With each new breast cancer patient diagnosed, there is the possibility of another population placed at higher-than-normal risk for breast cancer. These may be the patients’ daughters, sisters, and/or mothers—their first-degree relatives.
Emotional and behavioral effects have now been identified in studies of high-risk women. Valdimarsdottir et al.
(1) found that first-degree relatives had higher-than-normal levels of nonspecific distress, avoidance, and intrusive thoughts about breast cancer. In 1992
(2), we presented a profile of the distressed daughter of a breast cancer patient that included 1) having a mother die from breast cancer, 2) being an adolescent versus an adult or a preadolescent at the time of her mother’s illness, and 3) having long-term life plans altered by her mother’s illness. A study of women at high risk for breast cancer showed significantly higher levels of depressive symptoms and feelings of emotional alienation than did a standardized test group, with 27% of this population defined as having a level of psychological distress justifying psychological counseling
(3). A second study also documented an increase in distress among first-degree relatives of breast cancer patients
(4). An Israeli study found that first-degree relatives who have physical symptoms of breast pathology respond with more emotional distress than do women of normal risk in the same situation
(5). In an intervention study of women with family histories of breast cancer, a psychoeducational group significantly reduced the perception of risk and increased the adherence to screening behavior (getting mammograms and doing self-exams)—both considered to be anxiety-related
(6). This suggests that the group format can be an effective way to treat psychological distress in this population. To extend these limited data, we sought to design a more hybrid, short-term (six-session, 12-hour) group model that included elements of education, skills training, and emotional support.
METHOD
Thirty-three women ranging in age from 26 to 67 years were recruited for the study from the University of California at Los Angeles Revlon Breast Center’s High-Risk Program. Each subject had at least one biological first-degree relative with a history of breast cancer and did not herself have breast cancer. Approximately 66% (N=33 of 50) of the women who were approached accepted entry into the study. The women were divided into six groups: two by age, women over 42 years and women under 42 years (42 was the mean age of women in the High-Risk Program); two by survival status of the relatives with breast cancer (died versus survived); and two mixed in terms of age and survival status of the relatives with breast cancer. Eight subjects of 33 did not attend all six group sessions.
The subjects met for 6 consecutive weeks in the evening for 2.5 hours. The group consisted of an educational component that lasted for approximately 1 hour and a psychological component that lasted approximately 1 hour.
The first two educational sessions were devoted to the subject of genetics. The next two sessions were devoted to nutrition. The fifth group session was concerned with relaxation techniques. The sixth session covered medical information such as alternatives to hormone-replacement therapy, benign breast masses (different types of lumps), various forms of screening available (e.g., mammography, breast self-examination, clinical examination), and statistics on breast cancer. Each group was led by an expert in the particular area.
The psychological portion of each group session also had specific topics. The first session provided an opportunity for each person to tell her story about familial breast cancer. The second session focused on each subject’s relationship with her affected family member or members. In the third session, members read aloud letters that they had written during the week to their relatives and discussed the process of writing the letters. The fourth session focused on coping. The fifth session focused on anger/frustration management with respect to being at high risk. The sixth (final) session was a review of the entire experience.
At the end of the sixth group session, the subjects filled out all the baseline instruments again, along with a new one—a group evaluation questionnaire. All measures were collected at the end of the last session.
The subjects had been sent by mail a series of questionnaires to fill out before the first session. The data were received from about 3 weeks before the first night of the group sessions. There were four standardized psychological tests plus two measures developed specifically for the project. The subjects filled out these same questionnaires, along with a group evaluation questionnaire, immediately after the final group session. In this report, only the depression (Center for Epidemiologic Studies Depression Scale [CES-D Scale])
(7) and anxiety (State-Trait Anxiety Inventory)
(8) scores are reported. Future reports will discuss other measures and their results.
Changes in test scores after the intervention were analyzed with paired t tests. Paired t tests were selected because each test was performed by using the entire study group (N=33). No subgroup data were analyzed for this article.
RESULTS
The majority (88%, N=29) of the study subjects were Caucasian, with a mean age of 45.3 years (SD=10.3). Survival status of the first-degree relatives was balanced, with 45% (N=15) alive and 55% (N=18) deceased, due to chance. The majority (64%, N=21) of the study subjects’ own mothers were the relatives with breast cancer, with the minority (36%, N=12) having a sister, two sisters, or a mother and a sister as the affected relatives.
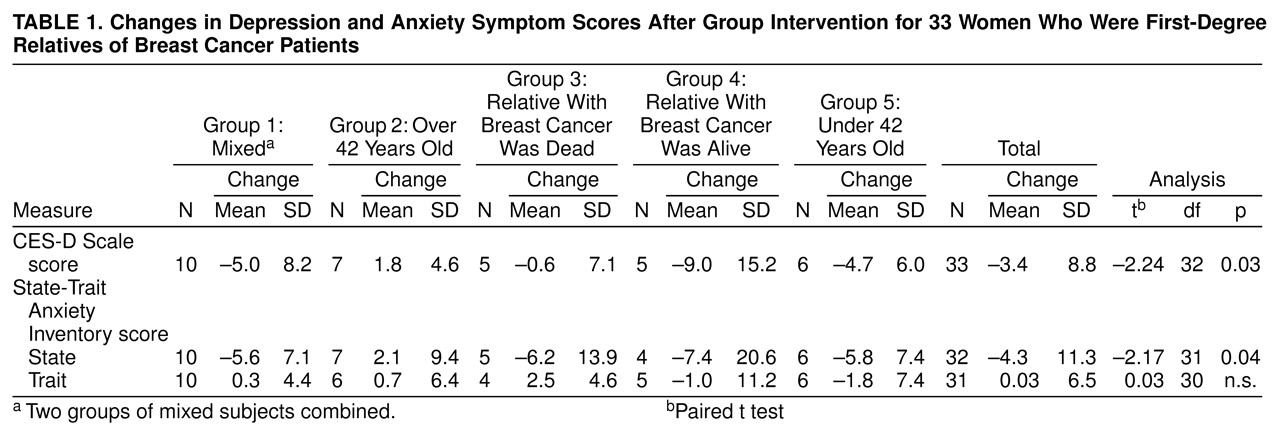
Table 1 shows changes on the depression and anxiety symptom scale scores after the group intervention. For the CES-D Scale scores, an overall significant reduction in depressive symptoms was reported after the group intervention . For the State-Trait Anxiety Inventory state scale scores, an overall significant reduction in anxiety symptoms was reported after the group intervention.
DISCUSSION
Three caveats to this research study need addressing. The first is that this was a nonrandomized trial; hence, it is impossible to say whether these improvements in symptoms would have occurred without this intervention. Second, the modest number of subjects in this study restricts the generalizability and strength of the data’s significance. Finally, the lack of follow-up data limits the evidence for the permanence of these benefits. A larger, randomized, controlled replication of this study, including follow-up data, is essential to confirm these preliminary findings.