The goal of the present investigation was to determine serial cerebrospinal fluid (CSF) and plasma concentrations of the dopamine metabolite homovanillic acid (HVA) in tobacco-smoking and nonsmoking combat veterans with posttraumatic stress disorder (PTSD) and in smoking and nonsmoking normal volunteers. It has been suggested that hyperresponsiveness of brain dopaminergic systems may contribute to the heightened arousal and increased sensitivity to both trauma-related and nonspecific stress seen in patients with PTSD
(1). Stimulation of central nervous system (CNS) dopaminergic activity has been associated with nicotine administration in experimental animals
(2–
4), and chronic cigarette smoking has been linked with both peripheral
(5–
7) and brain
(8,
9) monoamine oxidase inhibition in humans. We sought to extend these studies by examining a hypothesized association between tobacco cigarette smoking and perturbations in dopamine metabolism. We postulated that CSF concentrations of HVA would be abnormal in both smokers and patients with PTSD.
METHOD
The study was approved by the Institutional Review Board of the University of Cincinnati Medical Center and the Research Committee of the Cincinnati Department of Veterans Affairs Medical Center. Informed written consent was obtained from 13 healthy volunteers, without history of axis I psychiatric disorder, and 11 patients with combat-related PTSD. The lifetime-nonsmoking (N=9) and smoking (N=4) healthy volunteers had mean ages of 34 years (SD=10) and 42 years (SD=9), respectively, and weighed 76 kg (SD=9) and 70 kg (SD=13), respectively. The lifetime-nonsmoking (N=5) and smoking (N=6) patients with PTSD had mean ages of 43 years (SD=11) and 42 years (SD=8) and weighed 91 kg (SD=14) and 96 kg (SD=16), respectively. The smokers smoked 1.2 packets of cigarettes per day (SD=0.2, range=0.75–1.50). With the exception of one normal volunteer who had smoked for 13 years and one patient with PTSD who had smoked for 9 years, all had smoked for over 20 years. Six of the 11 patients with PTSD had histories of alcohol or drug abuse (excluding tobacco) but had been abstinent for 0.5 to 15.0 years. Seven of the 11 patients had taken psychoactive medication in the past but had discontinued taking all medications at least five half-lives before the study. The mean score of the patients on the Clinician-Administered PTSD Scale
(10) was 82 (SD=22).
CSF was collected into iced test tubes from 11:00 a.m. to 5:00 p.m. at the rate of 0.1 ml/min through an indwelling 20-gauge subarachnoid catheter that had been placed at 8:00 a.m. as previously described
(11), except that the seated rather than the lateral decubitus position was used. CSF samples were obtained at 10-minute intervals (37 samples total) for quantification of HVA. No smoking or oral intake was permitted after the midnight before the procedure. Blood was withdrawn at hourly intervals through an indwelling venous catheter, placed the night before, to obtain plasma for HVA quantification. CSF HVA was quantified by high-performance liquid chromatography with electrochemical detection, as previously described
(12); slight modifications of the system were used for plasma HVA assay.
Separate two-way analyses of variance were used to assess the effects of smoking and diagnosis on CSF HVA and plasma HVA concentrations.
RESULTS
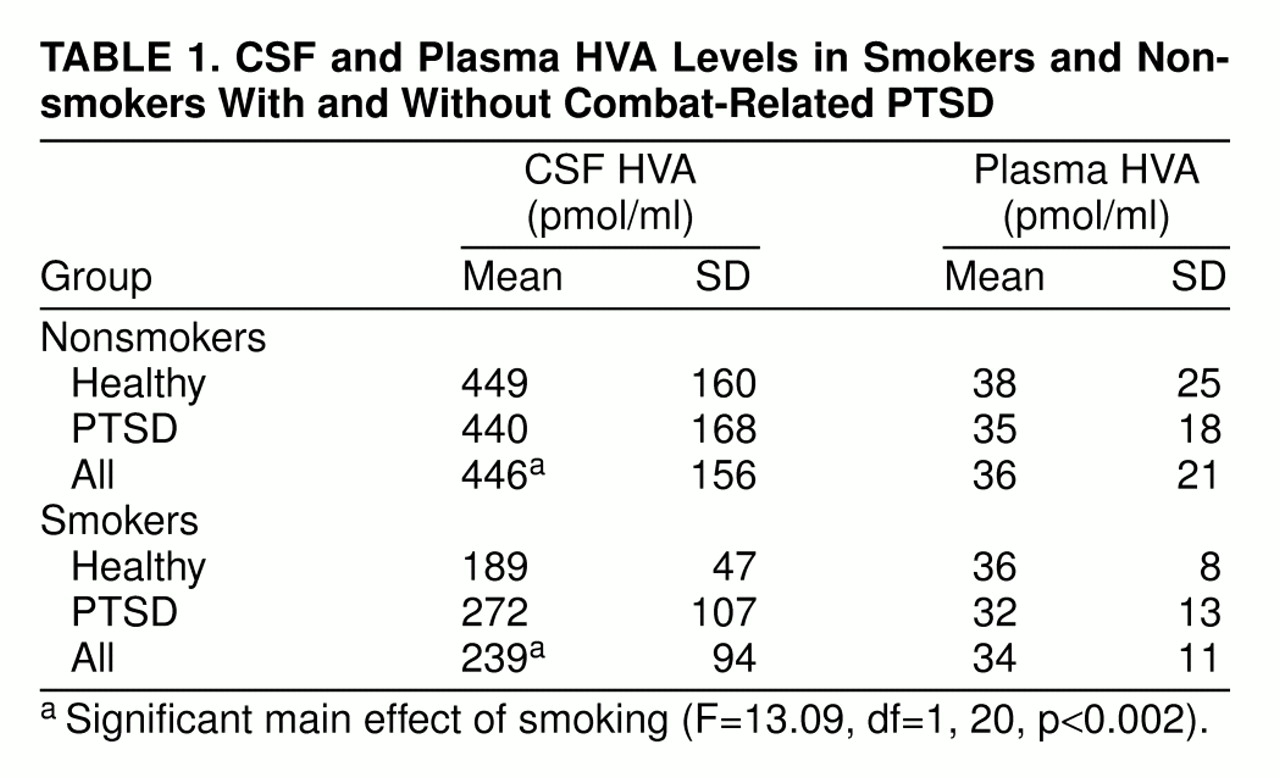
CSF HVA concentrations (
table 1) were lower in the smokers than in the nonsmokers (F=13.09, df=1, 20, p<0.002), but there was no significant effect of a diagnosis of PTSD (F=0.39, df=1, 20, n.s.). No significant interaction between smoking and diagnosis was present (F=0.61, df=1, 20, n.s.).
With regard to plasma HVA concentrations (
table 1), neither the main effects of diagnosis and smoking nor their interaction were significant (main effect of diagnosis: F=0.15, df=1, 16, n.s.; main effect of smoking: F=0.06, df=1, 16, n.s.; interaction: F=0.0004, df=1, 16, n.s.).
DISCUSSION
Using continuous sampling over 6 hours, we observed markedly low CSF levels of the major dopamine metabolite HVA in chronic tobacco-cigarette smokers, with CSF HVA concentrations averaging only 54% of those found in nonsmokers. Reduced dopamine synthesis or release, impaired metabolic conversion of dopamine to HVA, and enhanced efflux of HVA from CSF might all account for low levels of CSF HVA.
We cannot determine from the present data whether the low CSF HVA concentrations are related to cigarette smoking per se or to early withdrawal from the effects of tobacco smoke. Our patients were withdrawn for 11 hours at the time CSF sampling began and for 17 hours by the end of the sampling period. Thus, acute withdrawal of a dopaminergic stimulant (tobacco smoke) may have resulted in a rebound suppression of CNS dopaminergic activity. Accordingly, bupropion’s apparent efficacy in promoting smoking cessation
(13) may be related in part to its pro-dopaminergic effects
(14).
In contrast to the striking diminution in CSF HVA levels in cigarette smokers regardless of diagnosis, we failed to find any significant differences in CSF HVA concentrations between patients with PTSD and normal volunteers. It therefore appears that any possible basal abnormalities in CSF HVA concentrations in patients with PTSD, if present at all, are small relative to the dopaminergic abnormalities associated with tobacco smoking.
No significant differences in plasma HVA levels were detected in either patients with PTSD or smokers, again showing that plasma HVA concentrations alone cannot be used as a surrogate for CSF HVA concentrations. The majority of plasma HVA is derived from dopamine (also the precursor of norepinephrine) in noradrenergic neurons, and only about 25% of plasma HVA originates from brain dopaminergic pathways
(15).
In light of our current findings, it should be noted that previous studies of possible alterations in brain-derived HVA concentrations in psychotic patients, who tend to be heavy smokers
(16), did not control for tobacco use and thus may require reinterpretation in light of the major perturbations of the dopamine system associated with tobacco smoking.
Among the limitations of this study is the fact that the only dopamine metabolite quantified was HVA; we cannot rule out the possibility of abnormal metabolism of dopamine to 3,4-dihydroxyphenylacetic acid (DOPAC) or 3-
O-methyldopa in cigarette smokers. However, the levels of those metabolites are an order of magnitude lower than those of HVA in lumbar CSF
(17,
18). Also, only men were studied.
In conclusion, acutely abstinent tobacco smokers had greatly lower concentrations of the dopamine metabolite HVA in CSF, but not in plasma, than nonsmokers. The low CSF HVA levels may be the effects of smoking-related reductions in brain MAO concentrations
(8,
9) on CNS dopamine neurotransmission or may be due to alterations in dopamine synthesis, release, or clearance from CSF. In any event, the present results indicate that future studies of brain dopaminergic systems must take into account the tobacco-smoking histories of all experimental subjects.