI: 1.08, 2.30) for those receiving risperidone as compared with haloperidol. No significant difference was noted in the odds ratio for CVA in those receiving clozapine, olanzapine, or quetiapine as compared with haloperidol. Inclusion of individuals who did not have a diagnosis of dementia limits generalizability. | 0 | |||
3A | Subjects over 65 years of age who were identified via an Australian Government Department of Veterans’ Affairs database Design: observational, self-controlled case series Location: Australia | 10,638 subjects, with 514 receiving treatment that included initiation of an FGA and 564 receiving treatment that included initiation of an SGA | January 1, 2003– December 31, 2006 | In the first week after initiation of an FGA, there was an increased risk of hospital admission for stroke (IRR = 2.3; 95% CI: 1.3, 3.8), whereas no such risk was seen after initiation of an SGA. Inclusion of individuals who did not have a diagnosis of dementia limits generalizability. | 0 | |||
3 | Subjects over 65 years of age identified via data from a health search database of primary care patients in Italy Design: observational-retrospective cohort Location: Italy Funding: Health Authority of the Lombardia Region | 69,939 identified as non-users of antipsychotic medication; 4,223 had received an initial antipsychotic prescription during the study period (599 with atypicals, 749 with butyrophenones, 907 with phenothiazines, and 1,968 with substituted benzamides) | January 2000– June 2003 | Crude incidence of stroke was 12.0/1,000 person-years for subjects not exposed to antipsychotic as compared with 47.1, 72.7, 25.0, and 47.4 per 1,000 person-years for those prescribed butyrophenones, phenothiazines, substituted benzamides, and SGAs, respectively. Using multivariate Cox proportional regression analysis, the study authors found that the adjusted risk ratio for stroke as compared with subjects without antipsychotic exposure was 5.79 (95% CI: 3.07, 10.9), 3.55 (95% CI: 1.56, 8.07), and 2.46 (95% CI: 1.07, 5.65) for butyrophenones, phenothiazines, and SGAs, respectively. As compared with SGAs, the adjusted risk ratio for stroke was 1.44 (95% CI: 0.55, 3.76) for butyrophenones and 2.34 (95% CI: 1.01, 5.41) for phenothiazines.Inclusion of individuals who did not have a diagnosis of dementia limits generalizability. . | 0 | |||
3A | Subjects identified as being over 50 years of age based on data from a Health Search database of primary care patients in Italy Design: observational-retrospective cohort Location: Italy | 128,308 subjects (total who were identified as meeting inclusion criteria) | Not stated | Risk of stroke at the end of the first month of treatment was 12.4 times higher in individuals treated with antipsychotic as compared with those without antipsychotic exposure. However, absolute differences were small in terms of the cumulative proportion surviving (0.9921 [95% CI: 0.9899, 0.9943] with antipsychotic vs. 0.9995 [95% CI: 0.9979, 0.9983] without antipsychotic at 1 month; 0.9819 [95% CI: 0.9761, 0.9879] with antipsychotic vs. 0.9964 [95% CI: 0.9960, 0.9968] without antipsychotic at 6 months). | 0 | |||
Quality of the Body of Research Evidence for Harm Related to Cerebrovascular Accidents
Cardiovascular Events
Overview and Quality of Individual Studies
| Adverse effect | Drug | Number of studies | Drug (adverse events/sample size) | Placebo (adverse events/sample size) | OR | (95% CI) | NNH |
|---|---|---|---|---|---|---|---|
Cardiovascular | Aripiprazole | 1 | 42/366 | 12/121 | 1.18 | (0.58, 2.55) | NC |
Cardiovascular | Olanzapine | 5 | 40/778 | 9/440 | 2.33 | (1.08, 5.61) | 48 |
Cardiovascular | Quetiapine | 3 | 29/355 | 15/254 | 1.08 | (0.53, 2.30) | NC |
Cardiovascular | Risperidone | 6 | 119/1,757 | 34/1,010 | 2.08 | (1.38, 3.22) | 34 |
| Study type | Study | Subject/Method/Design/Location | N | Duration | Outcomes/Results | Rating of quality of evidence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
3 | Older community-dwelling patients who began treatment with a cholinesterase inhibitor treatment Subjects were identified via the Quebec, Canada, prescription claims database. Design: observational-retrospective cohort Location: Quebec, Canada | 37,138 subjects; 10,969 (29.5%) of the subjects started antipsychotic treatment during the follow-up period and were matched with a sample of individuals who did not use antipsychotic medications. | January 1, 2000– December 31, 2009 | Of individuals who started taking antipsychotic medication, 1.3% of them had an MI within the initial year of treatment. Hazard ratios were 2.19 (95% CI: 1.11, 4.32) for the first 30 days, 1.62 (95% CI: 0.99, 2.65) for the first 60 days, 1.36 (95% CI: 0.89, 2.08) for the first 90 days, and 1.15 (95% CI: 0.89, 1.47) for the first 365 days based on Cox proportional hazards models, with adjustment for age, sex, cardiovascular risk factors, psychotropic drug use, and propensity scores. A self-controlled case series study using Poisson regression in 804 instances of MI in new users of antipsychotic showed IRRs of 1.78 (95% CI: 1.26, 2.52) for 1–30 days, 1.67 (95% CI: 1.09, 2.56) for 31–60 days, and 1.37 (95% CI: 0.82, 2.28) for 61–90 days. | 0 | |||||||
3A | Subjects over 65 years of age who were being treated with an antipsychotic (risperidone, quetiapine, olanzapine, clozapine, or FGA) Subjects were identified via data from a British Columbia Ministry of Health Pharmanet database. Design: observational-retrospective cohort Location: British Columbia, Canada Funding: government funded | 37,241 subjects identified as meeting inclusion criteria; 12,882 of the identified subjects initiated treatment with an FGA, and 24,359 initiated treatment with an SGA | January 1, 1996 to December 31, 2004 | Using multivariable and propensity score–adjusted modeling, the study authors found that the adjusted hazard ratio for mortality within 180 days of FGA initiation relative to SGA initiation was 1.23 (95% CI: 1.10, 1.36) for cardiovascular mortality. Overall cardiovascular mortality and out-of-hospital cardiovascular mortality were each greater for doses of FGAs greater than the median prescribed dose. Hazard ratios for cardiovascular death with FGAs as compared with SGAs were also greatest in the initial days to weeks after treatment initiation. When data for individuals with dementia were analyzed separately, there was no difference in hazard ratios for overall cardiovascular mortality or for out-of-hospital cardiovascular mortality with FGAs as compared with SGAs (1.12 [95% CI: 0.80, 1.56] and 1.00 [95% CI: 1.22, 1.82], respectively). Inclusion of some individuals who did not have a diagnosis of dementia may limit generalizability. | 0 | |||||||
Quality of the Body of Research Evidence for Harm Related to Cardiovascular Events
Pulmonary-Related Adverse Events
Overview and Quality of Individual Studies
| Adverse effect | Drug | Number of studies | Drug (adverse events/sample size) | Placebo (adverse events/sample size) | OR | (95% CI) | NNH |
|---|---|---|---|---|---|---|---|
Pulmonary | Aripiprazole | 1 | 6/106 | 3/102 | 1.97 | (0.41, 12.54) | NC |
Pulmonary | Olanzapine | 1 | 0/204 | 3/94 | 0.00 | (0.00, 1.10) | NC |
Pulmonary | Risperidone | 1 | 6/196 | 3/94 | 0.96 | (0.20, 6.05) | NC |
| Study type | Study | Subject/Method/Design/Location | N | Duration | Outcomes/Results | Rating of quality of evidence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
3A | Nursing home residents age 65 years or older who had treatment with psychotropics initiated after admission Design: retrospective population-based cohort Location: British Columbia | 10,900 individuals; 1,942 of the subjects started taking an SGA, 1,902 started taking an FGA, 2,169 started taking an antidepressant, and 4,887 started taking a benzodiazepine | 1996–2006 | There was no difference observed in the risk of heart failure or pneumonia in individuals receiving FGAs, as compared with SGAs, with RR of 1.03 (95% CI: 0.62, 1.69) and 0.91 (95% CI: 0.41, 2.01), respectively. Inclusion of individuals who did not have a diagnosis of dementia may limit generalizability. | 0 | |||||||
3 | Community-dwelling subjects, age 65 or older, who had been exposed to antipsychotic medication Subjects identified via a national pharmacy database. Design: observational-retrospective nested case control study Location: Netherlands Funding: no industry funding | 22,944 subjects received an antipsychotic during the study period; 543 of these subjects had a hospitalization for pneumonia, and 2,163 randomly selected individuals served as controls | April 1985–December 2003 | Using multivariate logistic regression, the study authors found that current use of an antipsychotic as compared with no prior antipsychotic use was associated with an increased likelihood of hospitalization for pneumonia (adjusted odds ratio = 1.6; 95% CI: 1.3, 2.1), whereas past use of an antipsychotic did not show an effect. Lack of information about dementia diagnosis may limit generalizability. | 0 | |||||||
3* | Subjects over 65 years of age who were exposed to antipsychotic medication Subjects identified via the Australian Department of Veterans’ Affairs Health Care Claims Database. Design: observational-retrospective cohort Location: Australia Funding: Australian Government | 8,235 subjects had at least one hospitalization for hip fracture, and of these 494 had begun receiving an FGA and 1,091 had begun receiving an SGA; 13,324 had at least one hospitalization for pneumonia, and of these 807 had begun receiving an FGA and 1,107 had begun receiving an SGA during the study period | 2005–2008; median follow-up: 3.3–4.0 years | Using a self-controlled case-series design, the study authors found that the risk of hospitalization for pneumonia was increased during all postexposure periods for both FGA and SGA and remained significantly increased with more than 12 weeks of continuous exposure (IRR = 1.43; 95% CI: 1.23, 1.66). Risk of pneumonia was elevated for up to 12 weeks prior to the initiation of FGAs or SGAs. | 0 | |||||||
3 | Subjects age 65 years or older with dementia Subjects were identified via the German Pharmacoepidemiological Research Database. Design: nested case-control study Location: Germany Funding: no pharmaceutical funding | 72,591 individuals in total cohort, from which there were 1,028 VTE cases and 4,109 controls matched to each case according to age, sex, health insurance, and calendar time of the VTE | 2004–2007 | Using multivariate conditional logistic regression, the study authors found an increased risk of VTE for current users of antipsychotic medication (OR = 1.23; 95% CI: 1.01–1.50) and for users of a combination of an FGA and an SGA (OR = 1.62; 95% CI: 1.15, 2.27). In current users, only new use was associated with an increased risk (OR = 1.63; 95% CI: 1.10, 2.40). | 0 | |||||||
3A | Subjects over 65 years of age who were being treated with an antipsychotic (risperidone, quetiapine, olanzapine, clozapine, or FGA) Subjects were identified via data from a British Columbia Ministry of Health Pharmanet database. Design: observational-retrospective cohort Location: British Columbia, Canada Funding: government funded | 37,241 subjects identified as meeting inclusion criteria; 12,882 of the identified subjects initiated treatment with an FGA, and 24,359 initiated treatment with an SGA | January 1, 1996 to December 31, 2004 | Using multivariable and propensity score–adjusted modeling, the study authors found that the adjusted hazard ratio for mortality within 180 days of FGA initiation relative to SGA initiation was 1.71 (95% CI: 1.35, 2.17) for respiratory mortality other than that due to pneumonia. Inclusion of individuals who did not have a diagnosis of dementia may limit generalizability. | 0 | |||||||
3 | Subjects age 65 years or older who were taking an antipsychotic drug Subjects were identified via the Dutch Integrated Primary Care Information database as having incident community-acquired pneumonia. Design: population-based, nested case-control study Location: Netherlands Funding: none | 258 case subjects with incident pneumonia were matched to 1,686 control subjects on the basis of age, sex, and date of onset | 1996–2006 | Sixty-five (25%) of the case subjects died in 30 days with death attributable to pneumonia. Using conditional logistic regression, the study authors found that current use of either an FGA (OR = 1.76; CI: 1.22, 2.53) or SGA (OR = 2.61; 95% CI: 1.48, 4.61) was associated with a dose-dependent increase in the risk for pneumonia compared with past use of antipsychotic drugs. Current use of SGAs was not associated with an increase in odds ratio of pneumonia relative to FGAs (1.48; 95% CI: 0.84, 2.60). Only SGAs were associated with an increase in the risk for fatal pneumonia (OR = 5.97; CI: 1.49, 23.98). | 0 | |||||||
Quality of the Body of Research Evidence for Harm Related to Pulmonary Events
Neurological Side Effects: Cognitive Changes
Overview and Quality of Individual Studies
| Study type | Study | Subject/Method/ Design/Location | N | Duration | Outcomes/Results | Rating of quality of evidence | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
3 | Older nursing home residents with newly diagnosed Alzheimer’s disease or related dementias Subjects were identified based on Medicare enrollment and claims data linked to the Minimum Dataset 2.0. Design: prospective cohort study Location: United States | 18,950 subjects with a mean age of 83.6 years; 76% of the sample subjects were female. At baseline, 15% were taking antidementia medications, 40% antidepressants, 13% antipsychotics, and 3% mood stabilizers. | 2007–2008 | Using marginal structural models to account for time-dependent confounding, the study authors found that antipsychotic use was associated with a slower decline in cognition (slope difference: − 0.11 points/year on the CPS, 99% CI: − 0.17, − 0.06), with more rapid declines observed in females. However, the magnitude of these changes was not noted to be clinically significant, although it was statistically significant. | 0 | |||||||||||||
3 | Community-ascertained case patients from the Cache County Dementia Progression Study who had incident Alzheimer’s disease Design: prospective cohort Location: United States | 230 case subjects | Mean follow-up 3.7 years | At baseline, psychotropic medication use was associated with greater severity of dementia, and poorer medical status was associated with use of psychotropic medications (e.g., antidepressants, antipsychotics, benzodiazepines). Mixed-effects models showed that a higher proportion of observed time of medication exposure was associated with a more rapid decline in MMSE for all medication classes, including antipsychotic agents. In terms of FGAs, a higher proportion of observed time of medication exposure was associated with a more rapid increase in CDR Sum of Boxes and NPI total score. | 0 | |||||||||||||
1 | Ambulatory outpatients living at home or in an assisted-living facility whose symptoms met DSM-IV criteria for dementia of the Alzheimer’s type or NINCDS/ADRDA criteria for probable Alzheimer’s disease and who had delusions, hallucinations, agitation, or aggression nearly every day over the previous week or intermittently over 4 weeks Design: multiphase, multisite double-blind, randomized study. After initial treatment phase, subsequent phases and randomization were dependent on response to initial treatment assignment. Patients could be taking cholinesterase inhibitor medication but not antidepressants or anticonvulsants for mood disorder. Location: United States | 421 patients were randomly assigned in a double-blind fashion to receive olanzapine, quetiapine, risperidone, or placebo (randomized allocation 2:2:2:3). 342 subjects had at least one follow-up cognitive measure at 12 weeks, 320 at 24 weeks, and 307 at 36 weeks. Sample patients were 46% male, with a mean age of 77.6 years and a mean of 12.3 years of education ; 64% were taking cholinesterase inhibitors. | 36 weeks | Significant declines occurred in multiple cognitive measures, including the MMSE (P = 0.004), BPRS Cognitive subscale (P = 0.05), and a cognitive summary score summarizing change on 18 cognitive tests (P = 0.004). Declines were linear and significant over time (e.g., 2.4-point decrease in MMSE and a 4.4-point decrease in ADAS-Cog over 36 weeks) without effects of baseline MMSE, baseline BPRS score, or size of the study site. Patients taking an SGA for at least 2 weeks showed a greater rate of decline in cognitive function than those receiving placebo, although these declines were not statistically significant for all measures. | 1 | |||||||||||||
Quality of the Body of Research Evidence for Harm Related to Cognitive Changes
Sedation and Fatigue
Overview and Quality of Individual Studies
| Adverse effect | Drug | Number of studies | Drug (adverse events/sample size) | Placebo (adverse events/sample size) | OR | (95% CI) | NNH | |
|---|---|---|---|---|---|---|---|---|
Fatigue | Aripiprazole | 3 | 47/600 | 11/272 | 2.44 | (1.19, 5.43) | 22 | |
Fatigue | Olanzapine | 3 | 36/482 | 9/326 | 2.37 | (1.08, 5.75) | 34 | |
Fatigue | Quetiapine | 2 | 25/335 | 5/234 | 2.92 | (1.03, 10.26) | 34 | |
Fatigue | Risperidone | 2 | 20/281 | 4/236 | 3.56 | (1.13, 14.96) | 34 | |
Sedation | Aripiprazole | 4 | 116/706 | 22/374 | 2.62 | (1.57, 4.54) | 16 | |
Sedation | Olanzapine | 5 | 158/778 | 25/440 | 4.58 | (2.87, 7.55) | 9 | |
Sedation | Quetiapine | 4 | 84/446 | 18/353 | 5.16 | (2.93, 9.51) | 8 | |
Sedation | Risperidone | 6 | 265/1,260 | 102/922 | 2.33 | (1.79, 3.05) | 10 | |
Quality of the Body of Research Evidence for Harm Related to Sedation and Fatigue
Extrapyramidal Symptoms
Overview and Quality of Individual Studies
| Adverse effect | Drug | Number of studies | Drug (adverse events/sample size) | Placebo (adverse events/sample size) | OR | (95% CI) | NNH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
EPS | Aripiprazole | 4 | 39/706 | 16/374 | 1.29 | (0.68, 2.57) | NC | ||||||||
EPS | Olanzapine | 1 | 18/100 | 2/142 | 15.21 | (3.50, 138.55) | 10 | ||||||||
EPS | Quetiapine | 3 | 18/355 | 9/254 | 1.15 | (0.46, 3.08) | NC | ||||||||
EPS | Risperidone | 5 | 130/1,561 | 31/916 | 3.00 | (1.96, 4.70) | 20 | ||||||||
Akathisia | Olanzapine | 1 | 1/100 | 0/142 | inf+ | (0.04, inf+) | NC | ||||||||
Akathisia | Quetiapine | 2 | 1/114 | 1/162 | 1.23 | (0.02, 98.52) | NC | ||||||||
Akathisia | Risperidone | 1 | 0/85 | 0/142 | NC | NC | NC | ||||||||
TD | Olanzapine | 1 | 3/100 | 4/142 | 1.07 | (0.15, 6.46) | NC | ||||||||
TD | Quetiapine | 1 | 2/94 | 4/142 | 0.75 | (0.07, 5.36) | NC | ||||||||
TD | Risperidone | 4 | 4/949 | 14/713 | 0.31 | (0.07, 1.03) | NC | ||||||||
| Study type | Study | Subject/Method/Design/Location | N | Duration | Outcomes/Results | Rating of quality of evidence | |||
|---|---|---|---|---|---|---|---|---|---|
3 | Subjects age 66 years or older with a diagnosis of dementia who were identified via the Ontario Drug Benefits database as having initiated treatment with an antipsychotic agent Design: observational-retrospective cohort Location: Ontario, Canada Funding: Canadian Institutes of Health Research | 21,835 subjects; 12,045 of the subjects initiated treatment with an FGA, and 9,790 initiated treatment with an SGA | April 1, 1997–March 31, 2001 | TD or other movement disorder was documented as a diagnosis for 3.0% of subjects prescribed an FGA and 3.5% of subjects prescribed an SGA. Rates of TD or other drug-induced movement disorder with FGAs and SGAs were 5.24 and 5.19 cases per 100 person-years for treatment, respectively. Using Cox proportional hazards analysis, the study authors found that the relative risk of a drug-induced movement disorder did not differ for SGAs as compared with FGAs (relative risk = 0.99; 95% CI: 0.86, 1.15). | 0 | ||||
3 | Subjects with dementia who were newly prescribed quetiapine, olanzapine, or risperidone Subjects were identified with administrative database information. Design: observational-retrospective cohort Location: Ontario Canada | 51,878 subjects | 2002–2010 | From 15,939 person-years of observation, 421 patients developed parkinsonism. With low-dose risperidone as the reference group, the adjusted hazard ratios for developing parkinsonism were 0.49 (95% CI: 0.07, 3.53) for low-dose olanzapine and 1.18 (95% CI: 0.84, 1.66) for low-dose quetiapine. When comparisons were made across drugs within the most commonly prescribed dose ranges, the incidence of parkinsonism was higher in the medium-dose olanzapine group compared with the low-dose risperidone group (HR = 1.66; 95% CI: 0.23, 2.23). Adjusted hazard ratio for developing parkinsonism for men (compared with women) was 2.29 (95% CI: 1.88, 2.79). | 0 | ||||
1 | Subjects with Alzheimer’s disease or probable Alzheimer’s disease (MMSE scores 5–26), ambulatory and residing at home or in assisted living facilities, with moderate or greater levels of psychosis, aggression, or agitation Interventions: Placebo vs. masked, flexibly dosed olanzapine (mean dose: 5.5 mg/day), quetiapine (mean dose: 56.5 mg/day), or risperidone (mean dose: 1.0 mg/day) Stable doses of cholinesterase inhibitor were permitted. Design: Multicenter, federally funded CATIE-AD trial—Phase 1 | 421 subjects randomly assigned to treatment group, with 142 receiving placebo, 100 receiving olanzapine, 94 receiving quetiapine, and 85 receiving risperidone | Median duration on Phase 1 treatment was 7.1 weeks; clinical outcomes assessed for those who continued to take an antipsychotic at 12 weeks | Subjects treated with olanzapine and risperidone had higher rates of extrapyramidal signs (12% in each group) compared with subjects treated with quetiapine or receiving placebo (2% and 1%, respectively). Similar findings were noted in terms of SAS ratings of greater than 1, which were more frequent with olanzapine (14%) and risperidone (11%) than with placebo (2%). | 1 | ||||
3 | Residents of Manitoba, Canada age 65 years or older who had an antipsychotic medication dispensed for the first time during the study period Subjects were identified via Manitoba’s Department of Health administrative databases. Design: observational-retrospective cohort, population-based sample Location: Manitoba, Canada | 8,885 persons in the sample were identified as receiving an antipsychotic medication (accounting for values of 4.3% of males and 6.0% of females), with 4,242 persons in the group who received an FGA and 4,643 in the group who received risperidone | April 1, 2000–March 31, 2007 | Using Cox proportional hazards models to determine the risk of extrapyramidal symptoms in new users of risperidone compared with new users of FGAs, the study authors found that risperidone use was associated with a lower risk of EPS compared with FGAs at 30, 60, 90, and 180 days (adjusted HR = 0.38 [95% CI: 0.22, 0.67], 0.45 [95% CI: 0.28, 0.73], 0.50 [95% CI: 0.33, 0.77], 0.65 [95% CI: 0.45, 0.94], respectively) after controlling for potential confounders (demographics, comorbidity, and medication use). At 360 days, the strength of the association had weakened, with an adjusted HR of 0.75 (95% CI: 0.54, 1.05). | 0 | ||||
Quality of the Body of Research Evidence for Harm Related to Extrapyramidal Side Effects
Falls and Hip Fractures
Overview and Quality of Individual Studies
| Adverse effect | Drug | Number of studies | Drug (adverse events/sample size) | Placebo (adverse events/sample size) | OR | (95% CI) | NNH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Gait issues | Aripiprazole | 1 | 16/366 | 1/121 | 5.47 | (0.83, 231.93) | NC | |||||||
Gait issues | Olanzapine | 4 | 79/641 | 15/373 | 2.75 | (1.52, 5.29) | 21 | |||||||
Gait issues | Quetiapine | 3 | 18/426 | 6/333 | 2.36 | (0.85, 7.59) | NC | |||||||
Gait issues | Risperidone | 3 | 32/448 | 8/406 | 3.04 | (1.32, 7.84) | 33 | |||||||
| Study type | Study | Subject/Method/Design/Location | N | Duration | Outcomes/Results | Rating of quality of evidence |
|---|---|---|---|---|---|---|
3A | Nursing home residents age 65 years or older who had treatment with psychotropics initiated after admission Design: retrospective population-based cohort Location: British Columbia | 10,900 subjects; an SGA was begun in 1,942, an FGA in 1,902, an antidepressant in 2,169, and a benzodiazepine in 4,887 | 1996–2006 | Using proportional hazards models with propensity-score adjustments, the study authors found that users of FGAs had an increased risk of death (RR = 1.47, 95% CI: 1.14, 1.91), and an increased risk of femur fracture within 180 days after treatment initiation (RR = 1.61, 95% CI: 1.03, 2.51), as compared with users of SGAs. Users of benzodiazepines also had a higher risk of death (RR = 1.28, 95% CI: 1.04, 1.58) compared with users of SGAs. There was no difference observed in the risk of heart failure or pneumonia in individuals receiving FGAs, as compared with SGAs (RR = 1.03 [95% CI: 0.62, 1.69] and 0.91 [95% CI: 0.41, 2.01], respectively). Using subgroup adjusted propensity scores, the study authors found that individuals who started taking an FGA (as compared with users of an SGA) had an increased risk of mortality, with an RR of 1.37 (95% CI: 0.96, 1.95) for individuals with dementia and 1.61 (95% CI: 1.10, 2.36) for individuals without dementia. Among individuals with no history of antipsychotic treatment, the corresponding RR was 1.33 (95% CI: 0.99, 1.77) as compared with users of an SGA. | 0 | |
3 | Subjects age 65 years or older with a diagnosis of dementia and no record of a previous hip fracture; long-stay Medicaid-eligible residents living in one of 586 nursing homes in California, Florida, Illinois, New York, or Ohio Subjects were identified via Medicaid claims data. Excluded were individuals who were receiving hospice care, comatose, bedfast, paralyzed, or in a wheelchair. Design: nested case-control study Location: United States Funding: not explicitly stated | 69,027 individuals in total database; 764 of these individuals had experienced a hip fracture and were matched with up to 5 randomly selected controls (N = 3,582) | 2001–2002 | Current use of an antipsychotic was associated with a small increase in the risk of hospitalization for hip fracture (adjusted OR = 1.26; 95% CI: 1.05, 1.52). Risk of hip fracture was slightly higher for new users of antipsychotics (adjusted OR = 1.33; 95% CI: 0.95, 1.88) than for ongoing users (adjusted OR = 1.21; 95% CI: 0.99, 1.47). For current users of FGAs, risk was higher (adjusted OR = 1.44; 95% CI: 0.84, 2.47) than for SGAs (adjusted OR = 1.27; 95% CI: 1.05, 1.54). Corresponding odds ratios for current users of specific SGAs were olanzapine (adjusted OR = 1.41; 95% CI: 1.08, 1.84), risperidone (adjusted OR = 1.35; 95% CI: 1.07, 1.70), and quetiapine (adjusted OR = 1.30; 95% CI: 0.86, 1.96). Sample sizes were insufficient to calculate adjusted ORs for the other specific antipsychotics. Case and control subjects were similar on most measures, but case subjects had a greater frequency and severity of behavioral and psychological symptoms of dementia. | 0 | |
3 | Subjects over 65 years of age who were exposed to antipsychotic medication Subjects were identified via Australian Government Department of Veterans’ Affairs Health Care Claims Database. Design: observational-retrospective cohort Location: Australia Funding: Australian Government | 8,235 subjects had had at least one hospitalization for hip fracture; 494 of these subjects had started receiving an FGA and 1,091 had started receiving an SGA. 13,324 had had at least one hospitalization for pneumonia; 807 of these subjects had started receiving an FGA and 1,107 had started receiving an SGA during the study period. | 2005–2008; median follow-up: 3.3–4.0 years | Using a self-controlled case-series design, the study authors found a significantly increased risk of hip fracture with use of an FGA during all postexposure risk periods beginning at 1 week of exposure. Risk remained significantly increased with more than 12 weeks of continuous exposure (IRR = 2.19; 95% CI: 1.62, 2.95). After initiation of SGAs, the risk of hip fracture was highest in the first week (IRR = 2.17; 95% CI: 1.54, 3.06) and then declined but remained significantly raised with more than 12 weeks of continuous exposure (IRR = 1.43; 95% CI: 1.23, 1.66). The study also found a significantly increased risk of hospitalization for hip fracture up to 16 weeks prior to antipsychotic initiation. | 0 | |
1 | Subjects with Alzheimer’s disease or probable Alzheimer’s disease (MMSE scores 5–26), ambulatory and residing at home or in assisted living facilities, with moderate or greater levels of psychosis, aggression, or agitation Interventions: placebo vs. masked, flexibly dosed olanzapine (mean dose: 5.5 mg/day), quetiapine (mean dose: 56.5 mg/day), or risperidone (mean dose: 1.0 mg/day) Stable doses of cholinesterase inhibitor were permitted. Design: multicenter, federally funded CATIE-AD trial—Phase 1 | 421 subjects randomly assigned to treatment group, with 142 receiving placebo, 100 receiving olanzapine, 94 receiving quetiapine, and 85 receiving risperidone | Median duration on Phase 1 treatment was 7.1 weeks; clinical outcomes assessed for those who were continuing to take antipsychotic at 12 weeks | Falls, injuries, and fractures were reported together. No significant differences were found between subjects treated with SGAs and those receiving placebo with rates of 17% for olanzapine, 7% for quetiapine, and 12% for risperidone as compared with a rate of 15% for placebo. | 1 | |
3 | Nursing home residents with dementia who had data on drug use abstracted from a prescription database and falls identified using a standardized incident report system Design: observational-retrospective cohort Location: Netherlands | 248 subjects, accounting for 85,074 person-days, with an antipsychotic being used in 45.4% of these person-days | January 1, 2006– January 1, 2008 | Fall risk was increased with the use of antipsychotics (HR = 1.53; 95% CI: 1.17, 2.00). Fall risk was also increased with age (HR = 1.05; 95% CI: 1.02, 1.08) and with use of anxiolytics (1.60; 95% CI: 1.19, 2.16), hypnotics and sedatives (1.50; 95% CI: 1.04, 2.16), and antidepressants (2.28; 95% CI: 1.58, 3.29). There was a significant dose-response relationship between fall risk and use of antipsychotics (HR = 2.78; 95% CI: 1.49, 5.17). Also associated with a significant dose-response relationship and an increased risk of falls were anxiolytics (1.60; 95% CI: 1.20, 2.14), hypnotics and sedatives (2.58; 95% CI: 1.42, 4.68), and antidepressants (2.84; 95% CI: 1.93, 4.16). For antipsychotics, fall risk was increased even at low doses (25% of the average dosage of a drug taken by adults for the main indication as indicated by the World Health Organization); fall risk increased further with dose increments and with combinations of psychotropics. | 0 |
Quality of the Body of Research Evidence for Harm Related to Falls and Hip Fractures
Endocrine Adverse Events
Overview and Quality of Individual Studies
| Study type | Study | Subject/Method/Design/Location | N | Duration | Outcomes/Results | Rating of quality of evidence | |
|---|---|---|---|---|---|---|---|
3 | Nursing home residents age 65 years or older with dementia and no record of diabetes within 90 days of nursing home admission; long-stay Medicaid-eligible residents living in nursing homes in California, Florida, Illinois, New York, and Ohio Cases of incident diabetes were identified via MDS assessments and Medicaid claims, medication use was ascertained from Medicaid pharmacy files, and resident characteristics were obtained from MDS assessments Interventions: FGAs or SGAs vs. antipsychotic nonusers Design: observational-case control Location: United States Funding: unfunded study | 29,203 people; 762 incident cases of diabetes identified and up to 5 control cases randomly selected, with case and control subjects matched on nursing home and quarter of MDS assessment (N = 2,646) | Recruited from January 2001 to December 2002 | Relative to nonusers of antipsychotics, use of SGAs was not associated with diabetes onset (adjusted OR = 1.03; 95% CI: 0.84, 1.27) and risk of diabetes did not increase with length of time on treatment. FGA treatment was associated with diabetes onset, particularly when treatment duration was less than 30 days (adjusted OR = 2.70; 95% CI: 1.57, 4.65). | 0 | ||
3 | Subjects over 65 years of age without prior diabetes, who had treatment with an antipsychotic medication initiated Subjects were identified via a population-based health database; 42% of the sample had dementia. Design: nested case control Location: Ontario, Canada Funding: Canadian Institutes of Health Research | 44,121 subjects; 220 of the subjects had had a hospital visit for hyperglycemia and 2,190 served as matched control subjects | Recruited from April 1, 2002, and March 31, 2006, with an average follow-up duration of 2.2 years | Any current use of antipsychotic, use of an FGA, and use of an SGA were all associated with an increased adjusted odds ratio of hyperglycemia compared with use in the remote past (1.52 [95% CI: 1.07, 2.17], 1.44 [95% CI: 1.01, 2.07], and 2.86 [95% CI: 1.46, 3.59], respectively). | 0 | ||
1A | Micca et al. 2006 | Subjects over 65 years of age, who had been diagnosed with dementia Subjects identified via an olanzapine clinical trial database. Design: post hoc analysis of pooled data from clinical trials Location: not specified Funding: pharmaceutical (Eli Lilly) | 1,398 subjects; 835 of the subjects received olanzapine (mean modal dose across all studies was 4.87 mg/day), 223 received an active comparator (risperidone, haloperidol, or another FGA), and 340 received placebo | Not specified | No statistically significant increase in the risk of treatment-emergent diabetes (HR = 1.36), defined as 2 glucose values over 200 mg/dL after baseline (or 1 value at the final visit), initiation of antidiabetic medication, or clinical diagnosis of diabetes was noted. Other risk factors, such as BMI 25 kg/m2 or having at least 7% weight gain during the study, were also not significant (HR = 0.86 and HR = 2.26, respectively). | 0 | |
1 | Subjects with Alzheimer’s disease or probable Alzheimer’s disease (MMSE scores 5–26), ambulatory and residing at home or in assisted living facilities, with moderate or greater levels of psychosis, aggression, or agitation Interventions: Phase 1—placebo vs. masked, flexibly dosed olanzapine (mean dose: 5.5 mg/day), quetiapine (mean dose: 56.5 mg/day), or risperidone (mean dose: 1.0 mg/day); Phase 2—antipsychotic or citalopram; Phase 3—open label Stable doses of cholinesterase inhibitor were permitted. Design: multicenter, federally funded CATIE-AD trial—Phase 1 | 421 subjects randomly assigned in Phase 1, with 142 receiving placebo, 100 receiving olanzapine, 94 receiving quetiapine, and 85 receiving risperidone | Median duration on Phase 1 treatment was 7.1 weeks; total trial duration: 36 weeks | No treatment effects were noted for changes in blood pressure, glucose, and triglycerides, but olanzapine was significantly associated with decreases in high-density lipoprotein cholesterol (− 0.19 mg/dL/week) and increased girth (0.07 inches/week) relative to the placebo group. | 1 | ||
Quality of the Body of Research Evidence for Harm Related to Endocrine Effects
Appetite/Weight
Overview and Quality of Individual Studies
| Adverse effect | Drug | Number of studies | Drug (adverse events/sample size) | Placebo (adverse events/sample size) | OR | (95% CI) | NNH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Weight gain | Aripiprazole | 2 | 23/472 | 10/223 | 1.02 | (0.44, 2.49) | NC | |||||||
Weight gain | Olanzapine | 3 | 34/482 | 6/326 | 4.69 | (1.87, 14.14) | 24 | |||||||
Weight gain | Quetiapine | 1 | 5/94 | 4/142 | 1.93 | (0.40, 10.01) | NC | |||||||
Weight gain | Risperidone | 2 | 14/281 | 5/236 | 3.40 | (1.08, 12.75) | 24 | |||||||
| Study type | Study | Subject/Method/Design/Location | N | Duration | Outcomes/Results | Rating of quality of evidence | |
|---|---|---|---|---|---|---|---|
3A | Individuals over 65 years of age with dementia who were newly prescribed olanzapine Subjects identified via an olanzapine clinical trial database. Design: observational-retrospective cohort Location: United States Funding: Eli Lilly | 1,267 subjects | 20 weeks of follow-up | Estimated probability of gaining more than 7% of initial body weight was significantly greater with olanzapine as compared with placebo (P < 0.001). | 0 | ||
1 | Subjects with Alzheimer’s disease or probable Alzheimer’s disease (MMSE scores 5–26), ambulatory and residing at home or in assisted living facilities, with moderate or greater levels of psychosis, aggression, or agitation Interventions: Phase 1—placebo vs. masked, flexibly dosed olanzapine (mean dose: 5.5 mg/day), quetiapine (mean dose: 56.5 mg/day), or risperidone (mean dose: 1.0 mg/day); Phase 2—antipsychotic or citalopram; Phase 3—open label Stable doses of cholinesterase inhibitor were permitted. Design: multicenter, federally funded CATIE-AD trial—Phase 1 | 421 subjects randomly assigned in Phase 1, with 142 receiving placebo, 100 receiving olanzapine, 94 receiving quetiapine, and 85 receiving risperidone | Median duration on Phase 1 treatment was 7.1 weeks; total trial duration: 36 weeks | Clinically significant weight gain (i.e., 7% or more of body weight) was seen among patients with antipsychotic use relative to patients who did not use antipsychotics at all time periods during the trial (≤12 weeks: OR = 1.56 [95% CI: 0.53, 4.58]; 12 and 24 weeks: OR = 2.89 [95% CI: 0.97, 8.64]; >24 weeks OR = 3.38 [95% CI: 1.24, 9.23]). Significant weight gain was noted for women but not for men and for olanzapine and quetiapine but not other study medications. Monthly weight gains ranged from 0.4 to 1.0 lbs as compared with a monthly loss of 0.9 lbs for placebo. | 1 | ||
Quality of the Body of Research Evidence for Harm Related to Appetite and Weight Change
Urinary Symptoms
Overview and Quality of Individual Studies
| Adverse effect | Drug | Number of studies | Drug (adverse events/sample size) | Placebo (adverse events/sample size) | OR | (95% CI) | NNH | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Urinary | Aripiprazole | 3 | 115/603 | 44/348 | 1.37 | (0.92, 2.09) | NC | ||||||
Urinary | Olanzapine | 1 | 19/204 | 1/94 | 9.51 | (1.47, 401.07) | 36 | ||||||
Urinary | Quetiapine | 2 | 44/332 | 12/191 | 2.37 | (1.16, 5.15) | 16 | ||||||
Urinary | Risperidone | 4 | 164/1,060 | 71/665 | 1.55 | (1.13, 2.13) | 21 | ||||||
Quality of the Body of Research Evidence for Harm Related to Urinary Symptoms
Section I: Questions About Appropriate Use
| 1a. The agitation is a NEW SYMPTOM. Assessment SUGGESTS a short-term reversible cause of the agitation, such as acute delirium, medication side effects, or environmental causes. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aripiprazole (n = 203) | Haloperidol (n = 203) | Olanzapine (n = 202) | Quetiapine (n = 202) | Risperidone (n = 202) | Ziprasidone (n = 201) | |||||||
| Appropriateness of use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
1 (highly inappropriate) | 52 | 25.6 | 36 | 17.7 | 34 | 16.8 | 27 | 13.4 | 21 | 10.4 | 72 | 35.8 |
2 | 44 | 21.7 | 25 | 12.3 | 28 | 13.9 | 36 | 17.8 | 14 | 6.9 | 44 | 21.9 |
3 (uncertain) | 55 | 27.1 | 26 | 12.8 | 49 | 24.3 | 36 | 17.8 | 39 | 19.3 | 53 | 26.4 |
4 | 30 | 14.8 | 40 | 19.7 | 60 | 29.7 | 62 | 30.7 | 65 | 32 .2 | 19 | 9.5 |
5 (highly appropriate) | 22 | 10.8 | 76 | 37.4 | 31 | 15.4 | 41 | 20.3 | 63 | 31.2 | 13 | 6.5 |
| Median | 3 | 4 | 3 | 4 | 4 | 2 | ||||||
| Mean | 2.6 | 3.5 | 3.1 | 3.3 | 3.7 | 2.3 | ||||||
| SD | 1.3 | 1.5 | 1.3 | 1.3 | 1.3 | 1.2 | ||||||
| 1b. The agitation is a NEW SYMPTOM. Assessment DOES NOT FIND a short-term reversible cause. | ||||||||||||
| Aripiprazole (n = 198) | Haloperidol (n = 199) | Olanzapine (n = 201) | Quetiapine (n = 200) | Risperidone (n = 199) | Ziprasidone (n = 198) | |||||||
| Appropriateness of use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
1 (highly inappropriate) | 31 | 15.7 | 39 | 19.6 | 18 | 9.0 | 14 | 7.0 | 7 | 3.5 | 53 | 26.8 |
2 | 31 | 15.7 | 34 | 17.1 | 26 | 12.9 | 18 | 9.0 | 14 | 7.0 | 35 | 17.7 |
3 (uncertain) | 71 | 35.9 | 42 | 21.1 | 44 | 21.9 | 46 | 23.0 | 35 | 17.6 | 74 | 37.4 |
4 | 44 | 22.2 | 41 | 20.6 | 81 | 40.3 | 69 | 34.5 | 79 | 39.7 | 26 | 13.1 |
5 (highly appropriate) | 21 | 10.6 | 43 | 21.6 | 32 | 15.9 | 53 | 26.5 | 64 | 32.2 | 10 | 5.1 |
| Median | 3 | 3 | 4 | 4 | 4 | 3 | ||||||
| Mean | 3.0 | 3.1 | 3.4 | 3.6 | 3.9 | 2.5 | ||||||
| SD | 1.2 | 1.4 | 1.2 | 1.2 | 1 | 1.2 | ||||||
| 1c. The agitation is PERSISTENT or consists of repeated episodes. Assessment DOES NOT FIND a short-term reversible cause. | ||||||||||||
| Aripiprazole (n = 200) | Haloperidol (n = 201) | Olanzapine (n = 200) | Quetiapine (n = 201) | Risperidone (n = 199) | Ziprasidone (n = 198) | |||||||
| Appropriateness of use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
1 (highly inappropriate) | 34 | 17.0 | 60 | 29.9 | 20 | 10.0 | 12 | 6.0 | 13 | 6.5 | 57 | 28.8 |
2 | 30 | 15.0 | 32 | 15.9 | 20 | 10.0 | 20 | 10.0 | 12 | 6.0 | 37 | 18.7 |
3 (uncertain) | 54 | 27.0 | 39 | 19.4 | 43 | 21.5 | 39 | 19.4 | 36 | 18.1 | 61 | 30.8 |
4 | 58 | 29.0 | 40 | 19.9 | 79 | 39.5 | 70 | 34.8 | 74 | 37.2 | 32 | 16.2 |
5 (highly appropriate) | 24 | 12.0 | 30 | 14.9 | 38 | 19.0 | 60 | 29.9 | 64 | 32.2 | 11 | 5.6 |
| Median | 3 | 3 | 4 | 4 | 4 | 3 | ||||||
| Mean | 3.1 | 2.7 | 3.5 | 3.7 | 3.8 | 2.5 | ||||||
| SD | 1.3 | 1.4 | 1.2 | 1.2 | 1.1 | 1.2 | ||||||
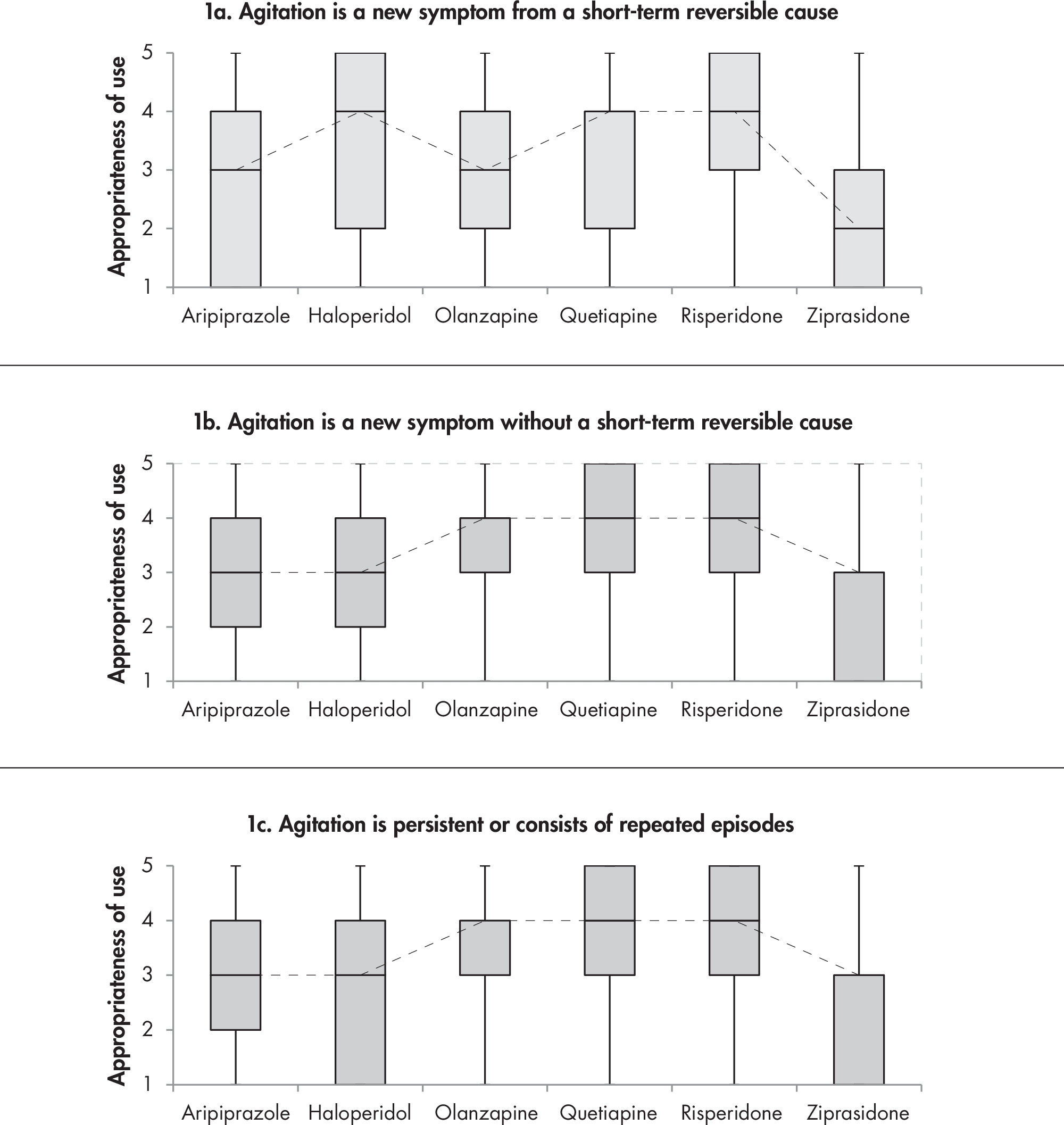

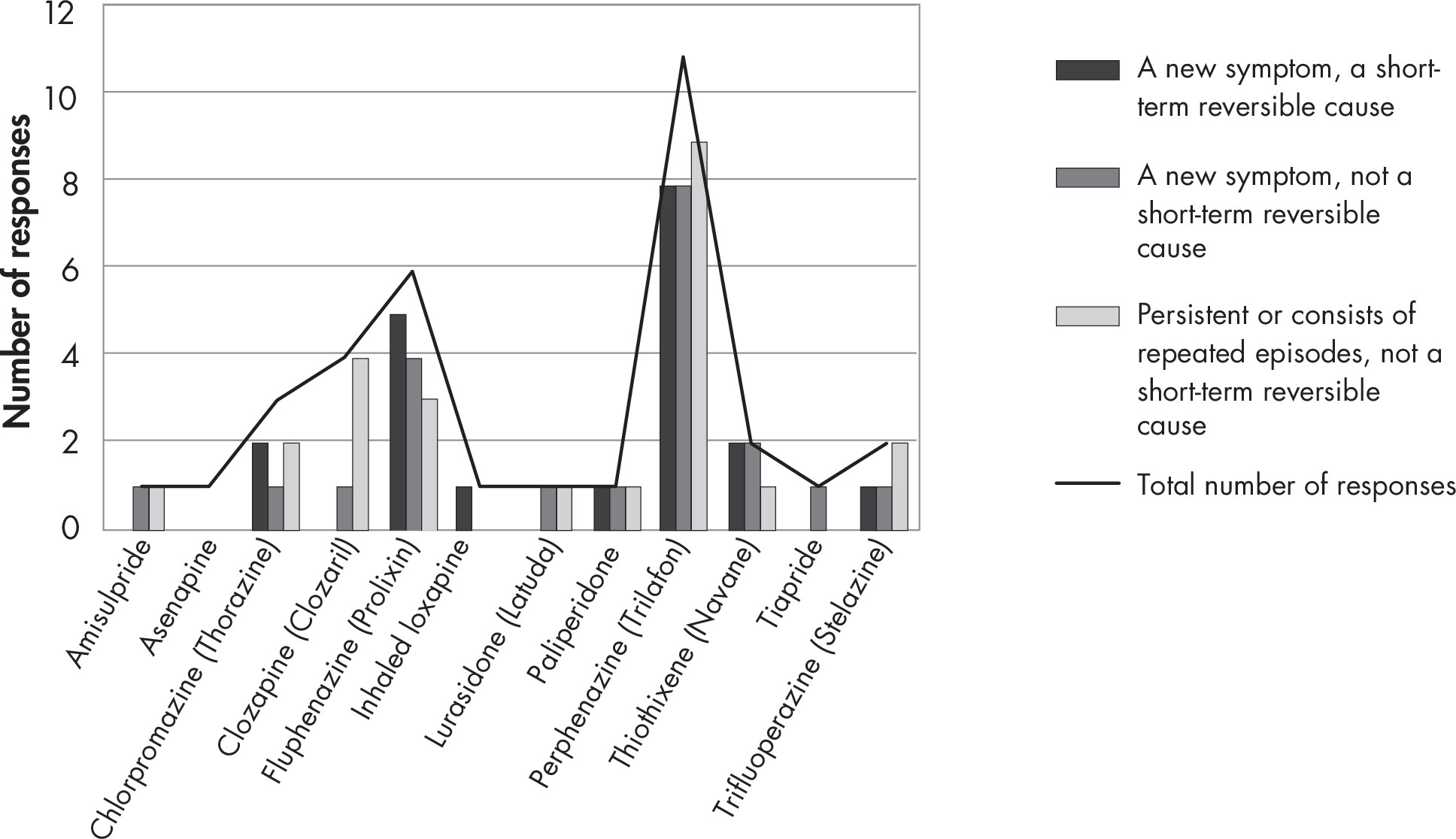
| 4a. The agitation is a NEW SYMPTOM. Assessment SUGGESTS a short-term reversible cause of the agitation, such as acute delirium, medication side effects, or environmental causes. | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aripiprazole (n = 198) | Haloperidol (n = 199) | Olanzapine (n = 198) | Quetiapine (n = 199) | Risperidone (n = 199) | Ziprasidone (n = 196) | |||||||||||||||||||||||||||
| Appropriateness of use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (highly inappropriate) | 97 | 49.0 | 78 | 39.2 | 76 | 38.4 | 64 | 32.2 | 58 | 29.2 | 115 | 58.7 | ||||||||||||||||||||
2 | 38 | 19 .2 | 41 | 20.6 | 37 | 18.7 | 36 | 18.1 | 38 | 19.1 | 32 | 16.3 | ||||||||||||||||||||
3 (uncertain) | 37 | 18.7 | 30 | 15.1 | 34 | 17.2 | 42 | 21.1 | 38 | 19.1 | 27 | 13.8 | ||||||||||||||||||||
4 | 18 | 9.1 | 29 | 14.6 | 39 | 19.7 | 32 | 16.1 | 44 | 22.1 | 15 | 7.7 | ||||||||||||||||||||
5 (highly appropriate) | 8 | 4.0 | 21 | 10.6 | 12 | 6.1 | 25 | 12.6 | 21 | 10.6 | 7 | 3.6 | ||||||||||||||||||||
| Median | 2 | 2 | 2 | 2 | 3 | 1 | ||||||||||||||||||||||||||
| Mean | 2.0 | 2.4 | 2.4 | 2.6 | 2.7 | 1.8 | ||||||||||||||||||||||||||
| SD | 1.2 | 1.4 | 1.3 | 1.4 | 1.4 | 1.1 | ||||||||||||||||||||||||||
| 4b. The agitation is a NEW SYMPTOM. Assessment DOES NOT FIND a short-term reversible cause. | ||||||||||||||||||||||||||||||||
| Aripiprazole (n = 193) | Haloperidol (n = 191) | Olanzapine (n = 192) | Quetiapine (n = 191) | Risperidone (n = 193) | Ziprasidone (n = 189) | |||||||||||||||||||||||||||
| Appropriateness of use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (highly inappropriate) | 71 | 36.8 | 74 | 38.7 | 59 | 30.7 | 48 | 25.1 | 45 | 23.3 | 96 | 50.8 | ||||||||||||||||||||
2 | 39 | 20.2 | 48 | 25.1 | 41 | 21.4 | 37 | 19.4 | 37 | 19.2 | 35 | 18.5 | ||||||||||||||||||||
3 (uncertain) | 53 | 27.5 | 33 | 17.3 | 41 | 21.4 | 44 | 23.0 | 46 | 23.8 | 38 | 20.1 | ||||||||||||||||||||
4 | 25 | 13.0 | 23 | 12.0 | 43 | 22.4 | 39 | 20.4 | 51 | 26.4 | 16 | 8.5 | ||||||||||||||||||||
5 (highly appropriate) | 5 | 2.6 | 13 | 6.8 | 8 | 4.2 | 23 | 12.0 | 14 | 7.3 | 4 | 2.1 | ||||||||||||||||||||
| Median | 2 | 2 | 2 | 3 | 3 | 1 | ||||||||||||||||||||||||||
| Mean | 2.2 | 2.2 | 2.5 | 2.7 | 2.8 | 1.9 | ||||||||||||||||||||||||||
| SD | 1.2 | 1.3 | 1.2 | 1.3 | 1.3 | 1.1 | ||||||||||||||||||||||||||
| 4c. The agitation is PERSISTENT or consists of repeated episodes. Assessment DOES NOT FIND a short-term reversible cause. | ||||||||||||||||||||||||||||||||
| Aripiprazole (n = 193) | Haloperidol (n = 191) | Olanzapine (n = 191) | Quetiapine (n = 191) | Risperidone (n = 192) | Ziprasidone (n = 189) | |||||||||||||||||||||||||||
| Appropriateness of use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (highly inappropriate) | 62 | 32.1 | 81 | 42.4 | 59 | 30.9 | 37 | 19.4 | 43 | 22.4 | 88 | 46.6 | ||||||||||||||||||||
2 | 33 | 17.1 | 39 | 20.4 | 31 | 16.2 | 36 | 18.9 | 32 | 16.7 | 28 | 14.8 | ||||||||||||||||||||
3 (uncertain) | 63 | 32.6 | 35 | 18.3 | 42 | 22.0 | 47 | 24.6 | 42 | 21.9 | 49 | 25.9 | ||||||||||||||||||||
4 | 30 | 15.5 | 27 | 14.1 | 46 | 24.1 | 44 | 23.0 | 56 | 29.2 | 20 | 10.6 | ||||||||||||||||||||
5 (highly appropriate) | 5 | 2.6 | 9 | 4.7 | 13 | 6.8 | 27 | 14.1 | 19 | 9.9 | 4 | 2.1 | ||||||||||||||||||||
| Median | 3 | 2 | 3 | 3 | 3 | 2 | ||||||||||||||||||||||||||
| Mean | 2.4 | 2.2 | 2.6 | 2.9 | 2.9 | 2.1 | ||||||||||||||||||||||||||
| SD | 1.2 | 1.3 | 1.3 | 1.3 | 1.3 | 1.2 | ||||||||||||||||||||||||||
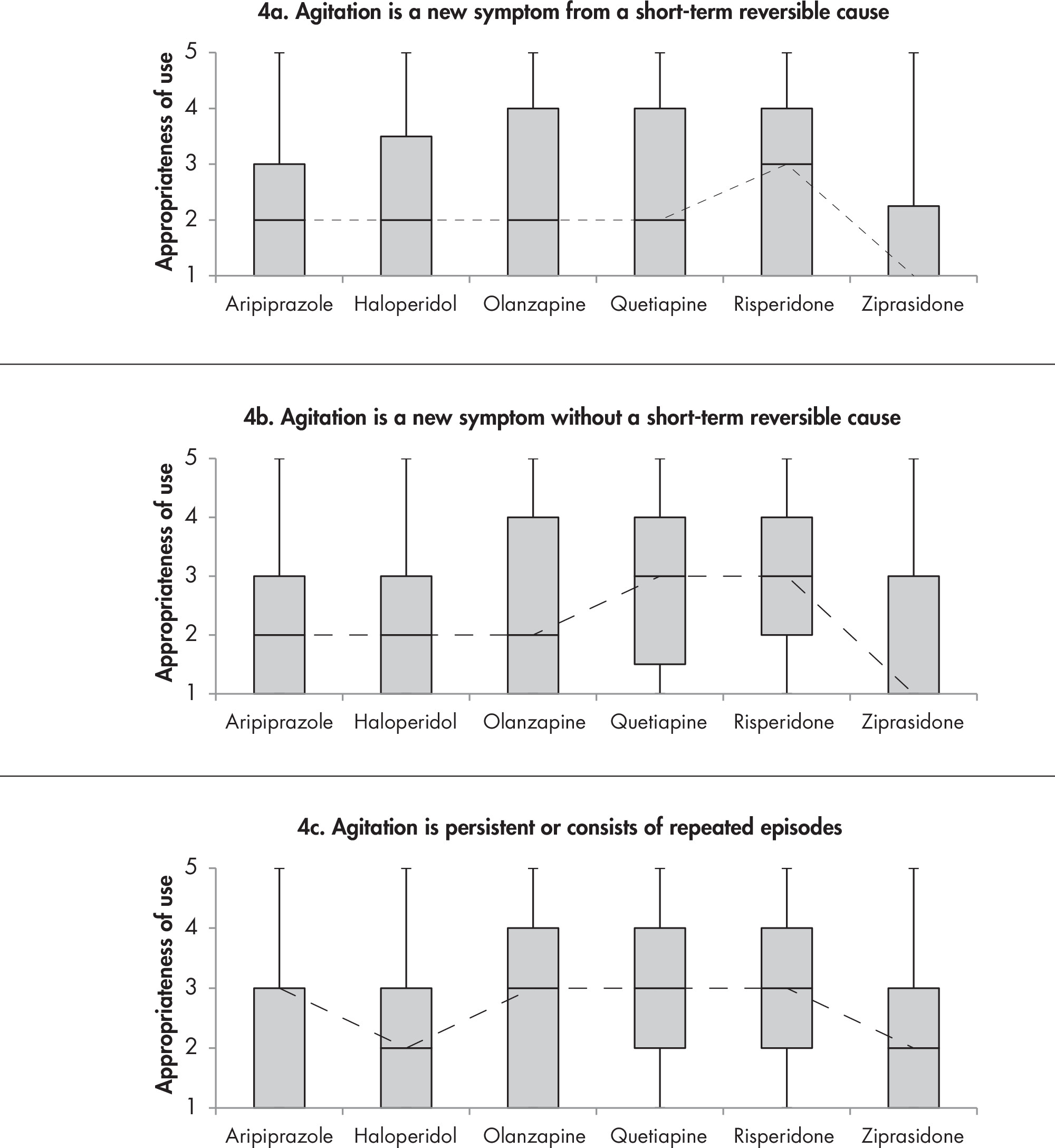
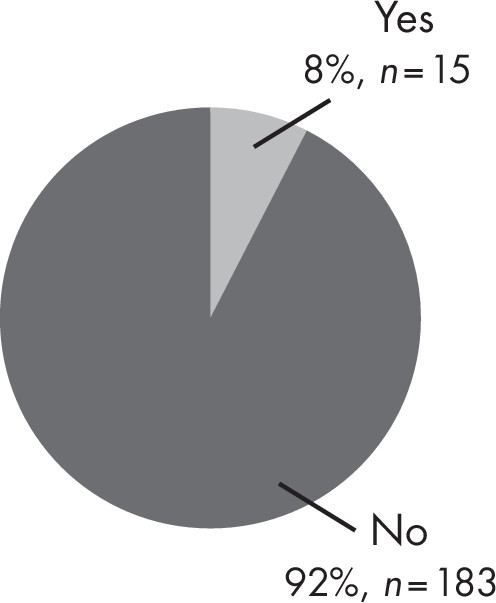
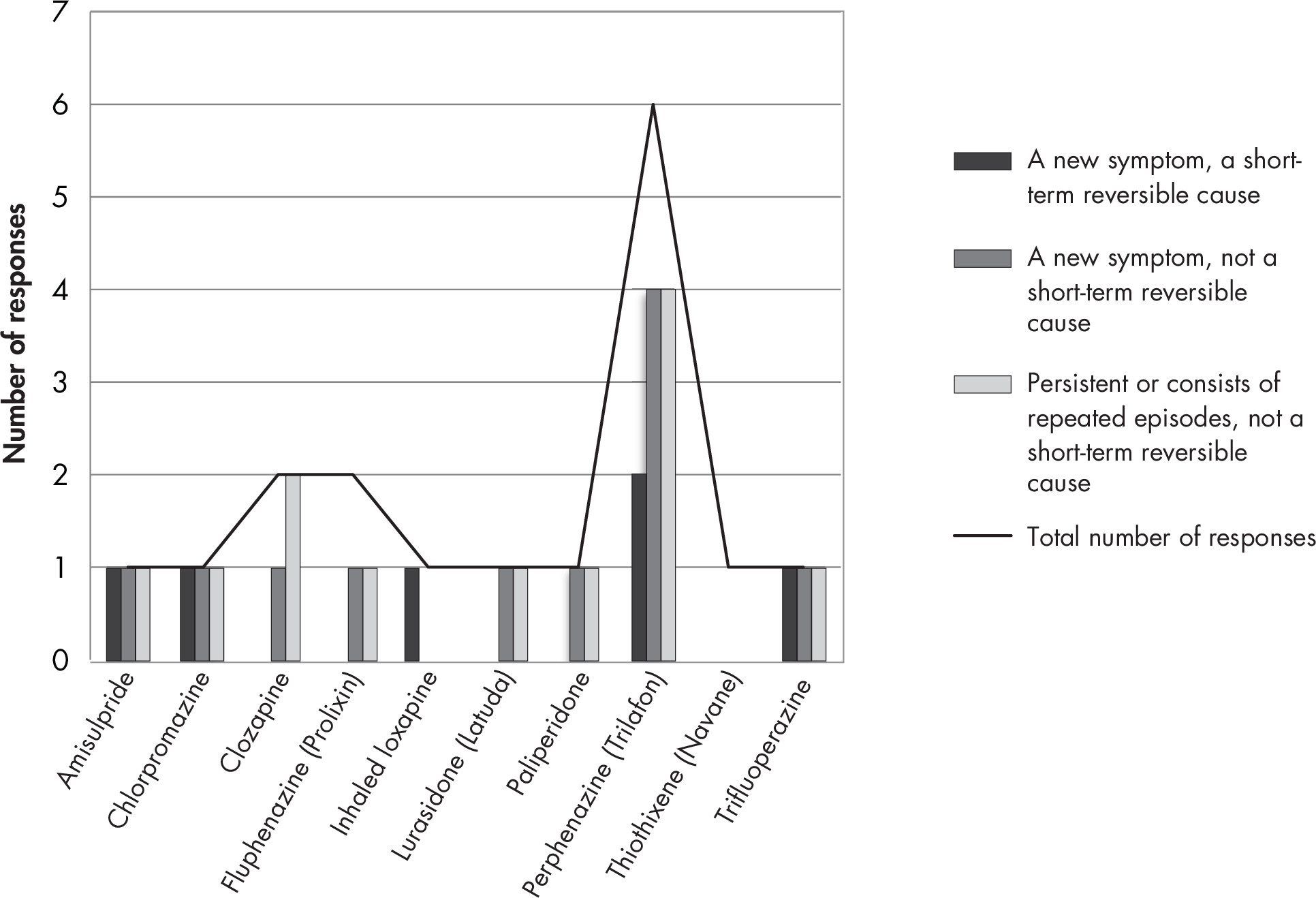
| 7a. The psychosis is a NEW SYMPTOM. Assessment SUGGESTS a short-term reversible cause of the agitation, such as acute delirium, medication side effects, or environmental causes. | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aripiprazole (n = 185) | Haloperidol (n = 187) | Olanzapine (n = 185) | Quetiapine (n = 186) | Risperidone (n = 187) | Ziprasidone (n = 182) | |||||||||||||||||||||||||||
| Appropriateness of use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (highly inappropriate) | 43 | 23.2 | 24 | 12.8 | 20 | 10.8 | 19 | 10.2 | 10 | 5.4 | 55 | 30.2 | ||||||||||||||||||||
2 | 25 | 13.5 | 15 | 8.0 | 14 | 7.6 | 19 | 10.2 | 9 | 4.8 | 32 | 17.6 | ||||||||||||||||||||
3 (uncertain) | 38 | 20.5 | 27 | 14.4 | 34 | 18.4 | 36 | 19.4 | 27 | 14.4 | 51 | 28.0 | ||||||||||||||||||||
4 | 38 | 20.5 | 36 | 19.3 | 61 | 33.0 | 54 | 29.0 | 48 | 25.7 | 22 | 12.1 | ||||||||||||||||||||
5 (highly appropriate) | 41 | 22.2 | 85 | 45.5 | 56 | 30.3 | 58 | 31.2 | 93 | 49.7 | 22 | 12.1 | ||||||||||||||||||||
| Median | 3 | 4 | 4 | 4 | 4 | 3 | ||||||||||||||||||||||||||
| Mean | 3.0 | 3.8 | 3.6 | 3.6 | 4.1 | 2.6 | ||||||||||||||||||||||||||
| SD | 1.5 | 1.4 | 1.3 | 1.3 | 1.1 | 1.3 | ||||||||||||||||||||||||||
| 7b. The psychosis is a NEW SYMPTOM. Assessment DOES NOT FIND a short-term reversible cause. | ||||||||||||||||||||||||||||||||
| Aripiprazole (n = 181) | Haloperidol (n = 186) | Olanzapine (n = 185) | Quetiapine (n = 183) | Risperidone (n = 181) | Ziprasidone (n = 183) | |||||||||||||||||||||||||||
| Appropriateness of use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (highly inappropriate) | 30 | 16.6 | 34 | 18.3 | 18 | 9.7 | 11 | 6.0 | 6 | 3.3 | 52 | 28.4 | ||||||||||||||||||||
2 | 25 | 13.8 | 17 | 9.1 | 8 | 4.3 | 13 | 7.1 | 7 | 3.9 | 24 | 13.1 | ||||||||||||||||||||
3 (uncertain) | 37 | 20.4 | 32 | 17.2 | 33 | 17.8 | 31 | 16.9 | 22 | 12.2 | 57 | 31.2 | ||||||||||||||||||||
4 | 41 | 22.7 | 38 | 20.4 | 59 | 31.9 | 57 | 31.2 | 53 | 29.3 | 27 | 14.8 | ||||||||||||||||||||
5 (highly appropriate) | 48 | 26.5 | 65 | 35.0 | 67 | 36.2 | 71 | 38.8 | 93 | 51.4 | 23 | 12.6 | ||||||||||||||||||||
| Median | 3 | 4 | 4 | 4 | 5 | 3 | ||||||||||||||||||||||||||
| Mean | 3.3 | 3.4 | 3.8 | 3.9 | 4.2 | 2.7 | ||||||||||||||||||||||||||
| SD | 1.4 | 1.5 | 1.2 | 1.2 | 1 | 1.4 | ||||||||||||||||||||||||||
| 7c. The psychosis is PERSISTENT or consists of repeated episodes. Assessment DOES NOT FIND a short-term reversible cause. | ||||||||||||||||||||||||||||||||
| Aripiprazole (n = 182) | Haloperidol (n = 187) | Olanzapine (n = 184) | Quetiapine (n = 182) | Risperidone (n = 183) | Ziprasidone (n = 182) | |||||||||||||||||||||||||||
| Appropriateness of use | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | ||||||||||||||||||||
1 (highly inappropriate) | 27 | 14.8 | 44 | 23.5 | 18 | 9.8 | 12 | 6.6 | 9 | 4.9 | 50 | 27.5 | ||||||||||||||||||||
2 | 17 | 9.3 | 24 | 12.8 | 14 | 7.6 | 5 | 2.8 | 6 | 3.3 | 21 | 11.5 | ||||||||||||||||||||
3 (uncertain) | 39 | 21.4 | 35 | 18.7 | 22 | 12.0 | 35 | 19.2 | 18 | 9.8 | 56 | 30.8 | ||||||||||||||||||||
4 | 45 | 24.7 | 33 | 17.7 | 59 | 32.1 | 48 | 26.4 | 56 | 30.6 | 29 | 15.9 | ||||||||||||||||||||
5 (highly appropriate) | 54 | 29.7 | 51 | 27.3 | 71 | 38.6 | 82 | 45.1 | 94 | 51.4 | 26 | 14.3 | ||||||||||||||||||||
| Median | 4 | 3 | 4 | 4 | 5 | 3 | ||||||||||||||||||||||||||
| Mean | 3.5 | 3.1 | 3.8 | 4.0 | 4.2 | 2.8 | ||||||||||||||||||||||||||
| SD | 1.4 | 1.5 | 1.3 | 1.2 | 1.1 | 1.4 | ||||||||||||||||||||||||||
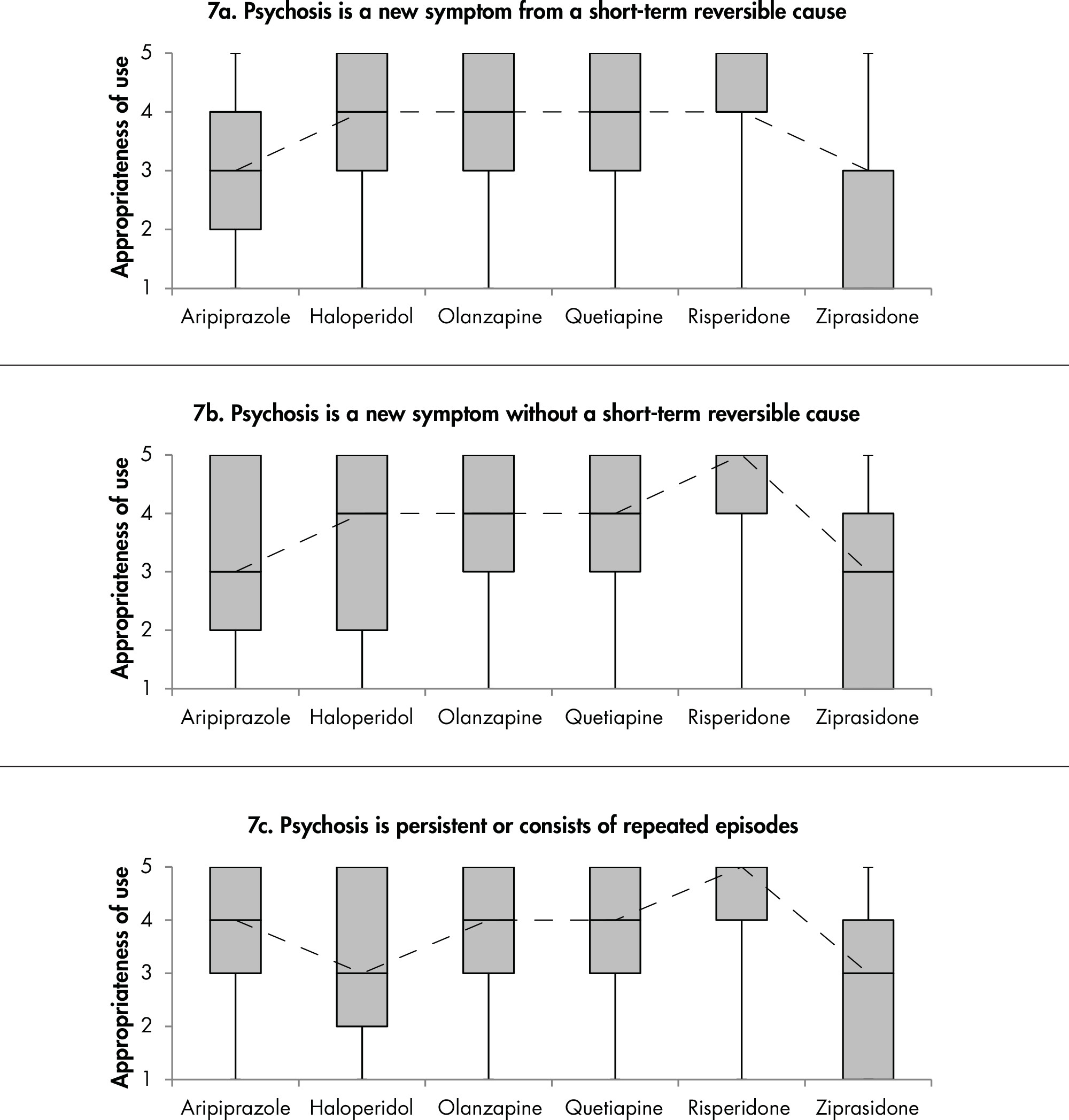
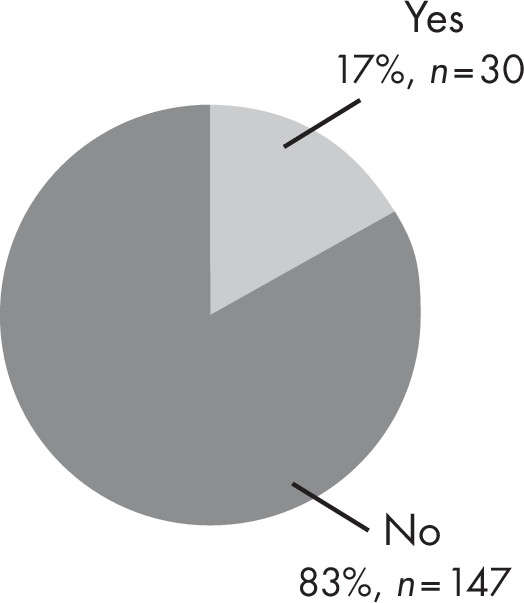
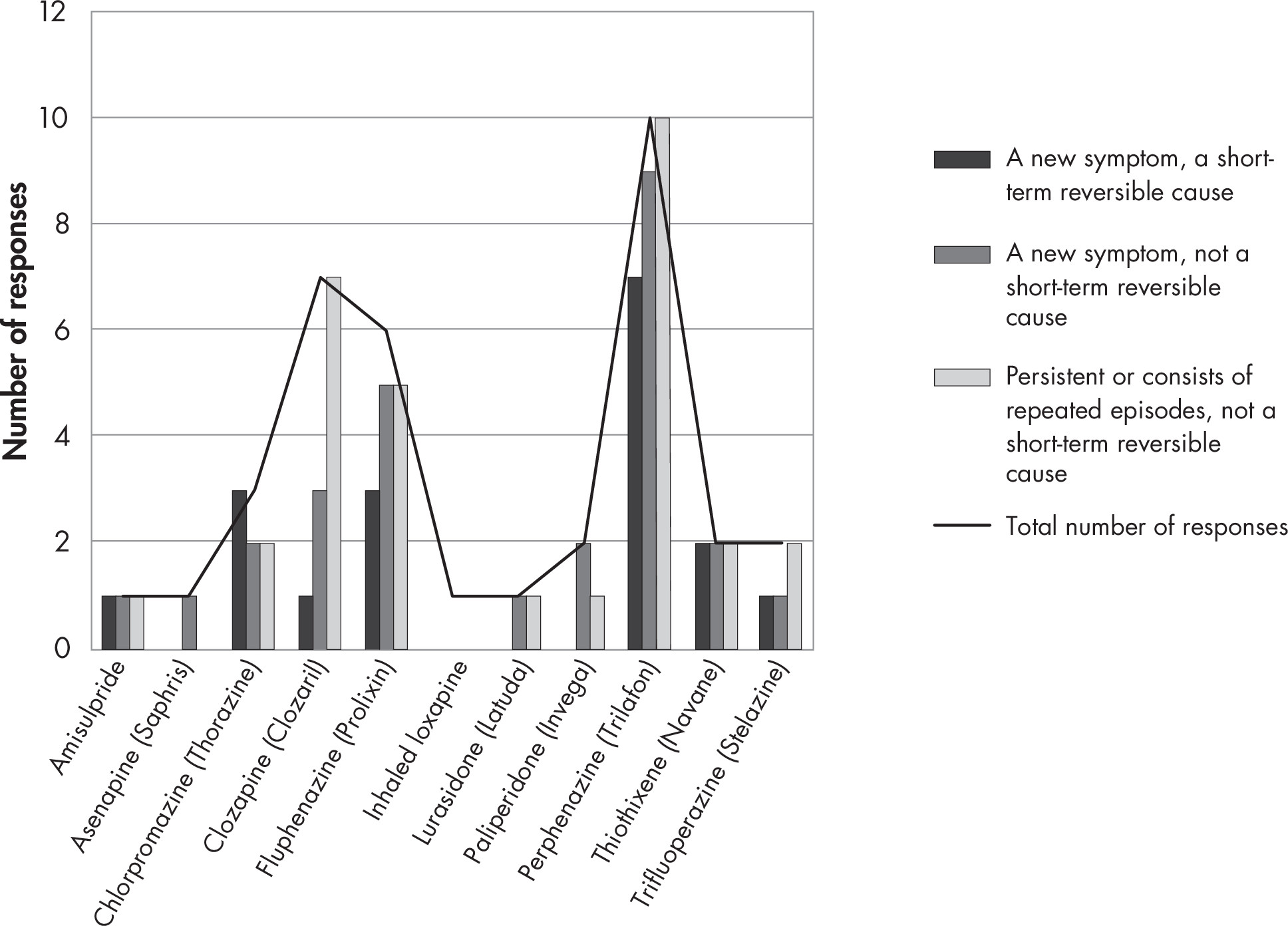
| 10a. The psychosis is a NEW SYMPTOM. Assessment SUGGESTS a short-term reversible cause of the agitation, such as acute delirium, medication side effects, or environmental causes. | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aripiprazole (n = 187) | Haloperidol (n = 188) | Olanzapine (n = 187) | Quetiapine (n = 187) | Risperidone (n = 186) | Ziprasidone (n = 181) | |||||||||||||||||||||||||||
| Appropriateness of use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (highly inappropriate) | 78 | 41.7 | 67 | 35.6 | 57 | 30.5 | 50 | 26.7 | 43 | 23.1 | 91 | 50.3 | ||||||||||||||||||||
2 | 25 | 13.4 | 36 | 19.2 | 34 | 18.2 | 37 | 19.8 | 33 | 17.7 | 30 | 16.6 | ||||||||||||||||||||
3 (uncertain) | 48 | 25.7 | 27 | 14.4 | 38 | 20.3 | 38 | 20.3 | 34 | 18.3 | 39 | 21.6 | ||||||||||||||||||||
4 | 22 | 11.8 | 35 | 18.6 | 39 | 20.9 | 39 | 20.9 | 45 | 24.2 | 11 | 6.1 | ||||||||||||||||||||
5 (highly appropriate) | 14 | 7.5 | 23 | 12.2 | 19 | 10.2 | 23 | 12.3 | 31 | 16.7 | 10 | 5.5 | ||||||||||||||||||||
| Median | 2 | 2 | 3 | 3 | 3 | 1 | ||||||||||||||||||||||||||
| Mean | 2.3 | 2.5 | 2.6 | 2.7 | 2.9 | 2.0 | ||||||||||||||||||||||||||
| SD | 1.3 | 1.4 | 1.4 | 1.4 | 1.4 | 1.2 | ||||||||||||||||||||||||||
| 10b. The psychosis is a NEW SYMPTOM. Assessment DOES NOT FIND a short-term reversible cause. | ||||||||||||||||||||||||||||||||
| Aripiprazole (n = 184) | Haloperidol (n = 183) | Olanzapine (n = 183) | Quetiapine (n = 184) | Risperidone (n = 181) | Ziprasidone (n = 182) | |||||||||||||||||||||||||||
| Appropriateness of use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (highly inappropriate) | 59 | 32.1 | 67 | 36.6 | 43 | 23.5 | 37 | 20.1 | 36 | 19.9 | 74 | 40.7 | ||||||||||||||||||||
2 | 23 | 12.5 | 34 | 18.6 | 32 | 17.5 | 33 | 17.9 | 27 | 14.9 | 31 | 17.0 | ||||||||||||||||||||
3 (uncertain) | 49 | 26.6 | 33 | 18.0 | 35 | 19.1 | 45 | 24.5 | 43 | 23.8 | 44 | 24.2 | ||||||||||||||||||||
4 | 36 | 19.6 | 29 | 15.9 | 51 | 27.9 | 42 | 22.8 | 45 | 24.9 | 20 | 11.0 | ||||||||||||||||||||
5 (highly appropriate) | 17 | 9.2 | 20 | 10.9 | 22 | 12.0 | 27 | 14.7 | 30 | 16.6 | 13 | 7.1 | ||||||||||||||||||||
| Median | 3 | 2 | 3 | 3 | 3 | 2 | ||||||||||||||||||||||||||
| Mean | 2.6 | 2.5 | 2.9 | 2.9 | 3.0 | 2.3 | ||||||||||||||||||||||||||
| SD | 1.4 | 1.4 | 1.4 | 1.3 | 1.4 | 1.3 | ||||||||||||||||||||||||||
| 10c. The psychosis is PERSISTENT or consists of repeated episodes. Assessment DOES NOT FIND a short-term reversible cause. | ||||||||||||||||||||||||||||||||
| Aripiprazole (n = 182) | Haloperidol (n = 183) | Olanzapine (n = 184) | Quetiapine (n = 184) | Risperidone (n = 182) | Ziprasidone (n = 179) | |||||||||||||||||||||||||||
| Appropriateness of use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (highly inappropriate) | 49 | 26.9 | 67 | 36.6 | 39 | 21.2 | 32 | 17.4 | 33 | 18.1 | 70 | 39.1 | ||||||||||||||||||||
2 | 29 | 15.9 | 44 | 24.0 | 37 | 20.1 | 37 | 20.1 | 27 | 14.8 | 38 | 21.2 | ||||||||||||||||||||
3 (uncertain) | 42 | 23.1 | 28 | 15.3 | 31 | 16.9 | 38 | 20.7 | 38 | 20.9 | 42 | 23.5 | ||||||||||||||||||||
4 | 44 | 24.2 | 23 | 12.6 | 53 | 28.8 | 44 | 23.9 | 50 | 27.5 | 15 | 8.4 | ||||||||||||||||||||
5 (highly appropriate) | 18 | 9.9 | 21 | 11.5 | 24 | 13.0 | 33 | 17.9 | 34 | 18.7 | 14 | 7.8 | ||||||||||||||||||||
| Median | 3 | 2 | 3 | 3 | 3 | 2 | ||||||||||||||||||||||||||
| Mean | 2.7 | 2.4 | 2.9 | 3.0 | 3.1 | 2.2 | ||||||||||||||||||||||||||
| SD | 1.3 | 1.4 | 1.4 | 1.4 | 1.4 | 1.3 | ||||||||||||||||||||||||||


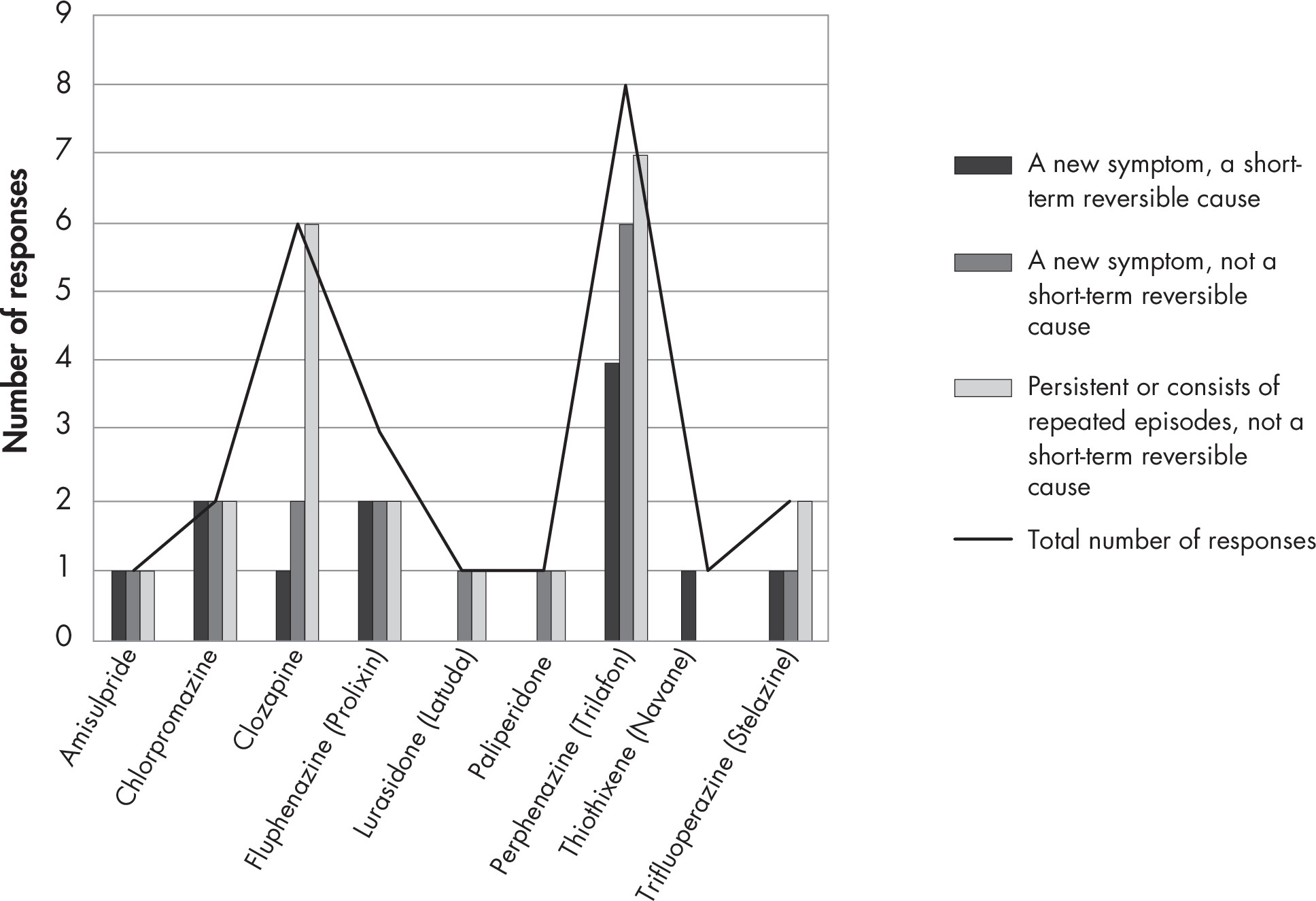
Section II: Duration of Treatment
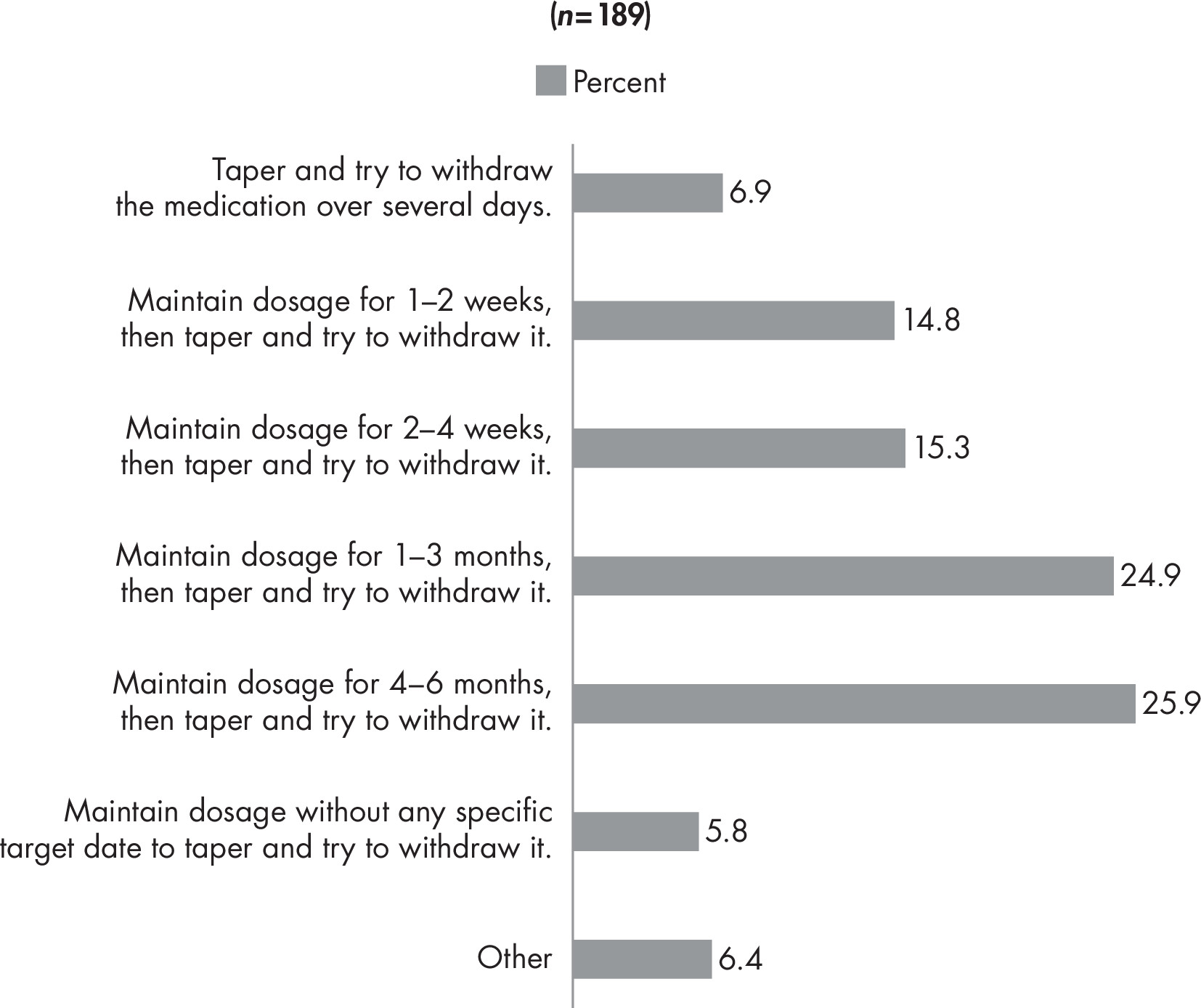
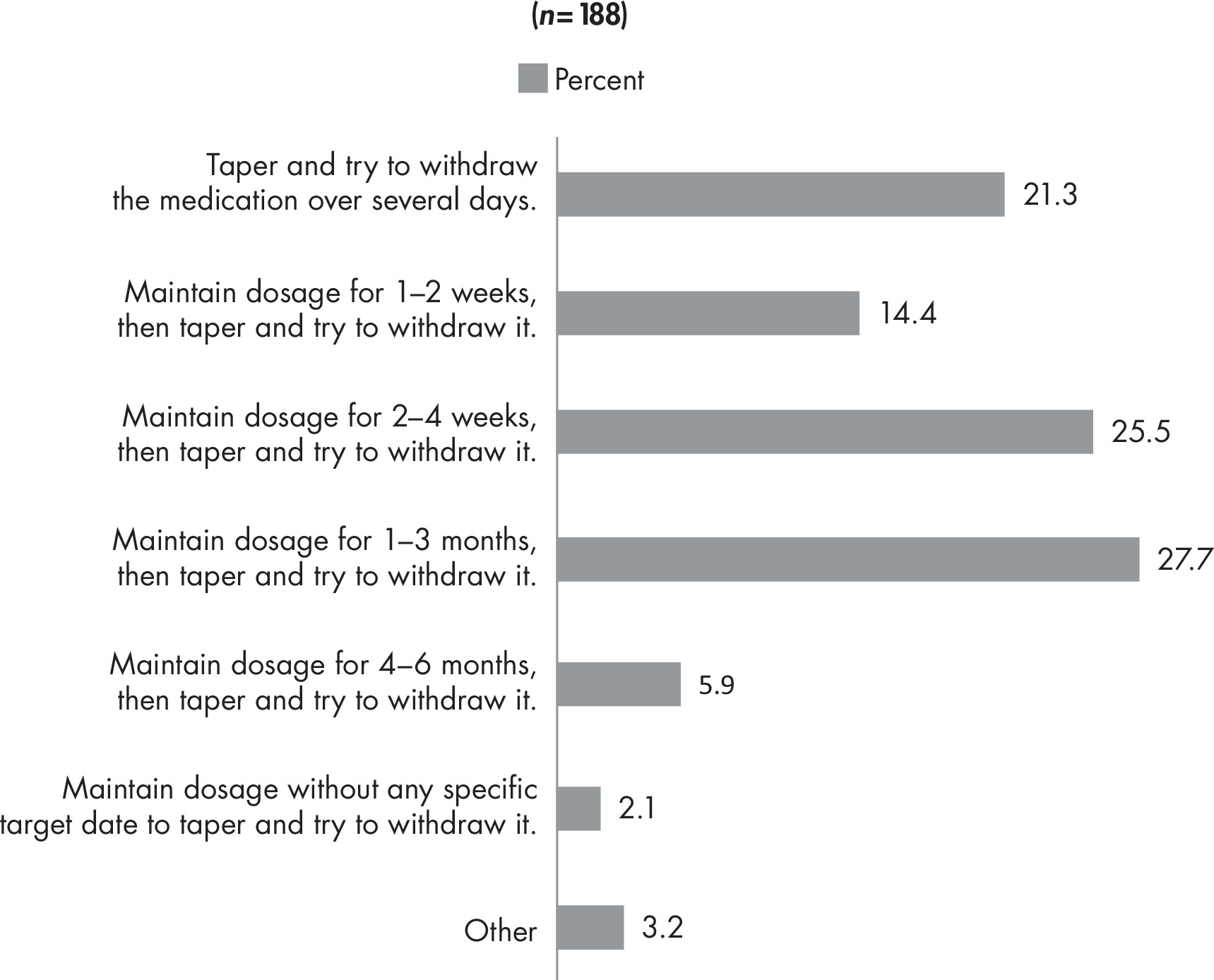
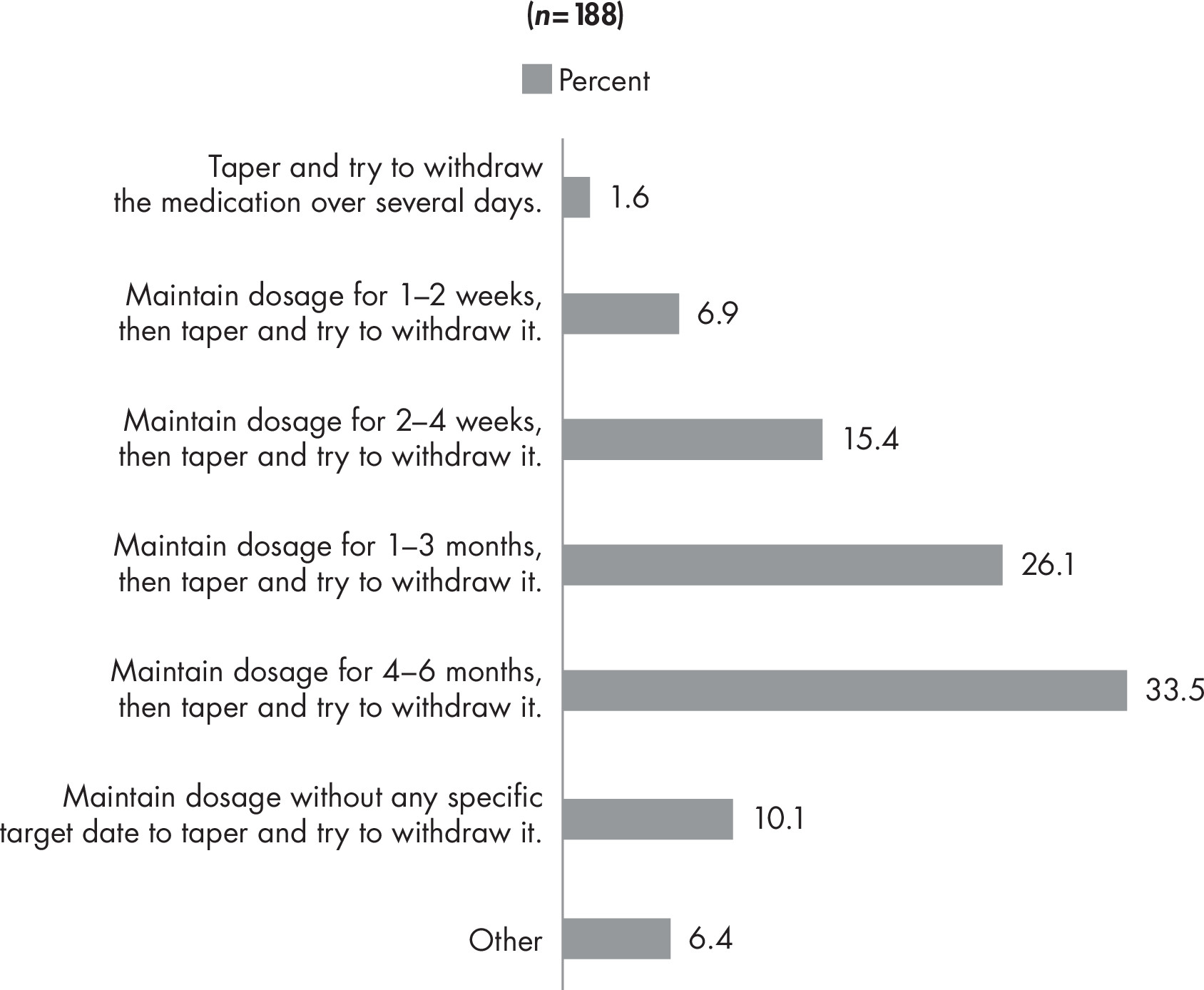

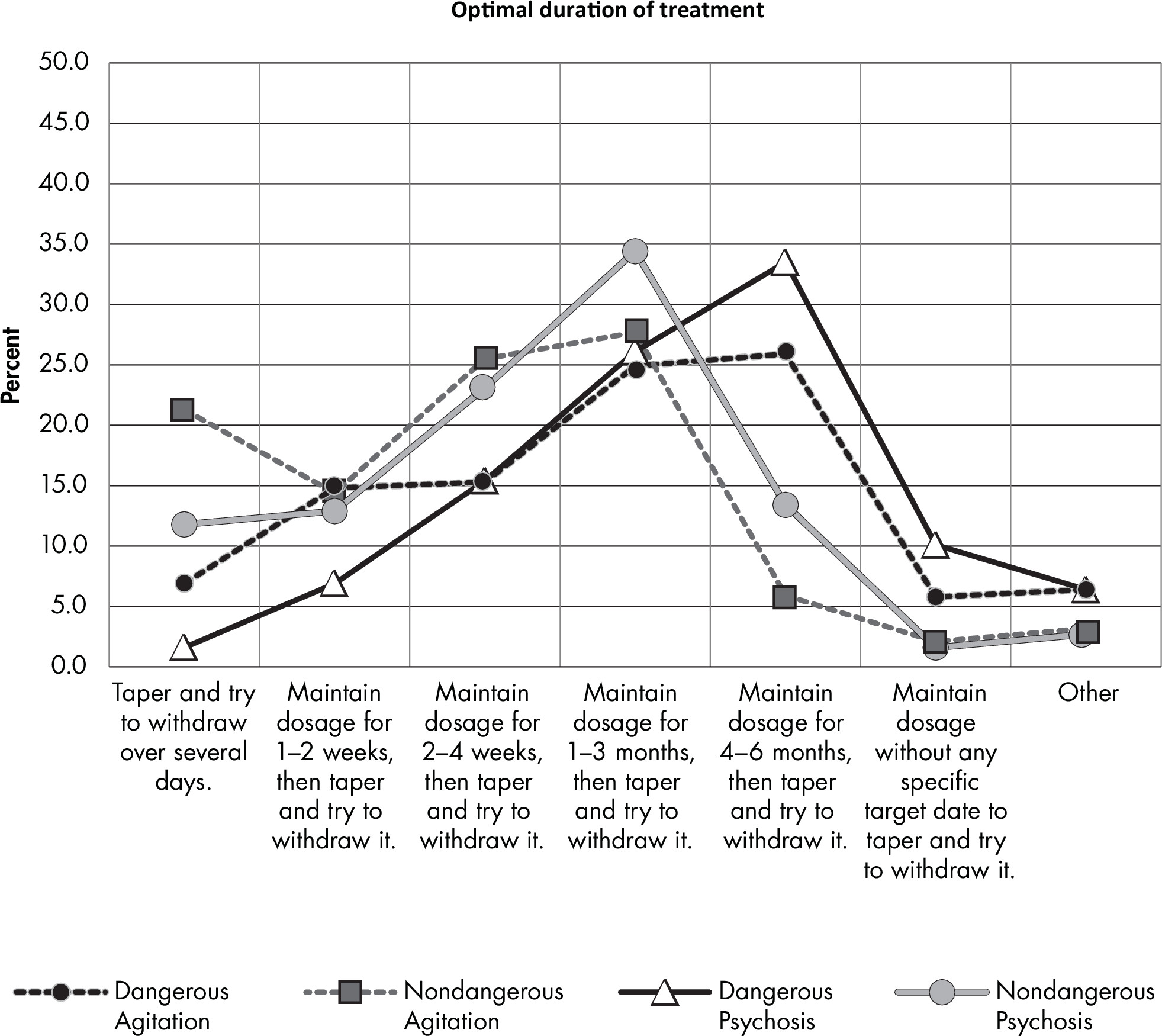
Section III: Clinical Experience Using Antipsychotics in Patients With Dementia




| 21a. AKATHISIA | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aripiprazole (n = 146) | Haloperidol (n = 147) | Olanzapine (n = 142) | Quetiapine (n = 145) | Risperidone (n = 145) | Ziprasidone (n = 142) | |||||||||||||||||||||||||||
| Extent of decreased use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (not at all) | 46 | 31.5 | 31 | 21.1 | 50 | 35.2 | 77 | 53.1 | 38 | 26.2 | 56 | 39.4 | ||||||||||||||||||||
2 | 21 | 14.4 | 16 | 10.9 | 38 | 26.8 | 34 | 23.5 | 29 | 20.0 | 24 | 16.9 | ||||||||||||||||||||
3 (somewhat) | 33 | 22.6 | 33 | 22.5 | 37 | 26.1 | 22 | 15.2 | 38 | 26.2 | 43 | 30.3 | ||||||||||||||||||||
4 | 34 | 23.3 | 39 | 26.5 | 11 | 7.8 | 10 | 6.9 | 30 | 20.7 | 12 | 8.5 | ||||||||||||||||||||
5 (very much) | 12 | 8.2 | 28 | 19.1 | 6 | 4.2 | 2 | 1.4 | 10 | 6.9 | 7 | 4.9 | ||||||||||||||||||||
| Median | 3 | 3 | 2 | 1 | 3 | 2 | ||||||||||||||||||||||||||
| Mean | 2.6 | 3.1 | 2.2 | 1.8 | 2.6 | 2.2 | ||||||||||||||||||||||||||
| SD | 1.4 | 1.4 | 1.1 | 1.0 | 1.3 | 1.2 | ||||||||||||||||||||||||||
| 21b. ANTICHOLINERGIC EFFECTS | ||||||||||||||||||||||||||||||||
| Aripiprazole (n = 145) | Haloperidol (n = 146) | Olanzapine (n = 143) | Quetiapine (n = 147) | Risperidone (n = 145) | Ziprasidone (n = 143) | |||||||||||||||||||||||||||
| Extent of decreased use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (not at all) | 94 | 64.8 | 72 | 49.3 | 41 | 28.7 | 60 | 40.8 | 66 | 45.5 | 82 | 57.3 | ||||||||||||||||||||
2 | 24 | 16.6 | 24 | 16.4 | 31 | 21.7 | 33 | 22.5 | 37 | 25.5 | 22 | 15.4 | ||||||||||||||||||||
3 (somewhat) | 16 | 11.0 | 24 | 16.4 | 39 | 27.3 | 31 | 21.1 | 23 | 15.9 | 29 | 20.3 | ||||||||||||||||||||
4 | 9 | 6.2 | 14 | 9.6 | 24 | 16.8 | 16 | 10.9 | 13 | 9.0 | 8 | 5.6 | ||||||||||||||||||||
5 (very much) | 2 | 1.4 | 12 | 8.2 | 8 | 5.6 | 7 | 4.8 | 6 | 4.1 | 2 | 1.4 | ||||||||||||||||||||
| Median | 1 | 2 | 2 | 2 | 2 | 1 | ||||||||||||||||||||||||||
| Mean | 1.6 | 2.1 | 2.5 | 2.2 | 2.0 | 1.8 | ||||||||||||||||||||||||||
| SD | 1.0 | 1.3 | 1.2 | 1.2 | 1.2 | 1.0 | ||||||||||||||||||||||||||
| 21c. CARDIAC EFFECTS | ||||||||||||||||||||||||||||||||
| Aripiprazole (n = 141) | Haloperidol (n = 144) | Olanzapine (n = 143) | Quetiapine (n = 143) | Risperidone (n = 142) | Ziprasidone (n = 143) | |||||||||||||||||||||||||||
| Extent of decreased use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (not at all) | 81 | 57.5 | 56 | 38.9 | 55 | 38.5 | 58 | 40.6 | 55 | 38.7 | 43 | 30.1 | ||||||||||||||||||||
2 | 16 | 11.4 | 27 | 18.8 | 24 | 16.8 | 27 | 18.9 | 27 | 19.0 | 13 | 9.1 | ||||||||||||||||||||
3 (somewhat) | 21 | 14.9 | 25 | 17.4 | 34 | 23.8 | 31 | 21.7 | 29 | 20.4 | 34 | 23.8 | ||||||||||||||||||||
4 | 15 | 10.6 | 21 | 14.6 | 21 | 14.7 | 19 | 13.3 | 21 | 14.8 | 25 | 17.5 | ||||||||||||||||||||
5 (very much) | 8 | 5.7 | 15 | 10.4 | 9 | 6.3 | 8 | 5.6 | 10 | 7.0 | 28 | 19.6 | ||||||||||||||||||||
| Median | 1 | 2 | 2 | 2 | 2 | 3 | ||||||||||||||||||||||||||
| Mean | 2.0 | 2.4 | 2.3 | 2.2 | 2.3 | 2.9 | ||||||||||||||||||||||||||
| SD | 1.3 | 1.4 | 1.3 | 1.3 | 1.3 | 1.5 | ||||||||||||||||||||||||||
| 21d. DEATH | ||||||||||||||||||||||||||||||||
| Aripiprazole (n = 148) | Haloperidol (n = 149) | Olanzapine (n = 148) | Quetiapine (n = 148) | Risperidone (n = 145) | Ziprasidone (n = 145) | |||||||||||||||||||||||||||
| Extent of decreased use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (not at all) | 73 | 49.3 | 62 | 41.6 | 59 | 39.9 | 63 | 42.6 | 59 | 40.7 | 58 | 40.0 | ||||||||||||||||||||
2 | 13 | 8.8 | 17 | 11.4 | 17 | 11.5 | 18 | 12.2 | 18 | 12.4 | 13 | 9.0 | ||||||||||||||||||||
3 (somewhat) | 31 | 21.0 | 25 | 16.8 | 36 | 24.3 | 36 | 24.3 | 32 | 22.1 | 37 | 25.5 | ||||||||||||||||||||
4 | 17 | 11.5 | 24 | 16.1 | 17 | 11.5 | 17 | 11.5 | 20 | 13.8 | 19 | 13.1 | ||||||||||||||||||||
5 (very much) | 14 | 9.5 | 21 | 14.1 | 19 | 12.8 | 14 | 9.5 | 16 | 11.0 | 18 | 12.4 | ||||||||||||||||||||
| Median | 2 | 2 | 2 | 2 | 2 | 3 | ||||||||||||||||||||||||||
| Mean | 2.2 | 2.5 | 2.5 | 2.3 | 2.4 | 2.5 | ||||||||||||||||||||||||||
| SD | 1.4 | 1.5 | 1.4 | 1.4 | 1.4 | 1.4 | ||||||||||||||||||||||||||
| 21e. DRUG-INDUCED PARKINSONISM | ||||||||||||||||||||||||||||||||
| Aripiprazole (n = 145) | Haloperidol (n = 148) | Olanzapine (n = 143) | Quetiapine (n = 146) | Risperidone (n = 147) | Ziprasidone (n = 141) | |||||||||||||||||||||||||||
| Extent of decreased use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (not at all) | 65 | 44.8 | 20 | 13.5 | 46 | 32.2 | 79 | 54.1 | 23 | 15.7 | 61 | 43.3 | ||||||||||||||||||||
2 | 23 | 15.9 | 12 | 8.1 | 30 | 21.0 | 35 | 24.0 | 18 | 12.2 | 30 | 21.3 | ||||||||||||||||||||
3 (somewhat) | 35 | 24.1 | 33 | 22.3 | 44 | 30.8 | 23 | 15.8 | 53 | 36.1 | 32 | 22.7 | ||||||||||||||||||||
4 | 15 | 10.3 | 34 | 23.0 | 16 | 11.2 | 6 | 4.1 | 34 | 23.1 | 11 | 7.8 | ||||||||||||||||||||
5 (very much) | 7 | 4.8 | 49 | 33.1 | 7 | 4.9 | 3 | 2.1 | 19 | 12.9 | 7 | 5.0 | ||||||||||||||||||||
| Median | 2 | 4 | 2 | 1 | 3 | 2 | ||||||||||||||||||||||||||
| Mean | 2.1 | 3.5 | 2.4 | 1.8 | 3.1 | 2.1 | ||||||||||||||||||||||||||
| SD | 1.2 | 1.4 | 1.2 | 1.0 | 1.2 | 1.2 | ||||||||||||||||||||||||||
| 21f. METABOLIC EFFECTS, EXCLUDING WEIGHT GAIN | ||||||||||||||||||||||||||||||||
| Aripiprazole (n = 143) | Haloperidol (n = 145) | Olanzapine (n = 149) | Quetiapine (n = 147) | Risperidone (n = 146) | Ziprasidone (n = 143) | |||||||||||||||||||||||||||
| Extent of decreased use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (not at all) | 75 | 52.5 | 79 | 54.5 | 28 | 18.8 | 43 | 29.3 | 45 | 30.8 | 74 | 51.8 | ||||||||||||||||||||
2 | 31 | 21.7 | 31 | 21.4 | 18 | 12.1 | 26 | 17.7 | 37 | 25.3 | 30 | 21.0 | ||||||||||||||||||||
3 (somewhat) | 22 | 15.4 | 20 | 13.8 | 32 | 21.5 | 38 | 25.9 | 39 | 26.7 | 29 | 20.3 | ||||||||||||||||||||
4 | 12 | 8.4 | 10 | 6.9 | 35 | 23.5 | 28 | 19.1 | 22 | 15.1 | 7 | 4.9 | ||||||||||||||||||||
5 (very much) | 3 | 2.1 | 5 | 3.5 | 36 | 24.2 | 12 | 8.2 | 3 | 2.1 | 3 | 2.1 | ||||||||||||||||||||
| Median | 1 | 1 | 3 | 3 | 2 | 1 | ||||||||||||||||||||||||||
| Mean | 1.9 | 1.8 | 3.2 | 2.6 | 2.3 | 1.8 | ||||||||||||||||||||||||||
| SD | 1.1 | 1.1 | 1.4 | 1.3 | 1.1 | 1.0 | ||||||||||||||||||||||||||
| 21g. NEUROLEPTIC MALIGNANT SYNDROME | ||||||||||||||||||||||||||||||||
| Aripiprazole (n = 148) | Haloperidol (n = 149) | Olanzapine (n = 144) | Quetiapine (n = 146) | Risperidone (n = 146) | Ziprasidone (n = 142) | |||||||||||||||||||||||||||
| Extent of decreased use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (not at all) | 93 | 62.8 | 72 | 48.3 | 86 | 59.7 | 95 | 65.1 | 82 | 56.2 | 87 | 61.3 | ||||||||||||||||||||
2 | 20 | 13.5 | 19 | 12.8 | 20 | 13.9 | 20 | 13.7 | 24 | 16.4 | 22 | 15.5 | ||||||||||||||||||||
3 (somewhat) | 18 | 12.2 | 22 | 14.8 | 24 | 16.7 | 23 | 15.8 | 21 | 14.4 | 24 | 16.9 | ||||||||||||||||||||
4 | 14 | 9.5 | 25 | 16.8 | 9 | 6.3 | 7 | 4.8 | 15 | 10.3 | 7 | 4.9 | ||||||||||||||||||||
5 (very much) | 3 | 2.0 | 11 | 7.4 | 5 | 3.5 | 1 | 0.7 | 4 | 2.7 | 2 | 1.4 | ||||||||||||||||||||
| Median | 1 | 2 | 1 | 1 | 1 | 1 | ||||||||||||||||||||||||||
| Mean | 1.7 | 2.2 | 1.8 | 1.6 | 1.9 | 1.7 | ||||||||||||||||||||||||||
| SD | 1.1 | 1.4 | 1.1 | 1.0 | 1.2 | 1.0 | ||||||||||||||||||||||||||
| 21h. STROKE | ||||||||||||||||||||||||||||||||
| Aripiprazole (n = 148) | Haloperidol (n = 150) | Olanzapine (n = 148) | Quetiapine (n = 148) | Risperidone (n = 148) | Ziprasidone (n = 145) | |||||||||||||||||||||||||||
| Extent of decreased use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (not at all) | 71 | 48.0 | 62 | 41.3 | 55 | 37.2 | 61 | 41.2 | 55 | 37.2 | 62 | 42.8 | ||||||||||||||||||||
2 | 22 | 14.9 | 21 | 14.0 | 21 | 14.2 | 26 | 17.6 | 29 | 19.6 | 23 | 15.9 | ||||||||||||||||||||
3 (somewhat) | 30 | 20.3 | 32 | 21.3 | 36 | 24.3 | 34 | 23.0 | 33 | 22.3 | 33 | 22.8 | ||||||||||||||||||||
4 | 17 | 11.5 | 21 | 14.0 | 26 | 17.6 | 21 | 14.2 | 23 | 15.5 | 18 | 12.4 | ||||||||||||||||||||
5 (very much) | 8 | 5.4 | 14 | 9.3 | 10 | 6.8 | 6 | 4.1 | 8 | 5.4 | 9 | 6.2 | ||||||||||||||||||||
| Median | 2 | 2 | 2 | 2 | 2 | 2 | ||||||||||||||||||||||||||
| Mean | 2.1 | 2.4 | 2.4 | 2.2 | 2.3 | 2.2 | ||||||||||||||||||||||||||
| SD | 1.3 | 1.4 | 1.3 | 1.2 | 1.3 | 1.3 | ||||||||||||||||||||||||||
| 21i. WEIGHT GAIN | ||||||||||||||||||||||||||||||||
| Aripiprazole (n = 145) | Haloperidol (n = 147) | Olanzapine (n = 147) | Quetiapine (n = 149) | Risperidone (n = 146) | Ziprasidone (n = 143) | |||||||||||||||||||||||||||
| Extent of decreased use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (not at all) | 86 | 59.3 | 92 | 62.6 | 32 | 21.8 | 48 | 32.2 | 51 | 34.9 | 90 | 62.9 | ||||||||||||||||||||
2 | 24 | 16.6 | 22 | 15.0 | 11 | 7.5 | 21 | 14.1 | 37 | 25.3 | 18 | 12.6 | ||||||||||||||||||||
3 (somewhat) | 23 | 15.9 | 20 | 13.6 | 33 | 22.5 | 39 | 26.2 | 36 | 24.7 | 27 | 18.9 | ||||||||||||||||||||
4 | 10 | 6.9 | 10 | 6.8 | 33 | 22.5 | 32 | 21.5 | 19 | 13.0 | 5 | 3.5 | ||||||||||||||||||||
5 (very much) | 2 | 1.4 | 3 | 2.0 | 38 | 25.9 | 9 | 6.0 | 3 | 2.1 | 3 | 2.1 | ||||||||||||||||||||
| Median | 1 | 1 | 3 | 3 | 2 | 1 | ||||||||||||||||||||||||||
| Mean | 1.7 | 1.7 | 3.2 | 2.6 | 2.2 | 1.7 | ||||||||||||||||||||||||||
| SD | 1.0 | 1.1 | 1.5 | 1.3 | 1.1 | 1.0 | ||||||||||||||||||||||||||
| 21j. OTHER | ||||||||||||||||||||||||||||||||
| Aripiprazole (n = 65) | Haloperidol (n = 65) | Olanzapine (n = 62) | Quetiapine (n = 63) | Risperidone (n = 63) | Ziprasidone (n = 60) | |||||||||||||||||||||||||||
| Extent of decreased use | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||||||||||||||||
1 (not at all) | 42 | 64.6 | 43 | 65.2 | 35 | 56.5 | 31 | 49.2 | 37 | 58.7 | 37 | 61.7 | ||||||||||||||||||||
2 | 4 | 6.2 | 4 | 6.1 | 5 | 8.1 | 2 | 3.2 | 9 | 14.3 | 7 | 11.7 | ||||||||||||||||||||
3 (somewhat) | 11 | 16.9 | 6 | 9.1 | 10 | 16.1 | 15 | 23.8 | 9 | 14.3 | 7 | 11.7 | ||||||||||||||||||||
4 | 4 | 6.2 | 4 | 6.1 | 4 | 6.5 | 6 | 9.5 | 4 | 6.4 | 3 | 5.0 | ||||||||||||||||||||
5 (very much) | 4 | 6.2 | 9 | 13.6 | 8 | 12.9 | 9 | 14.3 | 4 | 6.4 | 6 | 10.0 | ||||||||||||||||||||
| Median | 1 | 1 | 1 | 2 | 1 | 1 | ||||||||||||||||||||||||||
| Mean | 1.8 | 2.0 | 2.1 | 2.4 | 1.9 | 1.9 | ||||||||||||||||||||||||||
| SD | 1.3 | 1.5 | 1.5 | 1.5 | 1.2 | 1.4 | ||||||||||||||||||||||||||
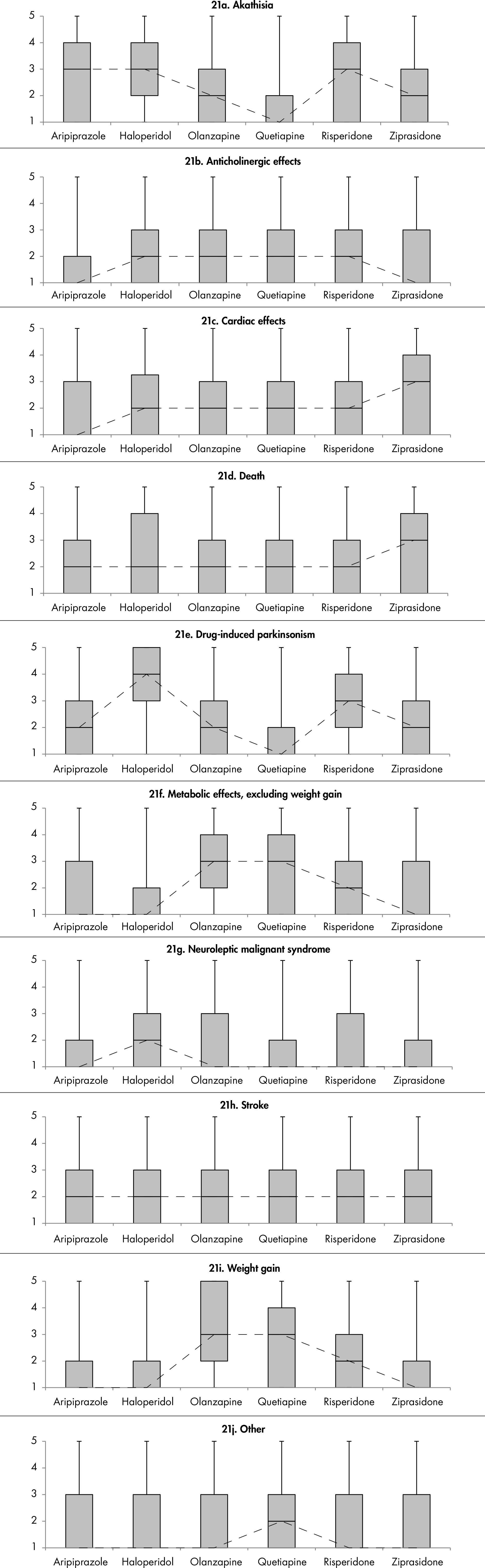
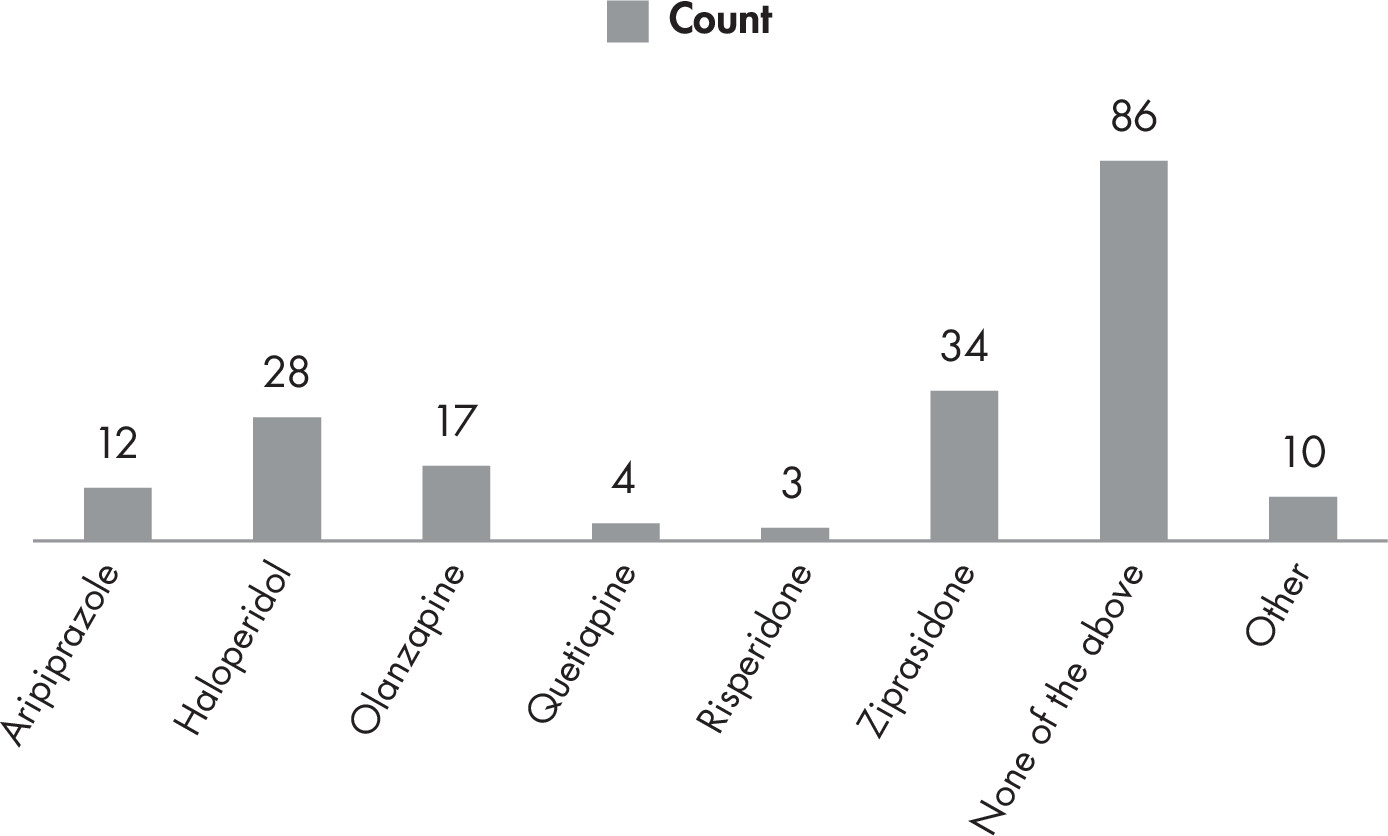
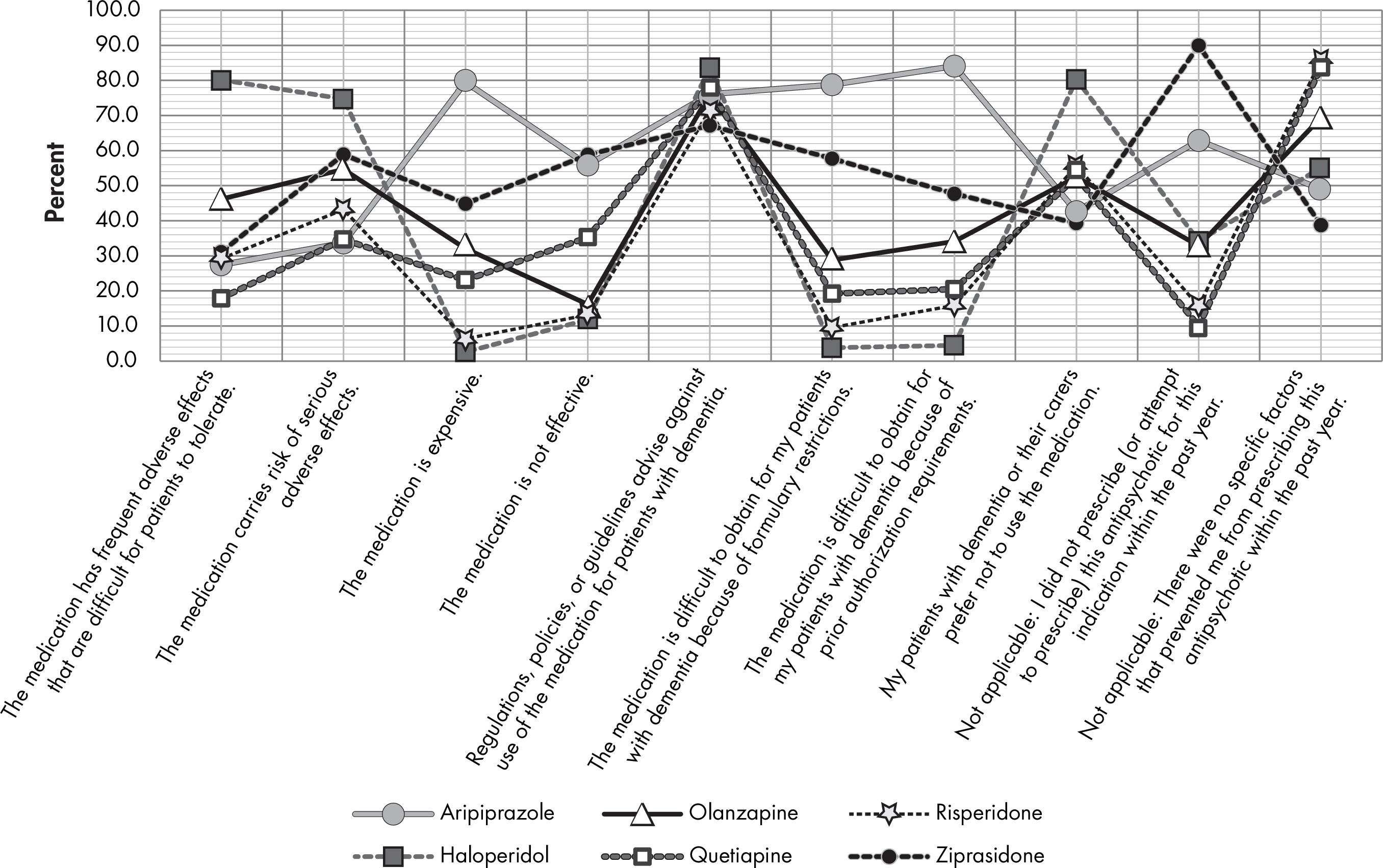
Footnote
Information & Authors
Information
Published In
Authors
Metrics & Citations
Metrics
Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
Login options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).
