An association of increased platelet membrane fluidity, as reflected by the fluorescence anisotropy of 1,6-diphenyl-1,3,5-hexatriene (DPH) in labeled membranes, with Alzheimer’s disease was originally reported for clinical populations recruited in Boston and Pittsburgh
(1,
2). This finding has been replicated by several research groups during the last decade, all of which recruited subjects from clinical populations in their respective locations on three continents
(3–
8). In aggregate, these published studies have included approximately 500 patients and control subjects and have incorporated strategies to control for the potential effects of age, sex, concurrent medical conditions, and medication exposure.
Available evidence indicates that increased platelet membrane fluidity is relatively specifically associated with Alzheimer’s disease among the mental disorders that produce dementia in late life
(2,
3,
9) and that patients who manifest this characteristic may represent a clinically distinct subset of those with Alzheimer’s disease
(2,
10–
12). This membrane phenotype appears to be a stable trait that is vertically transmitted in families of patients with Alzheimer’s disease
(13,
14). Complex segregation analysis suggests that increased platelet membrane fluidity is controlled by the inheritance of a Mendelian single major locus
(15). At the cellular level, evidence from ultrastructural
(5,
16), biochemical
(5,
17,
18), and biophysical studies
(6,
14) suggests that increased platelet membrane fluidity in Alzheimer’s disease results from the elaboration of an internal membrane compartment resembling endoplasmic reticulum that is functionally abnormal.
The association of the
APOE ε4 allele with Alzheimer’s disease has been widely established
(19–
28). In addition to their association with both sporadic and familial forms of Alzheimer’s disease that have typical ages at onset, other similarities between the
APOE and
PMF loci have emerged. The
APOE locus is the structural gene for apolipoprotein E
(29), a cholesterol-carrying protein, while the increased platelet membrane fluidity phenotype arises, at least in part, from a reduction in platelet membrane cholesterol
(17). Both the
APOE and the
PMF loci are autosomal genes, whose alleles are additively expressed in peripheral cells
(15,
29). Individuals who are heterozygous for
PMF alleles that confer increased or normal platelet membrane fluidity have fluidity values that are intermediate between those of either homozygous individual
(15). Increased platelet membrane fluidity
(2,
10,
30) and the number of
APOE ε4 alleles
(31,
32) have been reported to affect age at onset of prototypic forms of Alzheimer’s disease in most, but not all
(4,
26,
33), patient populations. Both antemortem platelet membrane fluidity values
(34) and the number of
APOE ε4 alleles
(26,
33,
35) appear to be correlated with the density of senile plaques in brain tissue obtained from the autopsies of patients with confirmed Alzheimer’s disease, with smaller or no associations with the density of neurofibrillary tangles. Finally, the ε4 allele has been reported to confer increased risk of developing Alzheimer’s disease-like dementia among elders with mild cognitive impairment
(36) and of developing dementia, but not necessarily Alzheimer’s disease, among the participants in the Framingham study
(37).
This body of evidence raised the possibility that increased platelet membrane fluidity might be associated with an increased risk of developing Alzheimer’s disease, at least during the earlier part of the typical age at onset (65<onset≤75 years). To test this hypothesis, we embarked on a prospective, longitudinal study of 330 cognitively intact individuals between the ages of 40 and 75 years, who were first-degree relatives of probands with Alzheimer’s disease, all of whom were typed for platelet membrane fluidity at the time of recruitment. Subject selection was designed to include individuals who represented a wide age range so that the effects of an age-specific risk factor would be observable. By recruiting a cohort of individuals whose overall incidence rate of developing Alzheimer’s disease would be expected to be severalfold higher than that in a community sample by virtue of their family history of Alzheimer’s disease
(38–
42), we anticipated that the total number of subject-years of follow-up required to test our hypothesis would be practical. Transformed cell lines from these individuals also permitted us to explore the relationship of the
APOE ε4 allele to the platelet membrane fluidity phenotype, as well as to determine whether the ε4 allele affected the risk of developing Alzheimer’s disease among these cognitively intact individuals. This report describes the results of this longitudinal study after 7.5 years of systematic follow-up.
METHOD
Recruitment of Alzheimer’s Disease High-Risk Cohort
The Alzheimer’s disease high-risk cohort consisted of 330 first-degree relatives of 189 demented probands who met the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association
(43) for possible (N=35), probable (N=103), or definite (N=6) Alzheimer’s disease or DSM-III-R criteria for primary degenerative dementia of the Alzheimer’s type (N=45) or both. Members of the cohort were between the ages of 40 and 75 and were cognitively intact at the time of recruitment, as determined by a review of medical and psychiatric history, a survey of current activities of daily living, a mental status examination, and the Mini-Mental State
(44). Subjects were excluded if they had any clinical evidence of cognitive decline or impairment, a Mini-Mental State score of less than 27, or medical problems or were taking medications known to affect the determination of platelet membrane fluidity
(2). For 303 of the 330 subjects, the Mini-Mental State was augmented by the more detailed Mattis Dementia Rating Scale
(45), and all individuals tested had Mattis Dementia Rating Scale scores in the range expected for healthy elderly control subjects (135 or higher). All members of the cohort provided written informed consent for participation in the study, which was approved by the Biomedical Institutional Review Board of the University of Pittsburgh.
Longitudinal Surveillance Protocol
Semistructured telephone assessments were initiated on March 1, 1989, and were performed approximately annually by a mental health nurse with specialized experience in geriatrics who remained blind to the laboratory data. The telephone assessments included a review of the items on the Family History Form of Huff and colleagues
(10,
42) and a survey of the subjects’ activities of daily living. Subjects and their identified “best” informants were encouraged to contact the research nurse if any evidence of a change in mental functioning occurred during the interim. To determine the sensitivity and specificity of the telephone assessments for the detection of individuals with newly emergent cognitive impairment, 202 members of the Alzheimer’s disease high-risk cohort were also evaluated by in-person interviews. The telephone assessments detected subjects whose Clinical Dementia Rating scores
(46) exceeded 0 with a sensitivity of 82% and a specificity of 98%. These cross-sectional determinations seem likely to underestimate the sensitivity and specificity of the telephone assessments for identifying cases of progressive dementia when administered longitudinally over a period of years.
In-person assessments were performed when evidence suggestive of emergent cognitive dysfunction was reported during the telephone screen. If the presence of cognitive impairment suggested by the telephone screen was supported by a Mini-Mental State score of less than 27, a Mattis Dementia Rating Scale score of less than 135, a Clinical Dementia Rating score greater than 0, or other clinical evidence obtained during the in-person evaluation, the subject was referred for a complete diagnostic evaluation. If no such evidence was detected, the frequency of the periodic telephone follow-up was increased to approximately every 6 months. Consensus clinical diagnoses of Alzheimer’s disease among incident cases of dementia were established according to DSM-III-R criteria, as applied by two board-certified psychiatrists with added qualifications in geriatric psychiatry. Autopsies were performed by board-certified neuropathologists, and neuropathologic diagnoses were made according to generally accepted criteria
(47–
49).
Laboratory Methods
Platelets were qualitatively isolated from blood samples of fasting patients; blood samples were obtained at the time of recruitment
(2,
16). Platelet membranes were prepared from coded samples, labeled with DPH, and were examined by fluorescence spectroscopy as previously described
(2,
16). Increased platelet membrane fluidity was defined by a steady-state anisotropy value of less than 0.1920 at 37.0˚C for DPH-labeled membranes.
Genomic DNA was isolated from lymphocytes or lymphoblasts by using minor modifications of standard methods
(50,
51). Genotyping of the
APOE locus was performed through use of polymerase chain reaction methodology and the oligonucleotide primers described by Hixson and Vernier
(52). The radiolabeled products were digested with endonuclease
HhaI, and the resulting DNA fragments were resolved by electrophoresis and visualized by autoradiography. Allele assignments were made as previously described
(26,
30). Subtyping for platelet membrane fluidity and genotyping for
APOE were performed by laboratory staff who were blind to sociodemographic and clinical information.
Statistical Analysis
The database for this project was frozen on Aug. 31, 1996. Continuous variables were compared through use of the t statistic. Categorical variables were compared with the chi-square statistic or Fisher’s exact test, as appropriate. Survival analysis employing the Kaplan-Meier product limit method and Cox proportional hazards models with covariates was performed by using the 1L and 2L programs from the BMDP Statistical Software package (1990 release).
RESULTS
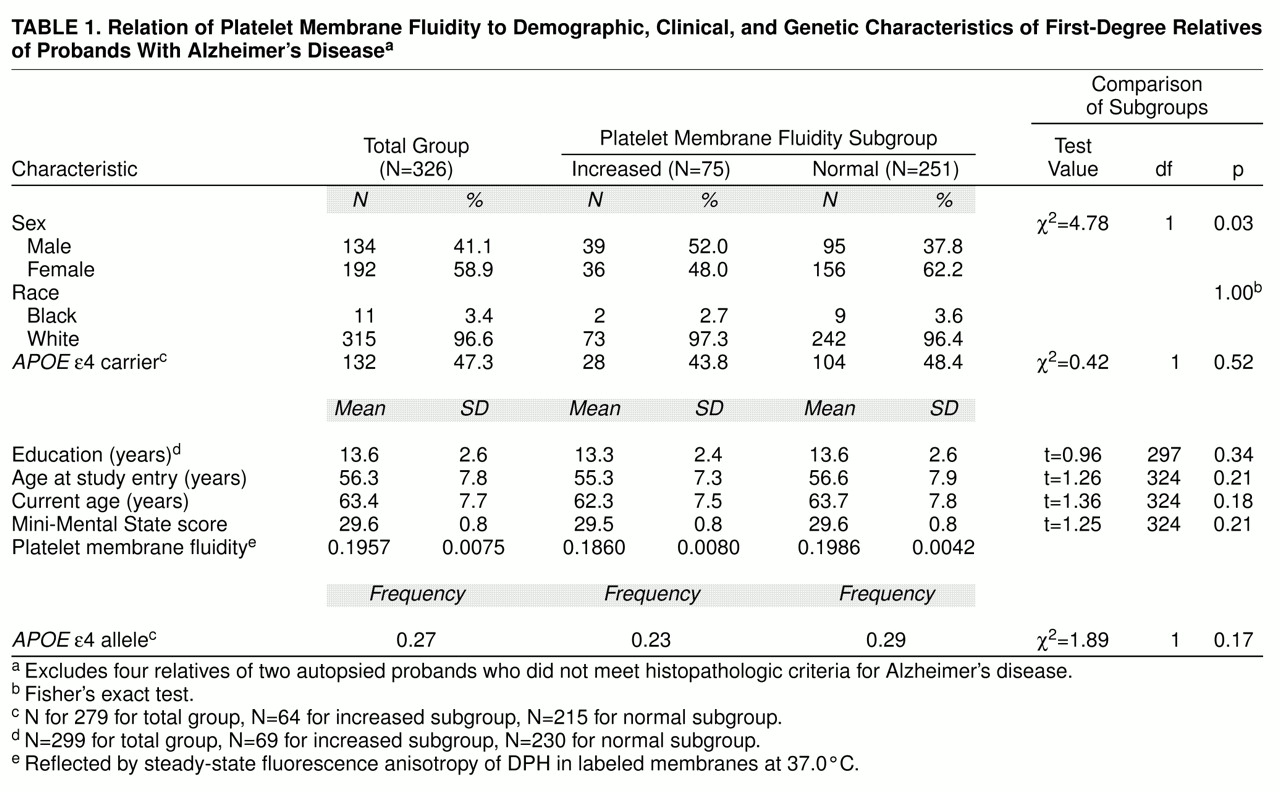
A description of the Alzheimer’s disease high-risk cohort and the subgroups with increased and normal platelet membrane fluidity is provided in
table 1. The cohort was composed almost entirely of Caucasians and included approximately equal proportions of men and women. The subjects had a mean age of 56.3 years at the time of entry into the longitudinal study and a mean age of 63.4 years at the time of their most recent follow-up assessment (effective Aug. 31, 1996; 7.5 years of follow-up). Consistent with the criteria for entry into the study, all subjects were cognitively intact as reflected by a mean Mini-Mental State score approaching 30. A total of 75 members (23.0%) of the cohort had increased platelet membrane fluidity. There were slightly more men than women in this subgroup; subjects in the subgroup were similar in mean age at entry and had completed a mean number of years of education that was similar to that of the subgroup with normal platelet membrane fluidity. The platelet membrane fluidity subgroups did not differ significantly with respect to their duration of follow-up or their cognitive performance as determined by mean Mini-Mental State scores.
As expected, the frequency of the
APOE ε4 allele in the Alzheimer’s disease high-risk cohort, 0.27 (
table 1), was midway between the frequencies previously reported for autopsy-confirmed cases of Alzheimer’s disease, 0.41, and elderly control subjects, 0.13, evaluated at our medical center. The ε4 allele frequencies, as well as the proportions of individuals who carried the ε4 allele, were indistinguishable between the platelet membrane fluidity subgroups. These findings provided no evidence of an association between the
APOE ε4 allele and increased platelet membrane fluidity. As a result, they were evaluated as independent variables in subsequent analyses of risk factors for Alzheimer’s disease.
A total of 51 of the 183 probands with clinically diagnosed Alzheimer’s disease were autopsied during the course of this study, and the diagnosis was histopathologically confirmed in 49 (96.1%) of the 51 cases. Of the two autopsied probands who did not meet histopathologic criteria for Alzheimer’s disease, one exhibited striatonigral degeneration and the other exhibited neuronal loss without findings to support a more specific diagnosis. To date, a total of 32 members (9.7%) of the Alzheimer’s disease high-risk cohort have been lost through attrition from all sources. Nineteen of these died, four were relatives of the two autopsied probands who did not meet histopathologic criteria for definite Alzheimer’s disease, five could not be reached for follow-up, and four subjects declined to participate further.
A total of 12 incident cases of perceived memory impairment were detected by our surveillance protocol during the first 2,220 subject-years of this longitudinal study. Three of these cases reflected subjective reports of memory impairment that occurred in the context of depressive or anxiety states, were not associated with objective evidence of cognitive impairment as determined by cognitive test performance or functional decline, and resolved concurrently with the remission of the transient emotional disturbances. Two of these individuals have remained actively engaged in business and community affairs, while the third died unexpectedly. The remaining nine individuals revealed objective evidence of cognitive impairment and developed progressive dementias that resulted in their inability to function independently. In all nine cases, medical and laboratory evaluations supported the diagnosis of Alzheimer’s disease. Following the death of one of these individuals, for whom concurrent cerebrovascular disease was detected clinically, an autopsy was performed, and the diagnosis of Alzheimer’s disease with multiple infarcts was determined by neuropathologic examination of the brain.
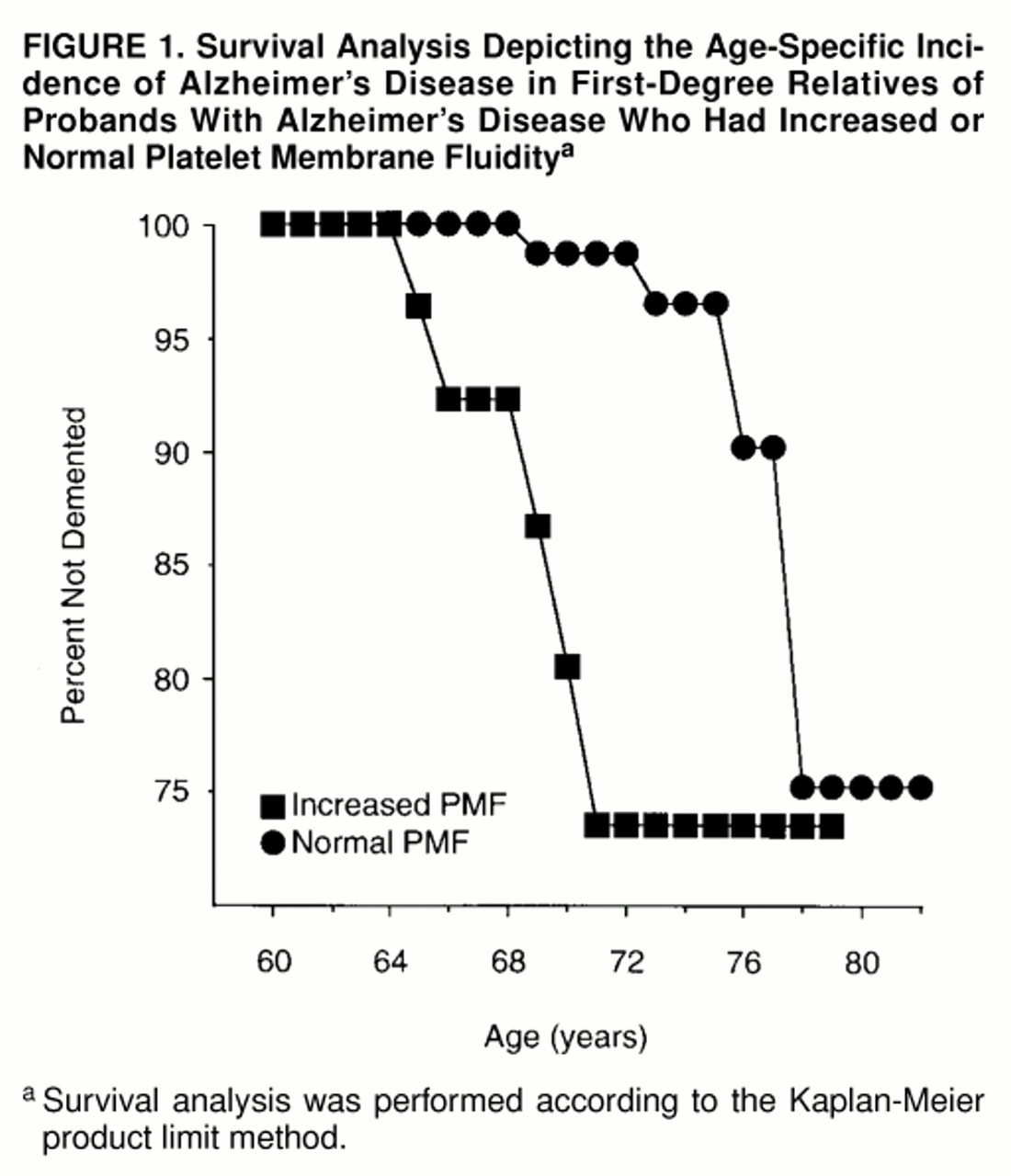
The age-specific cumulative incidence rates of Alzheimer’s disease for the platelet membrane fluidity subgroups are presented in
figure 1. Increased platelet membrane fluidity was associated with a 5- to 10-year reduction in the age at onset of Alzheimer’s disease (Mantel-Cox statistic=16.01, df=1, p=0.0001; Breslow statistic=35.87, df=1, p<0.0001). Similarly, the mean age at onset of dementia occurred approximately 5 years earlier for the five incident Alzheimer’s disease cases with increased platelet membrane fluidity (mean=67.2 years, SD=2.6) than for those with normal platelet membrane fluidity (mean=72.5 years, SD=3.3) (t=2.7, df=7, p=0.03). The effect of this risk factor was restricted to incident Alzheimer’s disease cases in which symptomatic onsets occurred between the ages of 64 and 71.
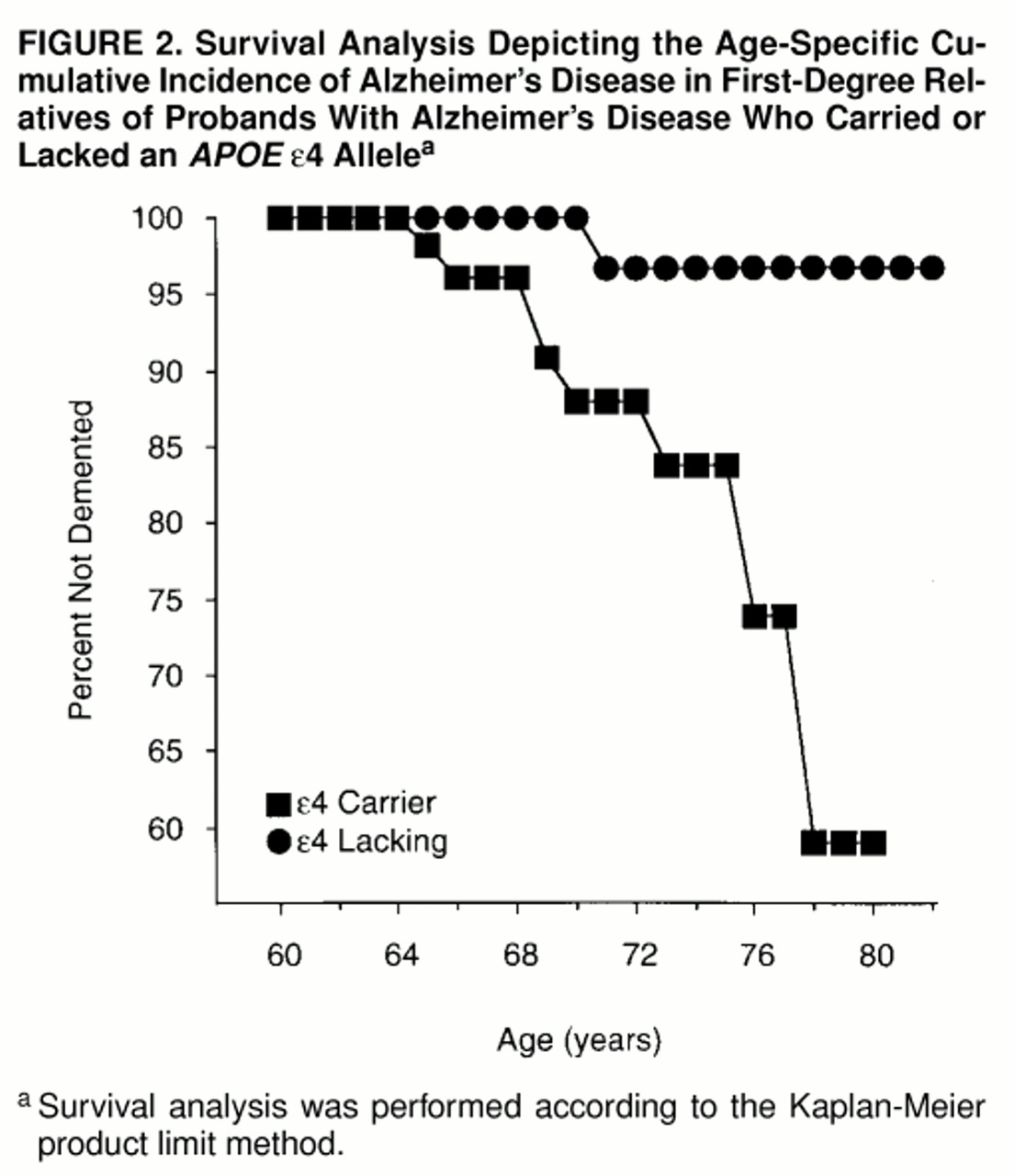
The age-specific cumulative incidence rates of Alzheimer’s disease for the
APOE ε4 carriers and noncarriers are presented in
figure 2. As with increased platelet membrane fluidity, the ε4 allele was associated with a significant increase in the age-specific cumulative incidence of Alzheimer’s disease in the high-risk cohort (Mantel-Cox statistic=6.23, df=1, p=0.01; Breslow statistic=5.44, df=1, p=0.02). In contrast to the findings for increased platelet membrane fluidity, the ε4 allele was associated with an increased incidence of Alzheimer’s disease over a wider age range, from 64 until at least 80 years of age.
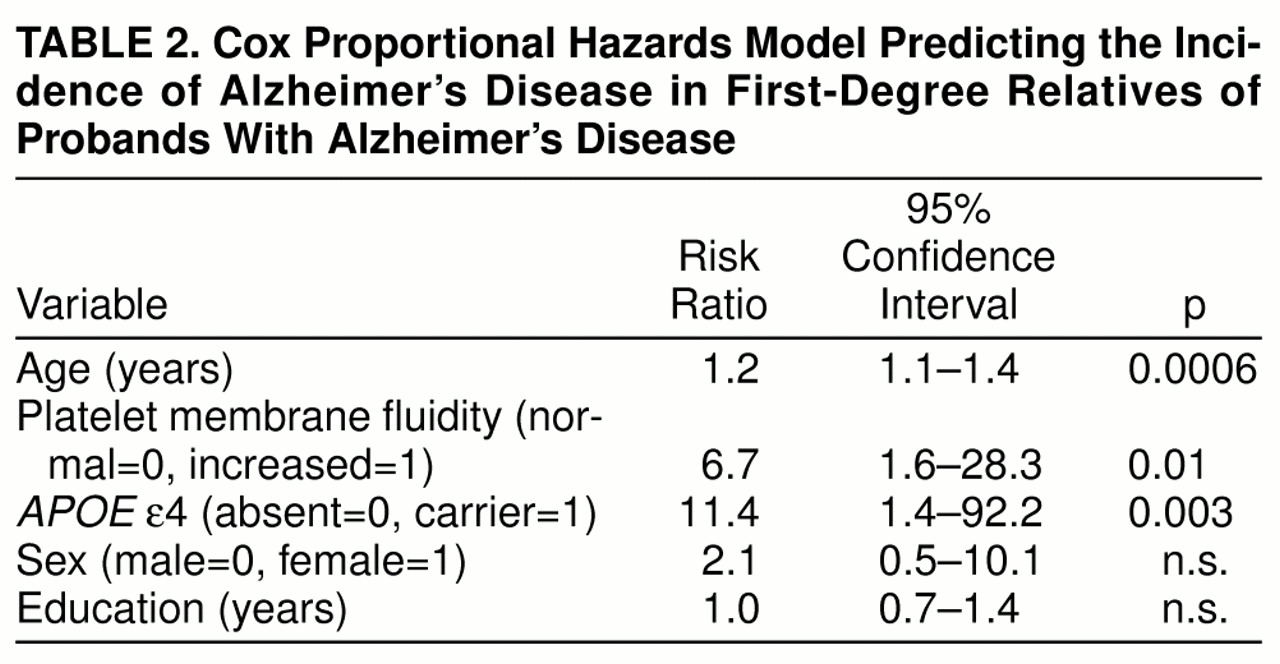
A best-fitting multivariate Cox proportional hazards model was developed to determine the independent effects of having increased platelet membrane fluidity and carrying an
APOE ε4 allele on the risk of developing Alzheimer’s disease, with control for the potential effects of age, sex, and education (global χ
2=25.57, df=5, p=0.0001). As shown in
table 2, age, platelet membrane fluidity subtype, and the ε4 genotype all made significant independent contributions to the prediction of Alzheimer’s disease in this population, with risk ratios of 1.2, 6.7, and 11.4, respectively. Although gender has sometimes been reported to be associated with greater risk for Alzheimer’s disease (for review see reference 53), sex did not emerge as a significant risk factor for Alzheimer’s disease in the high-risk cohort during the first 2,220 subject-years of follow-up. Similarly, years of education did not make a significant contribution to the prediction of Alzheimer’s disease, although limited educational experience has been reported to be a risk factor for Alzheimer’s disease in some
(54,
55) but not all
(56,
57) epidemiologic studies.
DISCUSSION
Increased platelet membrane fluidity and the inheritance of an
APOE ε4 allele emerged as significant independent risk factors for Alzheimer’s disease in our high-risk cohort during the first 2,220 subject-years of follow-up. Both factors appeared to be associated with substantial increases in risk, as reflected by risk ratios of 6.7 and 11.4, respectively. The risk ratio of 6.7 associated with increased platelet membrane fluidity is similar to the 7.7 value observed by the end of the first 5 years of follow-up
(30). The risk ratio of 11.4 associated with carrying an
APOE ε4 allele among the subjects in our study was approximately 2.5 times the corresponding value of 4.4 reported from a prospective study of elderly subjects with mild cognitive impairment
(36) and approximately three times the corresponding value of 3.7 for ε4/ε3 heterozygous individuals enrolled in the Framingham study
(37). These results suggest that the relative risk of developing Alzheimer’s disease associated with carrying an
APOE ε4 allele may be greater among the first-degree relatives of patients with Alzheimer’s disease, who may carry additional Alzheimer’s disease risk alleles, than among elderly subjects with mild cognitive impairment or individuals chosen at random from the community.
Age at onset of dementia has emerged as an important descriptive variable for classifying genes that influence the development of Alzheimer’s disease
(34). Although they are uncommon, the majority of cases of Alzheimer’s disease that produce symptoms before the age of 60 appear to arise from highly penetrant, autosomal dominant genetic lesions. These include mutations in the structural genes for the amyloid precursor protein located on chromosome 21
(58–
61), presenilin located on chromosome 14
(62–
70), and presenilin 2 located on chromosome 1
(71–
73), as well as trisomy 21
(74). The development of more typical, later-onset forms of Alzheimer’s disease appears to be influenced by at least four genes including
APOE (20,
21,
31,
75), an anonymous risk locus in the vicinity of the centromere on chromosome 12
(76), an anonymous risk locus on the long arm of the X chromosome
(77), and an anonymous locus that controls approximately 80% of the variance in platelet membrane fluidity
(15). Evidence from a recent genome survey employing batched genotyping methods for typing pools of DNA from autopsied Alzheimer’s disease cases and control subjects suggests that there may be several dozen chromosomal loci that influence the risk of developing Alzheimer’s disease
(78). Mutations in selected mitochondrial genes, including those that encode two catalytic subunits of cytochrome oxidase, may also contribute to the determination of age at onset and lifetime risk of Alzheimer’s disease
(79,
80).
The results of this study suggest that alleles that confer risk of developing Alzheimer’s disease at more typical ages of symptomatic onset after age 60 may not exert their effects evenly throughout the entire age of risk. Increased platelet membrane fluidity was associated with incident cases of Alzheimer’s disease with symptomatic onsets between ages 64 and 71, following which the increase in the age-specific cumulative incidence of Alzheimer’s disease associated with this phenotype appeared to dissipate. This observation recapitulates the results of previously published cross-sectional clinical studies and family history studies of the role of platelet membrane fluidity in Alzheimer’s disease
(2,
9,
10). In contrast, the
APOE ε4 allele was associated with incident cases of Alzheimer’s disease across the age of risk beginning at age 64 until at least 80. The latter observation is consistent with a previous study that found that the age at onset of memory problems had no effect on the association between the ε4 alleles and Alzheimer’s disease
(81). However, the results of Rebeck and co-workers
(33) reflect a weakening of this association among individuals with Alzheimer’s disease whose symptomatic onset occurs after age 80. These results suggest that the dichotomous classification of Alzheimer’s disease susceptibility genes into early- and late-onset categories may warrant revision, as additional risk alleles for typical-onset cases of Alzheimer’s disease are identified and characterized.
Several limitations of our study warrant further discussion. Our risk ratio estimates, while highly significant, rely on the emergence of nine incident cases of Alzheimer’s disease. Additional incident cases will be required to produce stable estimates of risk with narrower 95% confidence intervals and to permit the cohort to serve as a resource for detecting risk factors that have more modest but still clinically relevant effect sizes. Furthermore, the Alzheimer’s disease high-risk cohort was composed entirely of first-degree relatives who were almost exclusively Caucasians. These features may affect the generalizability of our results to other populations.