Selective attention, the ability to enhance the processing of information relevant to our goals and limit the processing of that which is irrelevant, is a fundamental cognitive capability that is essential for everyday functioning. Disturbances of selective attention are among the earliest described and most clinically apparent cognitive deficits that are present in schizophrenia (
1), and these disturbances appear to be related to behavioral disorganization in this illness (
2).
With the advent of functional brain imaging it has become possible to investigate the neural substrates of selective attention deficits. Positron emission tomography (PET) studies that employ continuous performance tasks have reported decreased metabolism in lateral and medial prefrontal cortex in patients with schizophrenia (
3,
4). These studies used continuous performance tasks to stabilize the subjects' psychological state and place demands upon systems involved in vigilance and attention. These studies did not include control tasks or seek to establish specific relationships between abnormal attentional processes and the metabolic response of neural systems.
With the development of the [
15O]H
2O PET brain mapping technique, in which repeated scans are performed during different psychological states, it has become possible to design experiments that associate discrete neural system responses with specific cognitive processes. This involves constructing control tasks that match activation tasks on sensory, motor, and cognitive processes that are not specifically of interest. Comparisons of regional cerebral blood flow (CBF) between activation and control states isolate the regional CBF responses that are related to the specific processes of interest. A recent application of this technique used a dichotic listening task to show impaired task-appropriate modulation of blood flow in the superior temporal gyrus in patients with schizophrenia (
5).
In studies of attentional processes analyzed by human brain mapping, considerable emphasis has been placed upon the role of the anterior cingulate gyrus (
6). The anterior cingulate gyrus is activated when subjects divide their attention across more than one feature of a stimulus (
7) but not when they attend to a single feature. During the Stroop task (
8), a paradigmatic measure of selective attention in which subjects must avoid reading a word (a pre-potent response) while naming its color, anterior cingulate gyrus activation has been reported in three independent studies (
9–
11); the precise rostro-caudal location of activation varied with the particular stimulus parameters employed (
11). In contrast, the dorsolateral prefrontal cortex and parietal cortex, but not the anterior cingulate gyrus, were activated when subjects maintained attention for the occurrence of visual and somatosensory stimuli (
12). It has been proposed that anterior cingulate gyrus activation is associated with the selection of one of several competing responses (
6,
13) and that it has an executive role in the control of selective attention (
6,
10).
Anterior cingulate gyrus abnormalities have been reported in morphometric and histopathological studies of schizophrenia (
14,
15). Medial frontal hypometabolism was found in patients at rest (
16) and during performance of a continuous performance task (
17,
18). Decreased medial frontal activation, probably in the anterior cingulate gyrus, was seen in patients during performance of the Tower of London task (
19), and impaired activation was seen in the anterior cingulate gyrus during paced verbal fluency, compared to word repetition, tasks in patients with schizophrenia (
20). We recently reported decreased activation in the anterior cingulate gyrus, dorsolateral prefrontal cortex, and superior temporal gyrus during supraspan memory performance (
21). Liddle and colleagues reported significant correlations between anterior cingulate gyrus regional CBF and disorganization in chronically symptomatic subjects (
22). Together these studies suggest that there are behaviorally relevant structural and functional anterior cingulate gyrus abnormalities in patients with schizophrenia. However the relationship between these abnormalities and specific functional deficits, such as impaired selective attention, remains unknown. The present study sought to address this question by using [
15O]H
2O PET and a single-trial Stroop task.
RESULTS
Task Performance
Because of initial technical difficulties in measuring verbal responses in the scanner, reliable reaction time data were available for only nine comparison subjects and 10 patients with schizophrenia. Accuracy ratings, scored from taped responses, were obtained for all but one subject in each group. Both groups were able to maintain the pace of responding to a stimulus every 1750 msec without omissions. Hence, differences between the groups' regional CBF response cannot be due to reduced rates of responding, which is a frequent confound in mapping studies of cognitively impaired subjects.
As in previous studies of single-trial Stroop task performance (
31), the patients with schizophrenia did not show greater interference, as measured by the difference in reaction times for color-incongruent stimuli (patients with schizophrenia: mean=958.6 msec [SD=186.4], comparison subjects: mean=902.3 msec [SD=229.4]) and neutral stimuli (patients with schizophrenia: mean=855.9 msec [SD=171.0], comparison subjects: mean=805.3 msec [SD=192.3]) (F=0.03, df=1,17, p<0.87). In the error analysis, overall error rates were low, which confirms that subjects understood the task and were performing as instructed. The patients with schizophrenia showed a larger increase in errors with color-incongruent stimuli (mean=11.1%, SD=9.2%) than with neutral stimuli (mean=2.2%, SD=1.9%) than did the comparison subjects (mean=1.9% [SD=3.4%] and mean=0.5% [SD=0.9%], respectively) (F=7.9, df=1,25, p<0.01). These errors invariably consisted of reading the word rather than naming its color. This greater error interference remained significant whether the comparison was made for all subjects or just those with reaction time data. Pearson's correlation coefficients between reaction times and error rates for color-incongruent stimuli for the whole group (r=0.10, df=18, p<0.34), the comparison subjects (r=0.36, df=8, p<0.34), and the patients with schizophrenia (r=–0.02, df=9, p<0.34) were not significant. The nonparametric Spearman's rank order correlation showed a similar result (whole group: r
s=0.14, N=19, p<0.26; comparison subjects: r
s=0.42, N=9, p<0.26; patients with schizophrenia: r
s=–0.12, N=10, p<0.26). Hence, the higher frequency of errors was not simply due to a speed-accuracy tradeoff by the patients with schizophrenia. Rather, the greater number of errors in the color-incongruent condition indicates attentional dysfunction in the patients with schizophrenia, which reflects a greater influence of the irrelevant dimension of the stimulus (the word) over naming the color (
32).
Performance during color-congruent blocks did not reveal differences between the patients with schizophrenia and the comparison subjects in either reaction times (color-congruent mean=723.7 msec [SD=209.7] and 665.7 msec [SD=160.0], respectively; neutral mean=790.8 msec [SD=218.5] and 751.2 msec [SD=176.2], respectively) or errors (color-congruent mean=0.1% [SD=0.3%] and 0.0% [SD=0.0%], respectively; neutral mean=1.4% [SD=1.2%] and 0.6% [SD=1.1%], respectively). Comparison subjects showed more reaction time facilitation than patients, but this was not significant. This would be expected if patients with schizophrenia have a deficit in the control of selective attention, since in the current design the color-congruent condition was a mix of neutral and color-congruent stimuli. Comparison subjects would be better able to strategically allow more reading, thus taking advantage of the presence of color-congruent color names to improve performance.
Regional CBF
The regional CBF analysis focuses on the comparison of the two groups' responses to color-incongruent versus neutral conditions, since it is in this comparison that patients showed significant evidence of impaired selective attention.
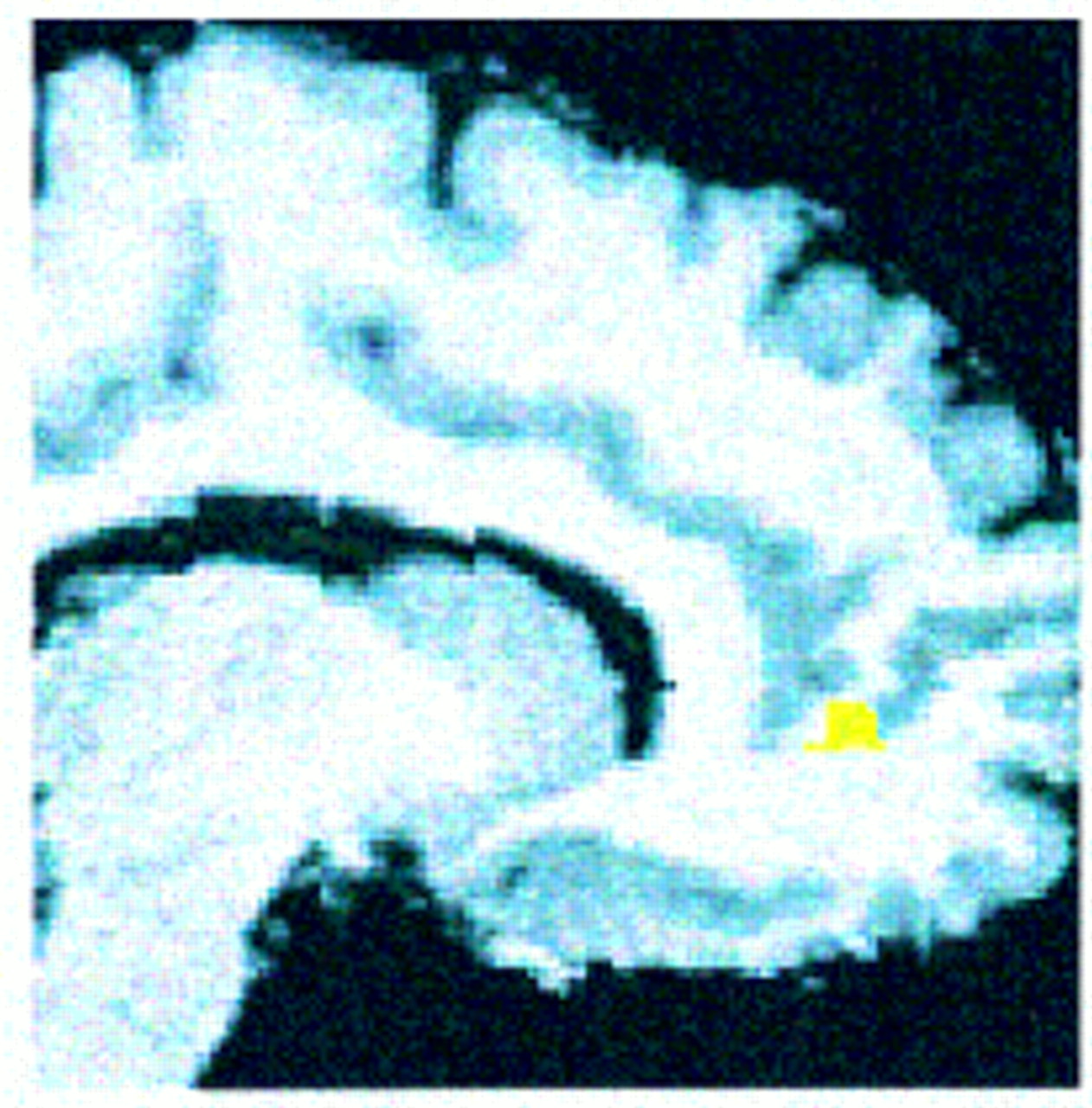
Consistent with our hypothesis, patients with schizophrenia showed significantly less activation than comparison subjects in the right anterior cingulate gyrus (pixel maxima at Talairach coordinates 12, 46, 4) (z=2.88, N=29, p<0.002). The location and extent of this group difference is shown in
figure 1.
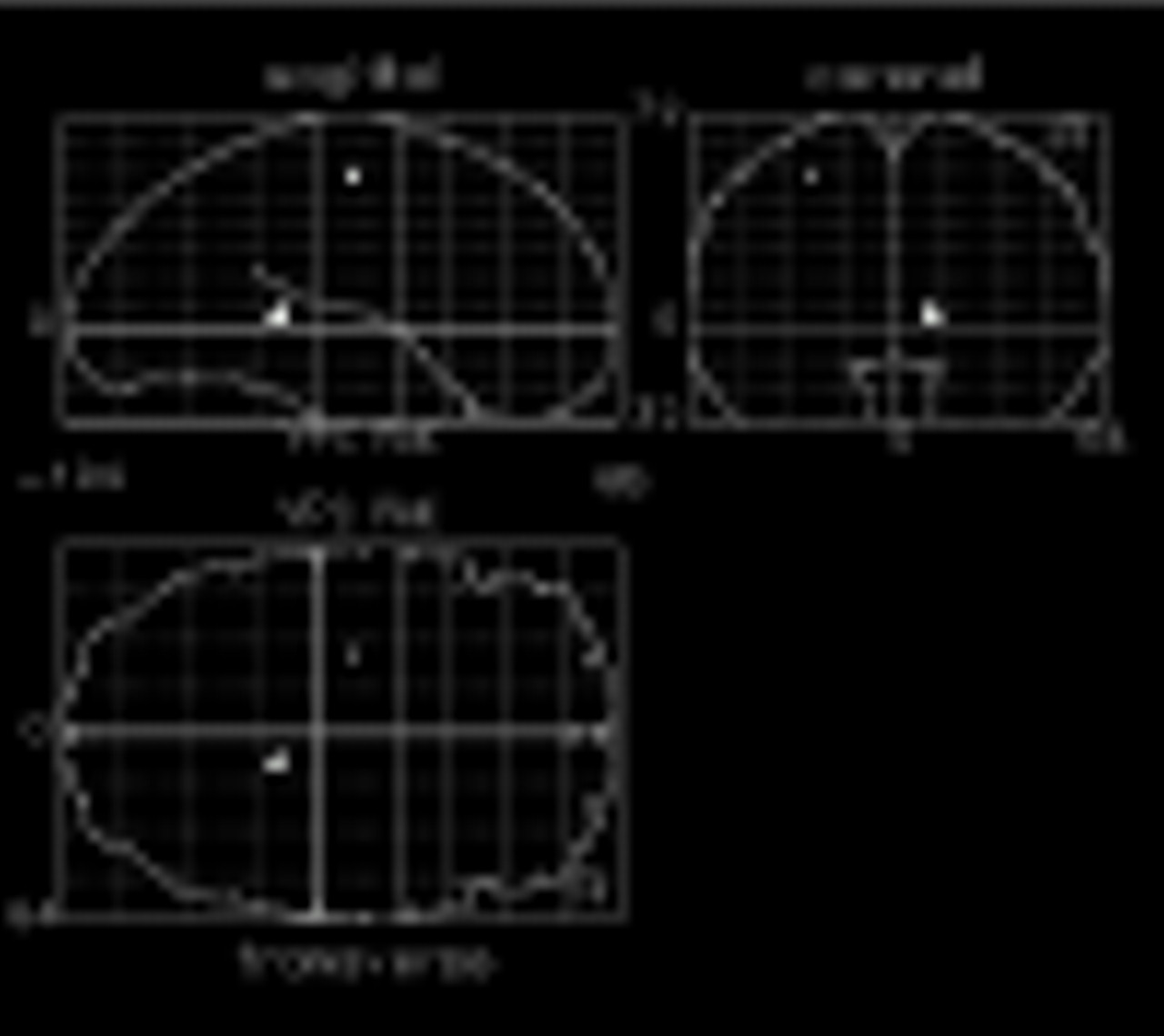
Figure 2 shows other regions of the brain that increased less in patients with schizophrenia than in normal comparison subjects in response to naming the color of color-incongruent stimuli. Differences are seen in the left precentral gyrus (Talairach coordinates –26, –14, 52) (z=3.24, N=29, p<0.001), and right hippocampal gyrus (Talairach coordinates 12, –38, 4) (z=3.45, N=29, p<0.001). Orthogonal projection views of these group differences in activation are shown in
figure 2.
Group differences in the magnitude of regional CBF responses seen in the anterior cingulate gyrus, left precentral gyrus, and right hippocampal region were not due to greater variability in these regions. The patients with schizophrenia showed smaller and generally less variable responses than comparison subjects in the anterior cingulate gyrus (patients with schizophrenia: mean change=1.2% [SD=2.4%], comparison subjects: 2.4% [SD=3.4%]) and the precentral gyrus (patients with schizophrenia: mean=–0.2% [SD=2.2%], comparison subjects: mean=3.3% [SD=3.4%]). The hippocampal gyrus difference reflected a region that decreased more in the patients with schizophrenia (mean=–2.4%, SD=3.7%) than in the normal comparison subjects (mean=0.3%, SD=4.3%).
Correlations Between Regional CBF Response and Behavior
Pearson's product moment correlation coefficients were calculated between errors in the color-incongruent condition and regional CBF response in the anterior cingulate gyrus. This correlation was positive and approached significance at a trend level in both groups (comparison subjects: r=0.48, df=13, p<0.09; patients with schizophrenia: r=0.48, df=12, p<0.10). To evaluate the specificity of this finding, correlations were computed between errors and the regional CBF response at the two other regions that distinguished patients with schizophrenia from comparison subjects: the left precentral gyrus (patients with schizophrenia: r=–0.52, df=12, p<0.07; comparison subjects: r=–0.23, df=13, p<0.16) and the right hippocampal region (patients with schizophrenia: r=0.44, df=12, p<0.14; comparison subjects: r=–0.40, df=13, p<0.16).
Within the schizophrenia group correlation coefficients were computed for anterior cingulate gyrus activation and positive, negative, and disorganization syndrome scores. None of these correlations approached significance. The correlation between errors in the color-incongruent condition and disorganization, which we have found in studies of larger groups of patients (
33), was also not significant.
DISCUSSION
Consistent with our hypothesis, patients with schizophrenia showed significantly less anterior cingulate gyrus activation than normal comparison subjects during the color-incongruent condition of the Stroop task. This is consistent with previous studies that suggested abnormal medial frontal physiology in schizophrenia (
16–
22). Because of our experimental design we are able to attribute functional relevance to this finding. We compared regional CBF under attentionally demanding conditions (response competition) to a state in which sensory, motor, and cognitive components unrelated to resolving this competition were identical. Hence, we conclude that failure to activate the anterior cingulate gyrus in the color-incongruent condition is related to the selective attention demands of the task.
The primary purpose of this study was to test a focal hypothesis: patients with schizophrenia would fail to activate the anterior cingulate gyrus during selective attention performance. Our results confirm this hypothesis while also showing that other regions respond abnormally to task performance in patients with schizophrenia. This result is consistent with recent PET findings that suggested that patients with schizophrenia fail to modulate cortical activity in distributed networks in a task-appropriate manner (
5,
34). One hypothesis that has been invoked to account for these findings is that corticocortical or corticostriatal-thalamic connectivity is disturbed in schizophrenia (
5,
35). The caveat to this interpretation is that a failure to activate a region involved in executive control would also have widely distributed ramifications within a neural network engaged by the task. The design of previous studies as well as the current one do not allow us to draw firm conclusions regarding which of these two interpretations is more accurate. Further studies with novel designs and quantitative methods for evaluating connectivity are needed to resolve these two competing views regarding the underpinnings of abnormal patterns of cortical activation during cognitive performance in schizophrenia.
In both groups, anterior cingulate gyrus activation correlated with errors in the color-incongruent condition. Similar correlations between poor performance and anterior cingulate gyrus activation have been previously reported during a Stroop-like task (
13) and during a response inhibition task (
36). A baseline level of task-related activation, with incremental increases with increasing error rates, is consistent with the hypothesized role of the anterior cingulate gyrus in monitoring for or detecting errors and in strategically reactivating selected circuits to compensate for their occurrence (
37,
38). Such a function would reflect the connectivity between the anterior cingulate gyrus and both the cortical systems involved in cognition and the limbic regions involved in emotion and motivation. Physiological dysfunction in this region could result in the lower regional CBF response and greater number of color-naming errors seen in patients in the current study, with preservation of the correlational relationship between these two measures.
The relationship between impaired selective attention performance and disorganization reported in other studies (
2,
31) was not found. There were no significant relationships between symptoms and cingulate regional CBF response, which may reflect a restricted range of patient symptoms. All patients were mildly ill and clinically stable outpatients.
All patients in the study were treated with neuroleptics. It is possible that the failure of the anterior cingulate gyrus to activate during Stroop performance in the patients with schizophrenia reflects the effects of antipsychotic medications rather than processes related to the pathophysiology of schizophrenia. Decreases in resting anterior cingulate gyrus regional CBF and metabolism have been reported 3–4 weeks after neuroleptic withdrawal in patients with schizophrenia (
39,
40), which confirms that increasing dopamine tone increases resting cingulate blood flow and metabolism. However, since previous studies that reported reduced anterior cingulate gyrus activation during Tower of London performance (
19) and verbal fluency (
34) found this result in unmedicated patients, we believe that this account of our results is unlikely. A definitive conclusion on this point must await a replication in an unmedicated group of patients.
The locus of the reduced regional CBF response associated with naming the color of color-incongruent stimuli is within the rostral anterior cingulate gyrus. Similar loci of activation have previously been reported during Stroop performance (
10). On the basis of animal studies and a limited review of human PET studies (
41), it has been proposed that within the anterior cingulate gyrus the more caudal region is involved with cognition and the more rostral region with emotions. The results of this and other Stroop studies suggest that a role in attention extends to the pregenual portion of the cingulate. Reports of caudal anterior cingulate gyrus activation in response to pain (
42) are also inconsistent with a simplified functional division of ventral and dorsal regions along cognitive versus emotional lines. Of course, one can always argue that activation associated with pain reflects attention to this sensation and that activation during a cognitive task reflects an emotional response. However, the association between performance and anterior cingulate gyrus activation in the present study, as well as those of Taylor et al. (
13) and Casey et al. (
36), argues against a nonspecific emotional response driving anterior cingulate gyrus activation. More functional imaging studies that focus on this region are needed before we will have a clear understanding of the nature of functional specialization within the human anterior cingulate gyrus.
While the precise pathophysiological mechanisms that underlie a failure to increase regional CBF in the anterior cingulate gyrus during selective attention in schizophrenia are unknown, recent histopathological studies have reported findings that may begin to illuminate this issue. Benes (
15) reported greater numbers of vertical glutamatergic fibers in layer II of the anterior cingulate gyrus in postmortem schizophrenic brains along with a reduction of GABAergic interneurones and up-regulation of postsynaptic GABA receptors. Benes proposed a model of a disturbed intrinsic circuitry of the anterior cingulate gyrus in which an increase in excitatory input and a reduction in GABAergic inhibition within layer II cause unmodulated pyramidal cell activity in this region and its distant projection sites. A similar model that emphasized disturbances in glutamatergic, dopaminergic, and GABAergic neurotransmission in the cingulate in schizophrenia has been also proposed by Olney and Farber (
43).
In the present study we found that patients with schizophrenia showed significantly less activation in the anterior cingulate gyrus, which was associated with impaired selective attention. This suggests that the functional significance associated with histopathological and physiological abnormalities that have been previously reported in this region of the brain in schizophrenia includes a relationship with deficits in selective attention. These results demonstrate the utility of the Stroop task as an activating procedure in functional imaging studies and invite further studies that evaluate the relationship of anterior cingulate gyrus function, attentional deficits, and associated clinical manifestations in schizophrenia.