Neurodevelopmental hypotheses of schizophrenia have found increasing support (
1,
2); however, the nature and site of neurodevelopmental abnormalities in this disorder remain subjects of debate. Childhood-onset schizophrenia is a rare and clinically severe form of schizophrenia and offers an important opportunity to examine abnormal neurodevelopment in this disorder. Studies of this population have demonstrated clinical continuity with later-onset schizophrenia (
3,
4), along with more severe premorbid language impairments (
5) and a more chronic course (
6), possibly reflecting a more extensive developmental brain lesion. Quantitative brain magnetic resonance imaging (MRI) of patients in the National Institute of Mental Health (NIMH) study of childhood-onset schizophrenia have revealed significantly smaller than normal midsagittal thalamic area, larger than normal lateral ventricular volume, which appears to be progressive, and no abnormality in the size of medial temporal lobe structures (
7–
9).
In humans the cerebellum undergoes intense neuronal proliferation and migration during the first 1.5 to 2.0 years of life, rendering this structure more susceptible to injury from infection, cytotoxic agents, or radiation during this period (
10). Studies have suggested that the maldeveloped neural circuitry producing schizophrenic symptoms may include the cerebellum. Many of the motor and eye-tracking abnormalities seen in schizophrenia and in children at risk for schizophrenia are characteristic of cerebellar disease (
11). The cerebellum is involved in higher cortical functions (
12–
14), including working memory, which is impaired in schizophrenia (
15), often in association with failure to activate frontal cortical regions normally during task performance (
16). Cerebellar activation during working memory tasks may be mediated by neurons in the ventral dentate nuclei that project to the prefrontal cortex via the thalamus (
17). Finally, abnormal cerebellar blood flow and metabolism have been demonstrated in both adult-onset (
18,
19) and childhood-onset (
20) schizophrenia.
The cerebellum has also been implicated in language processing (
21). Some patients with autistic disorder, a syndrome that includes impaired development of language and social skills, have associated hypoplasia of the cerebellar hemispheres and posterior vermis (
22,
23). In contrast, patients with Williams syndrome, who exhibit preservation of language and social skills in the context of global cognitive impairments (
24), have preserved cerebellar size despite hypoplasia of the cerebrum (
25).
Quantitative studies of cerebellar morphology in adult-onset schizophrenia have focused primarily on the midsagittal area of the vermis, with inconsistent results. Early computed tomography (CT) (
26) and postmortem (
27) studies yielded evidence of vermal hypoplasia in schizophrenic patients, but other CT studies (
28) did not replicate this finding. MRI studies of the midsagittal vermis in schizophrenia have similarly shown no abnormalities (
29,
30), larger than normal area (
31), and smaller area in male than in female schizophrenic patients (
32). Results from MRI examinations of cerebellar volume have included one finding of lower volume in female schizophrenic patients than in female comparison subjects (
33), one preliminary report of significantly lower white matter volume of both cerebellar hemispheres and lower volume of lobules IX and X of the vermis in male schizophrenic patients relative to male comparison subjects (
34), and another preliminary report of larger volume of a combined cerebellum/brainstem measure in male schizophrenic patients relative to male comparison subjects (
35). With one exception (
34), fourth ventricle size in schizophrenia has been estimated by using midsagittal area or maximum width; some studies have shown a larger than normal fourth ventricle (
26,
30,
34), and others have indicated no abnormalities (
29,
36–
38). While some of these inconsistencies may reflect differences between study groups across studies, failure to use intrastructural landmarks in determining the midsagittal plane of the cerebellum, which frequently deviates from the midsagittal plane of the cerebrum (
39), may also contribute to the lack of consensus.
In the present study midsagittal area of the vermis and volumes of the vermis, inferior posterior lobe, fourth ventricle, and total cerebellum were examined in the patients in the NIMH study of childhood-onset schizophrenia and were contrasted with those of a group of matched healthy children. Intrastructural landmarks were used to define the midsagittal plane. Given the evidence of cerebellar involvement in language development and processing (
21,
22,
25), together with the severe premorbid language deficits in this group (
5), we hypothesized that total cerebellar volume would be smaller than normal in subjects with childhood-onset schizophrenia. We further hypothesized that the vermis, and particularly the inferior posterior lobe of the vermis, would be smaller in schizophrenic patients, reflecting the role of these structures in oculomotor control (
40,
41) and, hence, potentially in the eye movement abnormalities seen in this disorder.
METHOD
Subjects
Schizophrenic children and adolescents were recruited nationally for an ongoing study of childhood-onset schizophrenia (
42). The inclusion criteria were a DSM-III-R diagnosis of schizophrenia with onset of psychotic symptoms by age 12, a premorbid full-scale IQ of at least 70, the absence of active medical or neurological disease, and a history of poor response to or inability to tolerate treatment with at least two different typical neuroleptics. The diagnosis was established by using previous records and clinical and structured interviews of the children and parents based on portions of the Schedule for Affective Disorders and Schizophrenia for School-Age Chidren—Epidemiologic Version (
43) and of the Diagnostic Interview for Children and Adolescents (DSM-III-R version) (
44).
The 24 patients with schizophrenia included 12 girls and 12 boys ranging in age from 9 to 18 years (mean=14.1, SD=2.2). The mean age at the onset of psychotic symptoms was 10.0 years (SD=1.9), and all of the patients had evidence of pubertal change upon admission to the study (mean Tanner score=3.9, SD=1.2). Most of these patients had substantial previous exposure to neuroleptics (mean exposure=25.0 months, SD=15.5) and hospitalization (mean=8.3 months, SD=13.0). There was no history of substance abuse, alcohol abuse, or ECT. The mean WISC-R (
45) vocabulary subscale score for 19 patients was 7.1 (SD=3.6), and the mean block design subscale score for 21 patients was 7.6 (SD=3.3). Five patients were not testable because of the severity of their psychotic symptoms. The mean height for 23 patients and mean weight for 24 patients were 158.4 cm (SD=10.8) and 62.1 kg (SD=18.8), respectively. A previously described method (
5) was used to review neuropsychological reports and school and medical records from the prepsychotic period of all patients, and this review indicated that nine patients (three girls, six boys) had been diagnosed with a language disorder during the prepsychotic period.
Fifty-two healthy children and adolescents, selected to be similar to the patients in age (mean=14.3, SD=2.0), sex (24 girls, 28 boys), and handedness, were recruited from the community. Screening included a telephone interview of the parents, completion of the Conners Preliminary Parent Report (
46) and the Achenbach Child Behavior Checklist (
47) by the parents, the Conners Teacher Preliminary School Report and Conners Teacher Questionnaire (
46,
48), physical and neurologic examinations of the child, and structured interview of the child and parent with the Diagnostic Interview for Children and Adolescents (
44). Psychiatric histories of all first- and second-degree relatives were obtained from the parents. Individuals with physical or neurologic abnormalities or lifetime histories of psychiatric problems and those for whom major psychiatric disorders were present in first-degree relatives or in more than 20% of second-degree relatives were excluded. The mean Tanner stage, height, and weight of the normal subjects were 3.6 (SD=1.4, N=51), 164.0 cm (SD=13.8), and 55.4 kg (SD=12.8), respectively, while their mean WISC-R vocabulary and block design subscale scores were 12.9 (SD=2.5, N=50) and 13.5 (SD=2.9, N=50), respectively.
Handedness was determined for both groups by using the 12 handedness items from the Revised Neurological Examination for Subtle Signs (
49). Eighteen of the schizophrenic patients and 45 of the healthy subjects were right-handed. The mean age, Tanner stage, height, and weight of the subgroup of 27 healthy subjects for whom total cerebellar volume was measured were 14.6 years (SD=2.2), 3.7 (SD=1.4), 164.4 cm (SD=13.3), and 55.9 kg (SD=14.5), respectively. Thirteen individuals in this subgroup of healthy subjects were female, and 20 were right-handed.
The parents of all subjects provided written informed consent, and the subjects provided assent for participation in this study. This study was approved by the NIMH Institutional Review Board.
MRI Image Acquisition
All subjects were scanned on the same GE 1.5-T Signa magnetic resonance scanner, as described in detail elsewhere (
50). We acquired 2-mm-thick contiguous slices in the coronal plane and 1.5-mm-thick contiguous slices in the axial plane, using three-dimensional spoiled gradient recalled echo in the steady state (TE=5 msec, TR=24 msec, flip angle=45°, acquisition matrix=192×256, number of excitations=1, and field of view=24 cm
2).
Image Analysis
All scans were read by a clinical neuroradiologist, who noted enlargement of the left lateral ventricle in one patient scan and a focal area of increased signal in the left frontal white matter in another.
As previously described (
50,
51), total cerebral volume was quantified by using an image analysis program that supplements characteristics of MRI signal intensity with a template based on a priori information about expected brain surface shape and location. The interclass correlation coefficient (ICC) for the interrater reliability of measurements of 10 brains by two raters was 0.99.
Midsagittal cerebellar areas and volumes of the vermis, inferior posterior lobe, fourth ventricle, and total cerebellum were measured by raters (L.K.J., P.C.B., A.L.K.) using NIH Image (
52) while blind to subject identity. For the midsagittal area measurements, a midsagittal image was reconstructed from the axial data set in the plane, perpendicular to the axial orientation, that bisected the pyramid of the vermis, the fourth ventricle, and the pyramid of the medulla. The midsagittal areas of the anterior, superior posterior, and inferior posterior lobes of the vermis were measured by manual tracing on this slice. The anterior superior fissure and the fourth ventricle demarcated the anterior lobe (comprising lobules I–V), while the anterior superior and prepyramidal fissures demarcated the superior posterior lobe (comprising lobules VI–VII). The inferior posterior lobe (comprising lobules VIII–X) was defined as the area between the prepyramidal fissure and the ventral edge of the fourth ventricle (
23,
53,
54). The cerebellar tonsils were not included in the measure of the cerebellar vermis. Ten brains were remeasured by a second rater to yield interrater reliabilities (ICC) of 0.83 for the anterior lobe, 0.85 for the superior posterior lobe, and 0.90 for the inferior posterior lobe.
The volumes of the vermis (lobules I–X) and inferior posterior lobe (lobules VIII–X) were measured by manually outlining these structures on all coronal slices in which they were visible for all subjects.
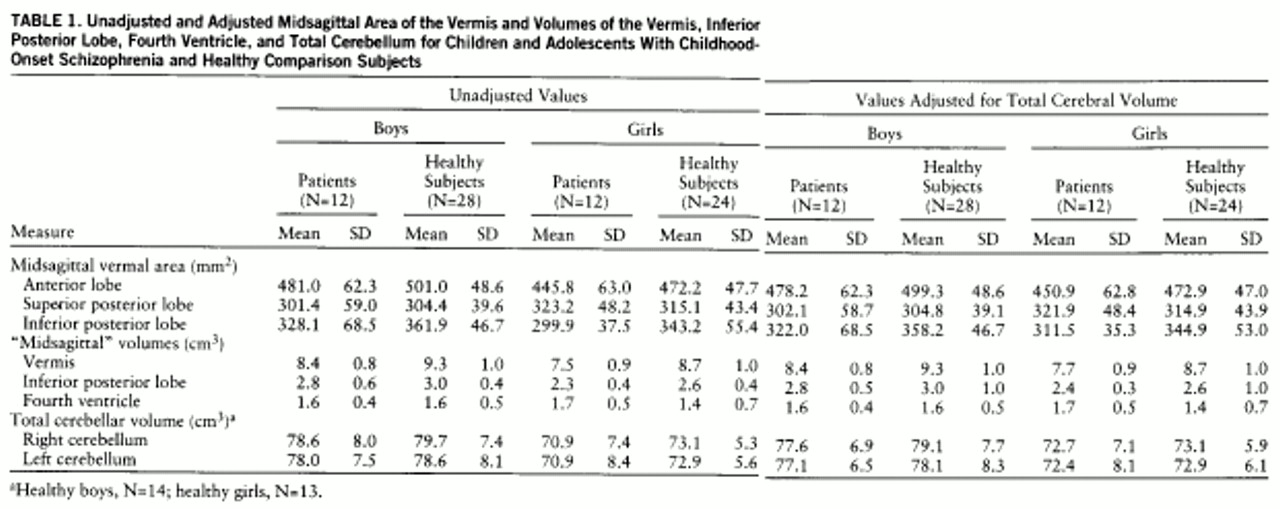
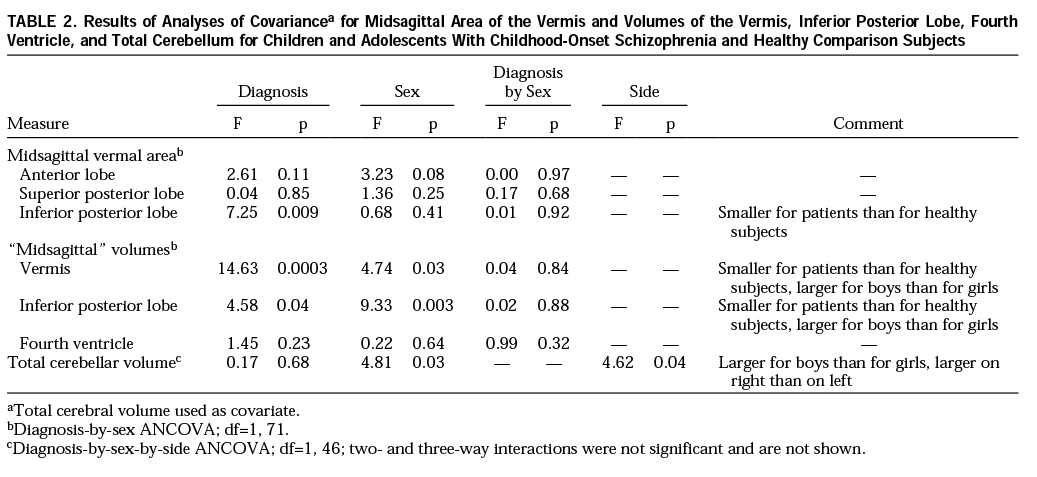
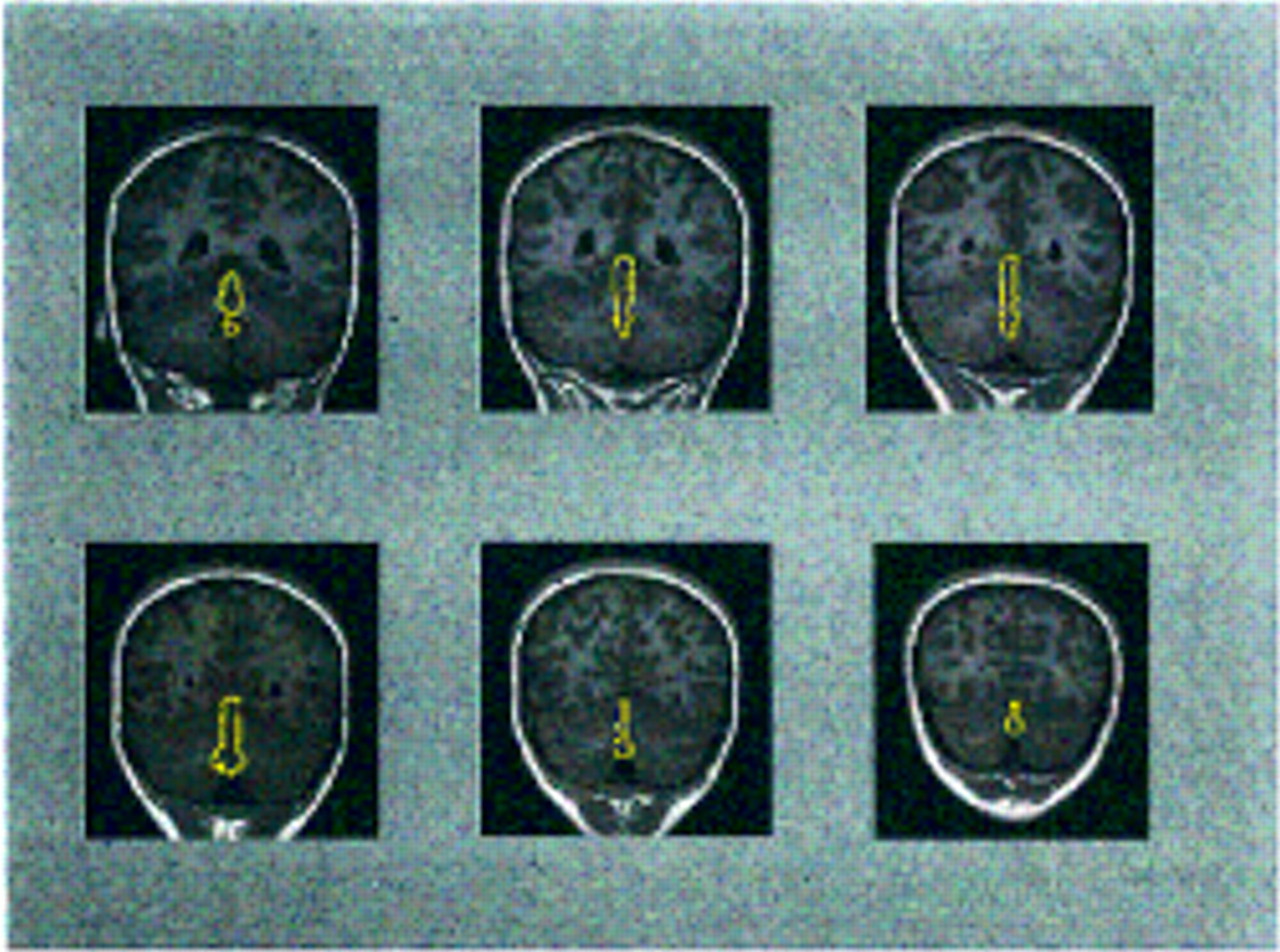
Figure 1 provides an example of outlines of the vermis on coronal slices from the brain MRI of a subject with schizophrenia. Total cerebellar volume was measured by manually outlining the cerebellum on all axial slices in which it was visible (
53,
54). The fourth ventricle and brainstem were excluded, while the cerebellar peduncles and vermis were included in this measure. The cerebellum was separated from the brainstem by drawing a straight line from the lateral point where the cerebellar hemispheres abut the pons to the sulcus limitans of the floor of the fourth ventricle (
55). Each cerebellar slice was bisected at the midline for a measure of right and left cerebellum. Because of the time-consuming nature of manually measuring this large structure, total cerebellar volume was measured for each subject with schizophrenia and for a subset of 27 healthy subjects selected to match the schizophrenic group in age, sex, and handedness. Fourth ventricle volume was measured by using an operator-supervised thresholding technique available in NIH Image (
52) for all slices in which this ventricle was visible. The volumes were calculated by multiplying the summed area by slice thickness. Ten cerebella were remeasured by a second rater, and the interrater reliability (ICC) was 0.94 for the vermis, 0.97 for the inferior posterior lobe, 0.91 for total cerebellar volume, 0.87 for the right cerebellum, 0.92 for the left cerebellum, and 0.97 for the fourth ventricle.
Statistical Analysis
Group differences in demographic measures and total cerebral volume were assessed with chi-square analyses and t tests for independent samples. The midsagittal vermal area and the volumes of the vermis, inferior posterior lobe, and fourth ventricle were examined by using repeated measures analysis of variance (ANOVA) with diagnosis and sex as between-subjects factors. Total cerebellar volume was examined by using an ANOVA with diagnosis and sex as between-subjects factors and side as a within-subjects factor. Because total cerebral volume was significantly correlated with the total, right, and left cerebellar volumes and with the vermis and inferior posterior lobe volumes in the patients, repeated measures analysis of covariance (ANCOVA) was also performed, with total cerebral volume used as a covariate.
We compared the effect size for the amount by which the midsagittal area of the inferior posterior lobe of the schizophrenic patients in the present study was smaller than that of the comparison subjects to the effect sizes in three previous MRI studies of adult schizophrenic patients for which means and standard deviations for this measure have been reported. The three studies of adults included, respectively, 36 schizophrenic patients and 51 healthy subjects (
30), 23 schizophrenic patients and 16 healthy subjects (
32), and 30 schizophrenic patients and 11 healthy subjects (
31). Comparisons were performed by computing a z score weighted for group size for each study (
56), subtracting the mean of these z scores from a z score derived from the present study, and dividing by the square root of 2 (the sum of the variances) to obtain a difference z score (
57).
For the schizophrenic group, Pearson correlation coefficients were used to examine the relationship of the morphology of cerebellar structures showing diagnostic differences to WISC-R vocabulary, block design, and digit span subscale scores, total number of months of neuroleptic exposure, and duration of illness. The relationship between presence of prepsychotic language disorder and morphology of cerebellar structures showing diagnostic differences was also examined with Pearson correlation coefficients, to approximate the point biserial correlation (
58). Statistical analyses were performed with SAS (
59), except for the ANCOVA, which was performed with BMDP (
60). All p values are two-tailed.
RESULTS
There were no significant differences between the schizophrenic group and either the total healthy comparison group or the subgroup of healthy subjects included in the assessment of total cerebellar volume in age, height, weight, Tanner stage, gender, handedness, or total cerebral volume. The mean total cerebral volume of the schizophrenic patients was 1076.8 cm3 (SD=129.8), and for the healthy subjects it was 1118.5 cm3 (SD=117.6) (t=1.39, df=74, p=0.17). The patients with schizophrenia had significantly lower scores on the WISC-R subscales for vocabulary (t=7.53, df=67, p<0.0001) and block design (t=7.61, df=69, p<0.0001) than the healthy comparison group. The differences in WISC-R scores for these variables between the schizophrenia group and the healthy subgroup involved in the analysis of cerebellar volume were similar in magnitude and were also significant.
Midsagittal Vermal Area
The mean midsagittal areas of the vermal lobes and the volumes of the vermis, inferior posterior lobe, fourth ventricle, and left and right cerebellum are shown in
table 1. ANOVAs indicated that the midsagittal area of the inferior posterior lobe of the vermis was significantly smaller in the schizophrenic patients than in the comparison subjects (F=8.94, df=1,72, p=0.004), while the midsagittal area of the anterior lobe was smaller in the female subjects in each group (F=5.96, df=1,72, p=0.02). There were no significant diagnosis-by-sex interactions.
The results of the ANCOVAs for all structures are shown in
table 2. The ANCOVA for the midsagittal vermal areas indicated that, after adjustment for total cerebral volume, all sex differences were lost while the area of the inferior posterior lobe remained significantly smaller in the schizophrenic patients than in the comparison subjects. Comparison of effect sizes to assess whether the difference in midsagittal inferior posterior lobe area between these patients with childhood-onset schizophrenia and the comparison subjects was greater than for patients with adult-onset schizophrenia (
30–
32) resulted in a difference z score of 2.38, which was significant at p=0.02 (two-tailed).
Volume of Total Cerebellum, Vermis, Inferior Posterior Lobe, and Fourth Ventricle
ANOVAs revealed no significant diagnostic differences for total, right, or left cerebellar volume. Female subjects had significantly smaller cerebellar volumes across sides and diagnostic groups (F=11.17, df=1,47, p=0.002), and this difference persisted after adjustment for total cerebral volume with ANCOVA. Although the mean volumes for left and right cerebellar hemispheres were similar across groups, a significant right-greater-than-left asymmetry was demonstrated for this structure. There were no significant interactions.
ANOVAs indicated that the vermis and inferior posterior lobe volumes were significantly smaller in the schizophrenic patients (F=16.66, df=1,72, p=0.0001, and F=5.91, df=1,72, p=0.02, respectively) and in the female subjects across groups (F=9.69, df=1,72, p=0.003, and F=18.52, df=1,72, p<0.0001). After adjustment for total cerebral volume, the vermal and inferior posterior lobe volumes remained significantly smaller in the schizophrenic patients and in the female subjects across groups. Because mean values for the volumes of these structures in adult schizophrenic patients and comparison subjects have not been published, comparisons of effect sizes could not be performed. ANOVA and ANCOVA revealed no significant diagnostic or sex differences and no significant diagnosis-by-sex interaction for the fourth ventricle.
Relationship Between Cerebellar Morphology and Clinical Variables
Within the schizophrenic group, no significant relationships were observed between volume of the vermis or volume or midsagittal area of the inferior posterior lobe and scores on WISC-R vocabulary, block design, or digit span subscales, number of months of neuroleptic exposure, duration of illness, or history of prepsychotic language disorder.
DISCUSSION
In what we believe to be the first study of cerebellar morphology in childhood-onset schizophrenia, predicted group differences were found for the volume of the vermis and the midsagittal area and volume of the inferior posterior lobe. Vermis volume was 11.7% smaller than normal in patients with childhood-onset schizophrenia, while midsagittal inferior posterior lobe area was 10.9% smaller and midsagittal inferior posterior lobe volume was 8.9% smaller. These findings are consistent with reports of small vermal size in adults with schizophrenia (
26,
27,
32) and with evidence of abnormal cerebellar function in childhood-onset and adult-onset schizophrenia (
18–
20). The observation of a greater difference between schizophrenic and comparison subjects in midsagittal inferior posterior lobe area in the present study than has been observed in studies of adult schizophrenic patients suggests that persons with childhood-onset schizophrenia may sustain a more severe neurodevelopmental lesion at this structure.
While effects of previous neuroleptic medications on vermal or inferior posterior lobe size in the present study cannot be ruled out, the absence of significant correlations between measures of neuroleptic exposure and the size of these structures suggests that the observed diagnostic differences may not reflect medication effects. The absence of a diagnostic difference in fourth ventricle volume is consistent with some observations of adult-onset schizophrenia (
29,
36–
38).
Although our failure to observe significant diagnostic differences in total cerebellar volume may reflect lack of statistical power, the right and left cerebellar volumes were only 2.2% and 2.0% smaller in the patients with childhood-onset schizophrenia than in the comparison subjects. To detect differences of this magnitude as significant, by assuming a power of 80% and significance level of 0.05, groups containing more than 200 subjects would be required (
61). The absence of a significant diagnostic difference in total cerebellar size does not preclude abnormalities of neural organization or function within the cerebellar hemispheres in schizophrenia. Subnormal activation of prefrontal-thalamic-cerebellar circuitry has been demonstrated in unmedicated adults with schizophrenia performing practiced and novel memory tasks, suggesting that “cognitive dysmetria,” or poor coordination of retrieval, processing, and expression of information, may be a fundamental deficit in schizophrenia (
18). Cerebellar glucose metabolism in medicated adult schizophrenic patients at rest has been found to be lower than normal (
19), while unmedicated adolescents with childhood-onset schizophrenia performing an auditory continuous performance task were found to have greater than normal cerebellar and thalamic glucose metabolism (
20). These observations are consistent with animal studies demonstrating that cerebellar glucose metabolism decreases after administration of dopamine antagonists and increases after administration of dopamine agonists (
62,
63), and they suggest that neuroleptics may attenuate abnormally activated cerebellar neurons in schizophrenia. Given the absence of dopamine receptors in the cerebellum, this effect of neuroleptic medication may occur through cortical-cerebellar neural circuits (
64).
The lack of a relationship between block design, vocabulary, or digit span performance and the size of the vermis or inferior posterior lobe is consistent with observations of healthy children (Giedd et al., in preparation) and healthy adults (
65), as is the persistence of sex differences in the size of the total cerebellum after adjustment for total cerebral volume (Giedd et al., in preparation). Our failure to observe a significant relationship between WISC-R vocabulary subtest score or presence of prepsychotic language disorder and the morphology of the vermis or inferior posterior lobe does not preclude the possibility that abnormal function in these brain regions contributes to impaired language function.
While the inclusion criterion of previous nonresponse to neuroleptic medication may have resulted in a nonrepresentative group of patients with childhood-onset schizophrenia, phenomenologic similarities between this study group and other groups of patients with childhood-onset schizophrenia described in the literature (
3,
4,
66) suggest that the present findings are relevant to childhood-onset schizophrenia in general.
Preliminary data from a 2-year follow-up rescan study of 11 schizophrenic and 22 healthy subjects from the present study suggest that the cerebellar abnormalities in childhood-onset schizophrenia may not be progressive. However, ongoing longitudinal rescanning of these subjects will continue to more definitively address this question.