The introduction of chlorpromazine by Delay and Deniker
(1) represented a major advancement in the treatment of schizophrenia and other psychoses. In the following 20 years, other, similar neuroleptic agents with different chemical structures became available. Some were more potent than others, but all shared a common mechanism of action that centered on dopamine 2 receptor antagonism. None appeared to be superior to the others in terms of clinical efficacy, and all of the neuroleptics produced an array of distressing extrapyramidal symptoms that varied in form and severity. These early antipsychotic drugs were introduced without systematic multiple-dose studies, and, unfortunately, it took many years to understand that high equivalent doses were associated with more side effects and, in some cases, a poorer treatment response
(2).
The Kane et al. study led to worldwide interest in clozapine, treatment-refractory schizophrenia, and the development of other atypical neuroleptic agents. When clozapine was reintroduced into the United States, little was known about either the optimal dose or optimal length of treatment. However, it soon became clear that, as with earlier neuroleptic agents, U.S. psychiatrists tended to use higher clozapine doses than did European psychiatrists
(6). The Kane et al. 6-week study used flexible dosing up to 900 mg/day of clozapine, with mean peak doses exceeding 600 mg/day. Longer treatment trials were suggested by Meltzer
(7), who found in an open study that 6 months of clozapine treatment yielded a 50% response rate. A recent double-blind study by Rosenheck et al.
(8) suggested a lower response rate for clozapine than that reported by either Kane et al. or Meltzer and a higher response rate for the typical neuroleptic haloperidol. The respective response rates at 6 weeks for clozapine and haloperidol were 24% and 13%, and 37% and 32% after 1 year, respectively.
Our study was designed to provide information about the appropriate dose of clozapine. In a double-blind manner, we examined patients with treatment-refractory schizophrenic symptoms in an initial trial of at least 4 months, using three different clozapine doses: 100, 300, and 600 mg/day. Patients who completed the 4-month study but did not respond to clozapine were further studied with the two other double-blind clozapine doses, each for a 4-month period.
RESULTS
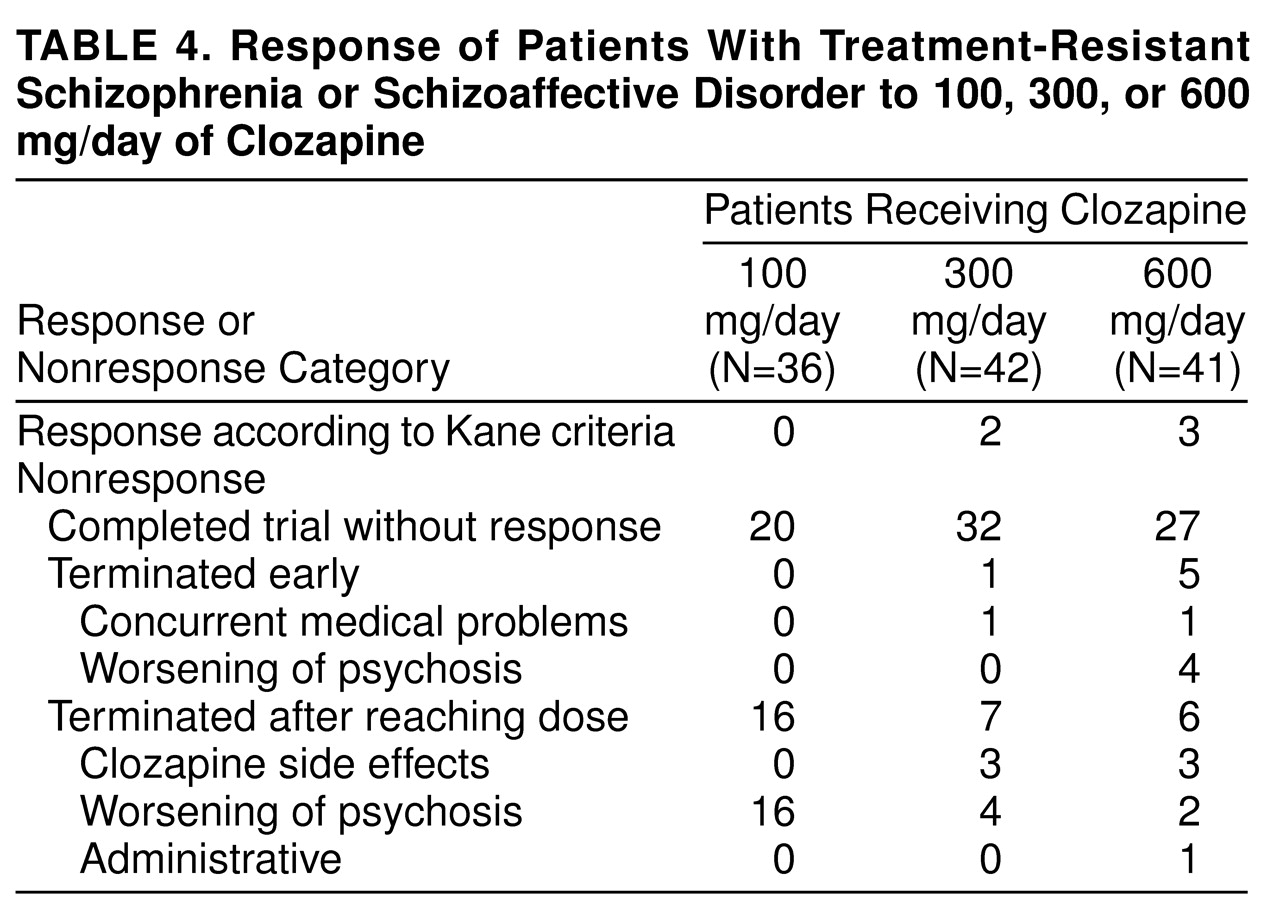
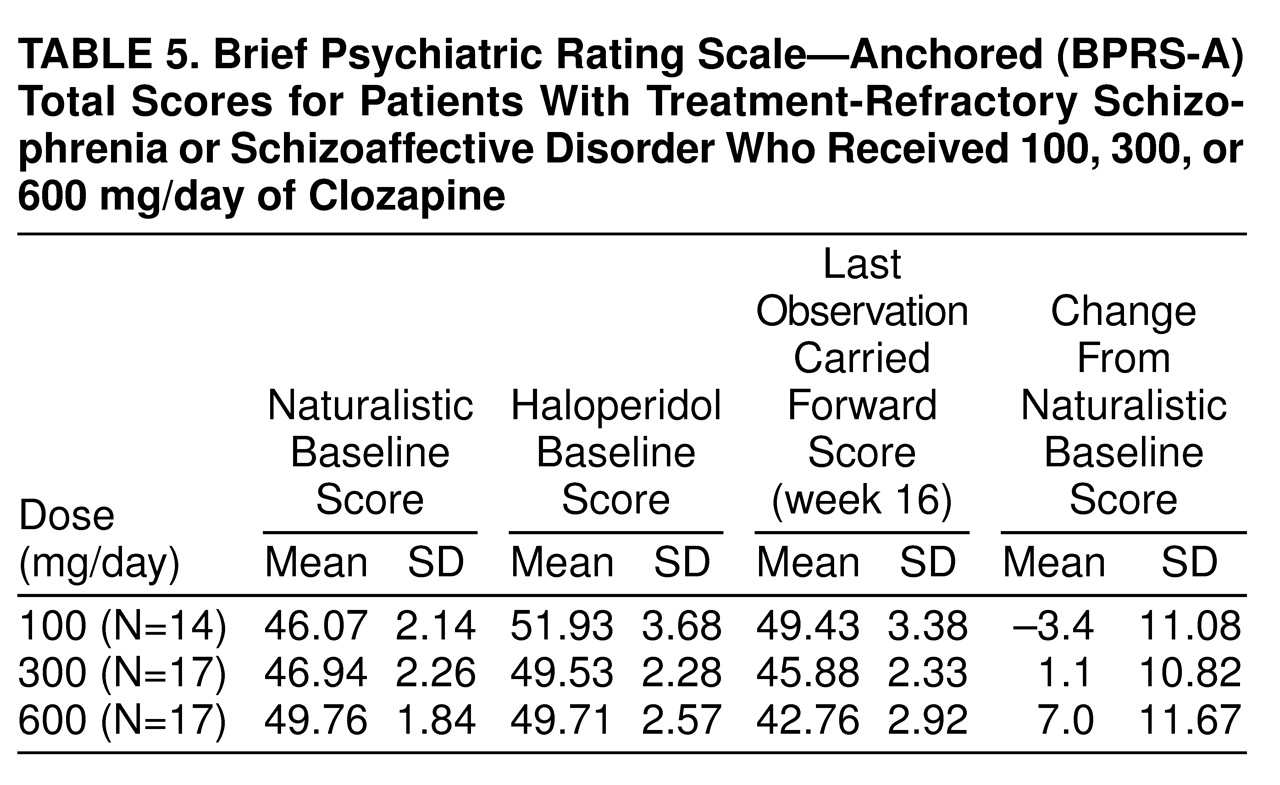
None of the patients responded to treatment with haloperidol, confirming the selection of patients with treatment-refractory symptoms for this study. It is interesting that, as shown in
table 5, many of them showed a worsening of symptoms during the standardized lead-in period with haloperidol treatment. When we used the criteria of Kane et al., four patients (8%) responded to the first clozapine dose and one subject (2%) to the next clozapine dose. None of the patients responded to the third clozapine dose. Of the five responders, two subjects responded to a dose of 300 mg/day, three to 600 mg/day, and none to 100 mg/day. A chi-square test comparing rates of responders and nonresponders during the first 16-week period across the three dose groups was nonsignificant (χ
2=3.5, df=2, n.s.).
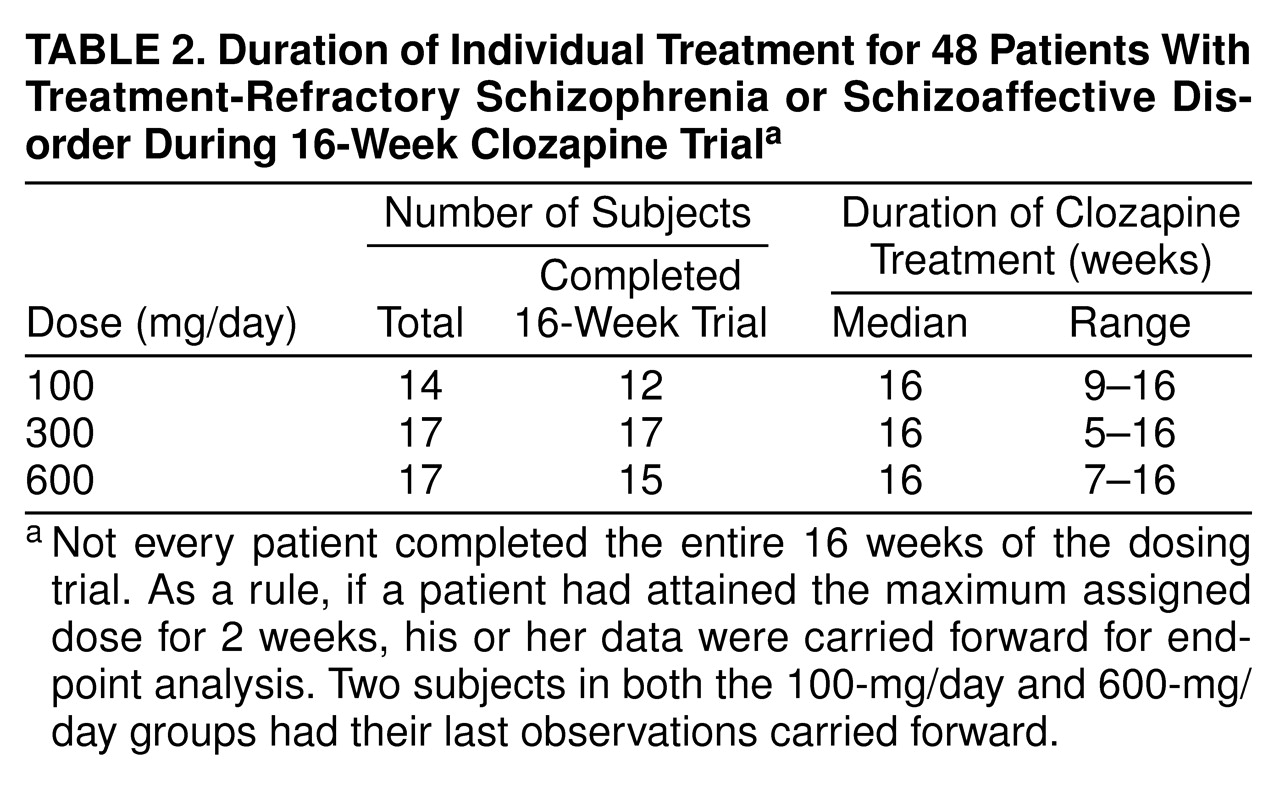
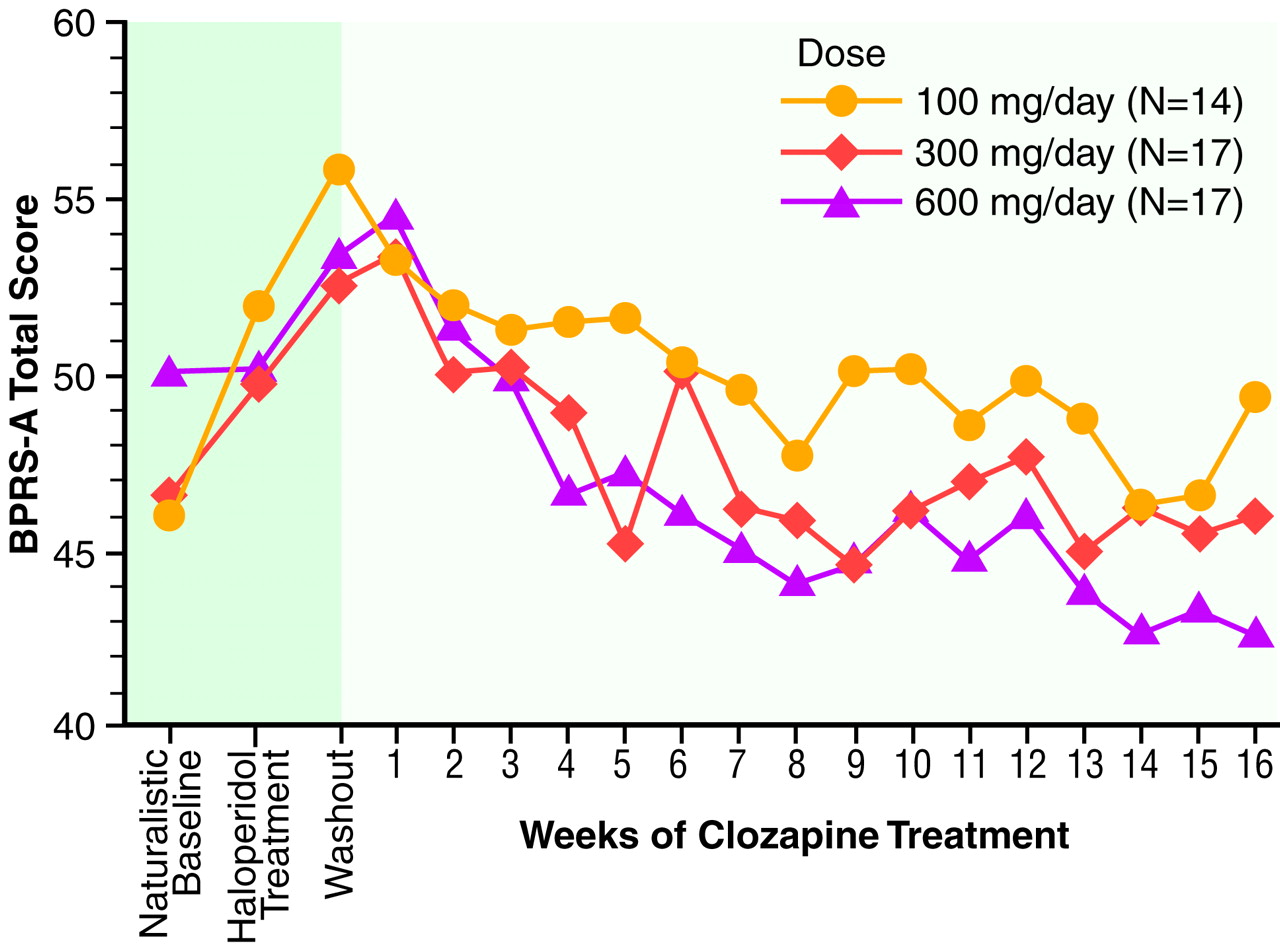
Results of the 16-week study (N=48). Mean BPRS-A total scores across the first full 16-week study are displayed in
figure 1.
table 5 summarizes mean scores. No significant naturalistic baseline differences were observed between the treatment groups. Levene’s test of equality of error variance showed that the error variance for the change scores from the naturalistic baseline to the last observation carried forward was equivalent across groups (F=0.05, df=2, 45, p>0.93). An ANOVA of between-group differences in BPRS-A total change scores from baseline to the last observation carried forward revealed a statistically significant dose effect (F=3.35, df=2, 45, p<0.04). Two-way comparisons by means of Tukey’s honestly significant difference test (p<0.05 criterion) revealed that the 600-mg/day group had a better clinical response than the 300-mg/day and 100-mg/day groups. The comparison between the 300-mg/day and 100-mg/day groups failed to reach statistical significance (p=0.13).
We wondered whether gender contributed to the overall variance in a systematic fashion. Therefore, another ANOVA was performed with both group (three levels: 100 mg/day, 300 mg/day, and 600 mg/day) and gender (two levels) as main effects, as well as the group-by-gender interaction. First, Levene’s test of equality of error variance showed that the error variance with gender included was equivalent across groups (F=0.74, df=5, 41, p>0.64). In an ANOVA of between-group differences from baseline to the last observation carried forward, a significant group effect was again obtained (F=5.08, df=2, 41, p<0.01). Gender was not statistically significant (F=1.54, df=1, 41, p>0.22), and the group-by-gender interaction failed to reach statistical significance (F=0.56, df=2, 41, p>0.57). Two-way comparisons by means of Tukey’s honestly significant difference test again revealed that the 600-mg/day group had a better clinical response than both the 300-mg/day (p<0.14) and 100-mg/day (p<0.01) groups. The difference between the 300-mg/day and 100-mg/day groups failed to reach statistical significance (p=0.51).
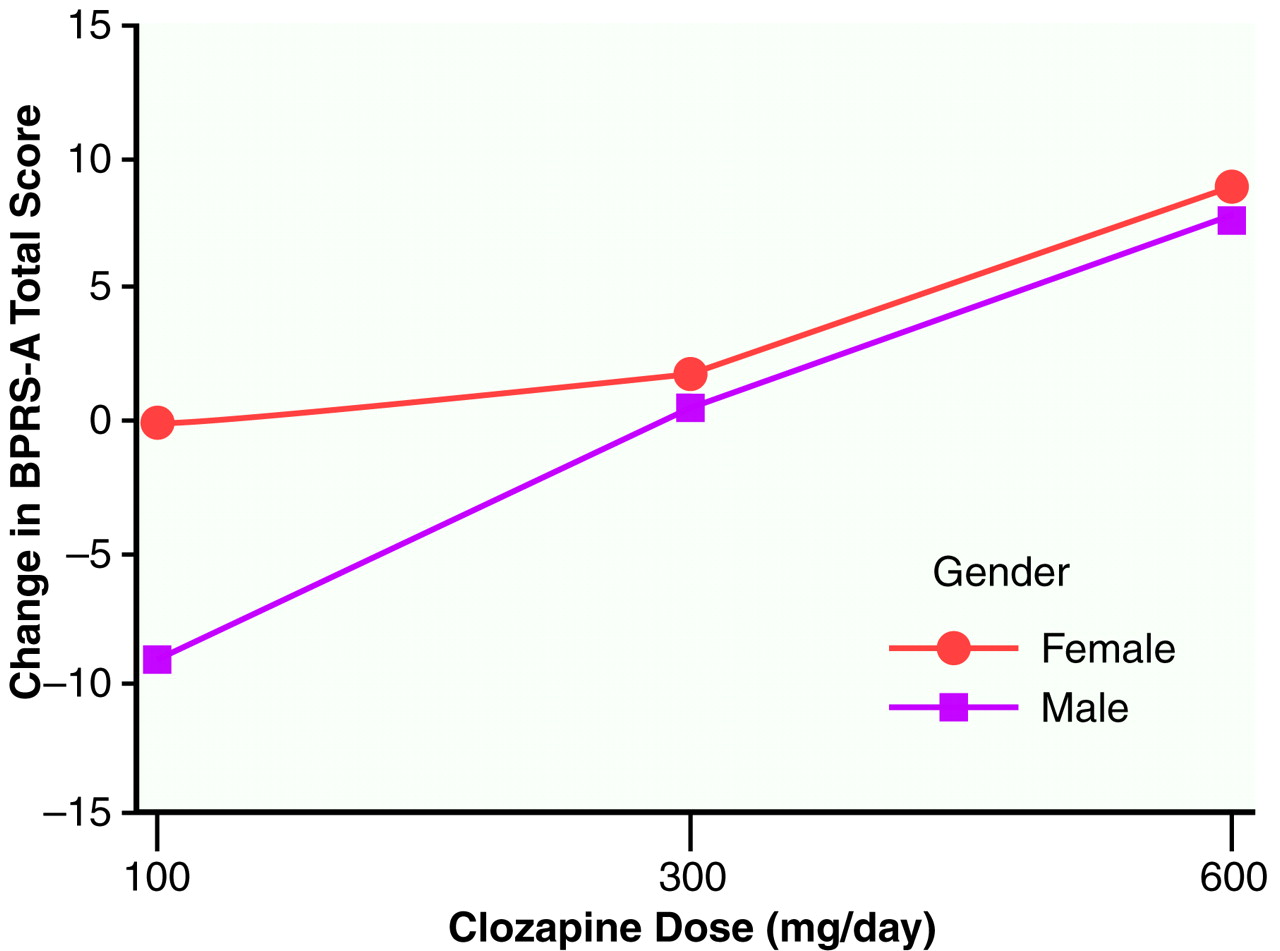
Figure 2 plots the mean BPRS-A total change scores across dose groups with scores separated by gender. It is interesting that for the 100-mg/day dose, a difference in clinical response was observed between men and women (women responded more than men), which was not seen for the other two doses.
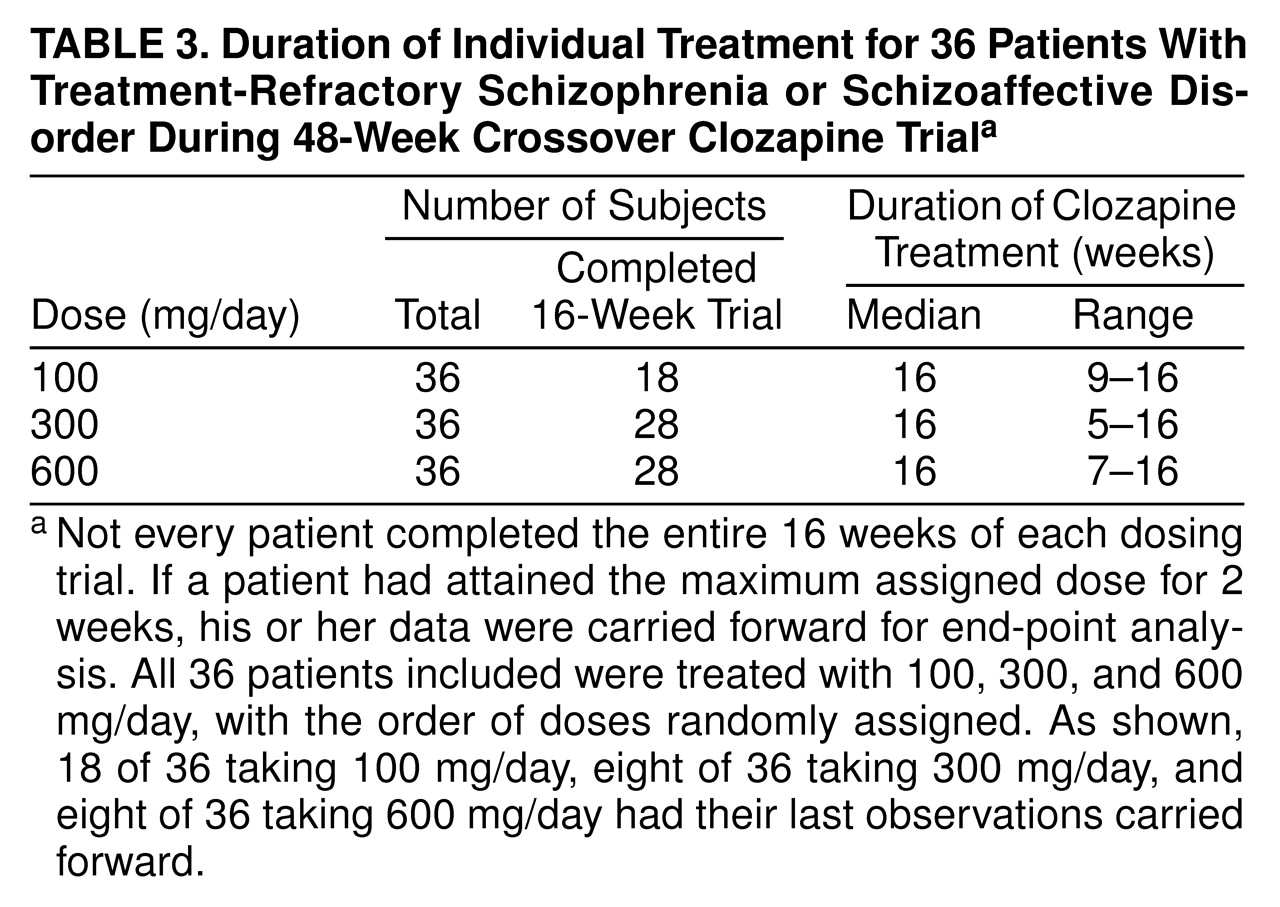
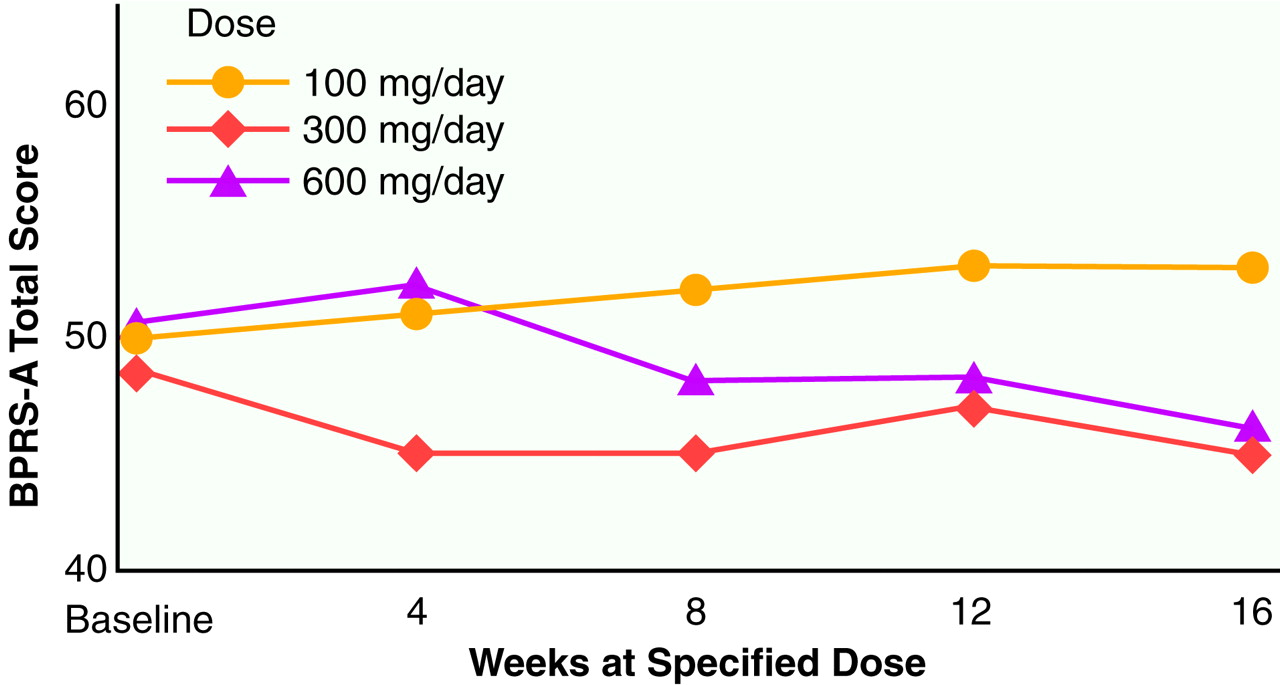
Results of the 48-week study (N=36). Mean BPRS-A total scores across the entire 48-week study are shown in
figure 3. Each of the three dosing phases across the 48 weeks were 16 weeks in length, with data from each dosing period represented as week 4, 8, 12, or 16. This analysis included a total of 36 subjects. In the crossover analysis, the main effect for group was highly significant (F=9.91, df=2, 34, p<0.0001), with the 100-mg/day dose showing the highest (poorest) values and the values of the 300-mg/day and 600-mg/day doses being approximately equal. The main effect for time was also significant (F=6.47, df=3, 34, p<0.01). There was no group-by-time interaction term in this crossover model.
Because of the limited range of response, no attempt was made to study the relationships of plasma levels of clozapine. At a descriptive level, however, all responders had levels that were greater than 350 ng/ml of clozapine (not including norclozapine). Extrapyramidal symptom results from this group have been reported elsewhere and essentially showed that clozapine did not cause rigidity or akathisia
(14,
15).
DISCUSSION
A group of state hospital patients was treated with three different doses of clozapine in a 48-week, double-blind study. The study initially began as a 16-week cross-sectional investigation (N=48) that was extended into a 48-week longitudinal study (N=36), with individual patients receiving a trial with each of the three doses (16 weeks each). The number of patients who improved to the point that they were judged to be responders by using the Kane et al. criteria was small compared to other studies of the efficacy of clozapine. If we consider all the patients treated across the entire 48-week, double-blind study, approximately 10% (five of 48) demonstrated sufficient clinical improvement to be considered treatment responders. Four additional patients responded to clozapine when they received open medication at higher doses (800–900 mg/day), but even adding these additional patients brought the number of responders to only 19% (nine of 48). Clearly, these results, based on the Kane et al. response criteria, are at variance with the bulk of the findings on clozapine efficacy.
The reasons for this lower response rate are not clear, but several factors need to be considered. 1) The patients enrolled in this study were chronically ill, perhaps more chronically ill than other groups reported in the literature. 2) The Kane et al. criteria for treatment response are categorical and, perhaps, insensitive to modest clinical gains. 3) The demonstration of clinical response was strongly influenced by the choice of baseline measure. 4) The raters may have been highly conservative in their assessment of symptoms across time. Each of these possibilities will be considered in turn.
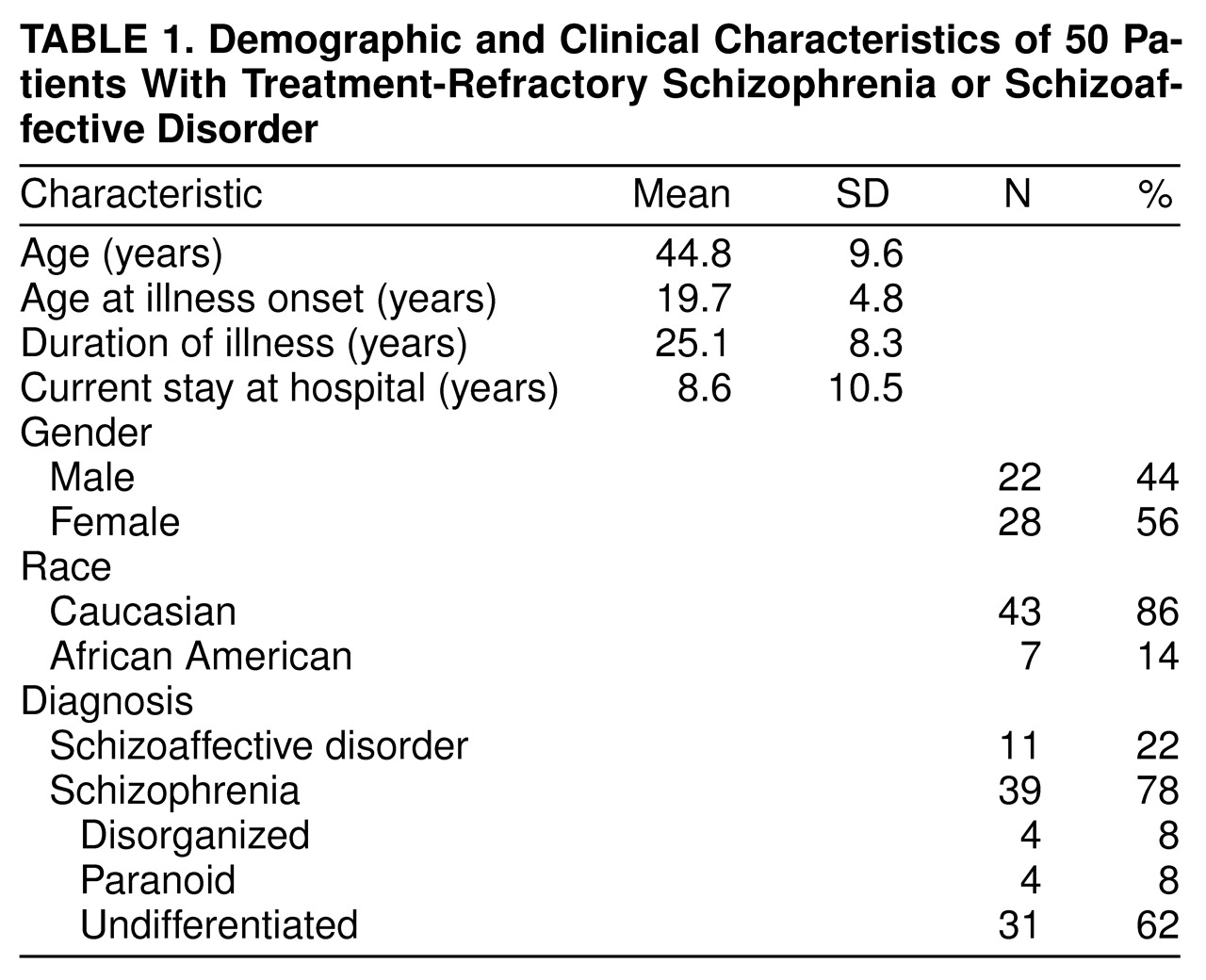
The first relates to the general problem of patient selection and subject variance across hospital sites. As shown in
table 1, for our group, mean age was 44.8 years; mean duration of illness was 25.1 years. The mean duration of the current hospitalization was 8.6 years (range=1–38); the group had an estimated median of five hospitalizations (range=1–25). The pivotal Kane et al. sample of 319 patients (5) was nearly a decade younger; the mean age was 35.7 years. The Lindenmayer et al. sample
(16) had a mean age of 34 years and a duration of illness of 15 years, and the Lieberman et al.
(17) study group had a mean age of 28 years and a duration of illness of 9 years. Clearly, the patients enrolled in this study were older and extremely chronically ill, indeed, to our knowledge, more chronically ill than other subject groups reported to date. Lieberman et al.
(17) found poorer clozapine response was associated with age at onset (19 years or less) and, to a lesser extent, duration of illness (9 or more years). In addition, at the time of our study, treatment with clozapine had commenced in the state hospital. More than 100 state hospital patients had already begun treatment with clozapine and, therefore, were not eligible for our study. This biases our study group in the sense that the majority of the remaining patients were much more difficult to manage clinically and many had additional comorbid medical problems. Perhaps these opaque subject factors combined to result in a lowering of the rate of clinical response.
The second possibility relates to the issue of defining treatment response. According to Kane et al., the a priori definition of treatment response to clozapine is categorical: patients with either a CGI of less than 3 or a BPRS-A total score of less than 35 who showed a 20% reduction in their BPRS-A total score. It is possible that some of our chronically ill patients responded to clozapine at a level that was clinically meaningful but was below the threshold of improvement sufficient to meet these a priori criteria. This would be consistent with general clinical observations that the majority of patients treated with clozapine, who are not terminated because of side effects, tend to improve according to ward staff and relatives, although these impressions are often found to be made on the basis of an improved level of social function, a reduction in level of aggression, and fewer periods of isolation and restraint. Our group showed a significant decline in BPRS-A total scores across both the 16-week and the 48-week evaluations, although the level of improvement was below the Kane et al. threshold. It is not surprising that a preliminary analysis of part of these data with less stringent response criteria showed much more of a response rate than reported here
(18).
A third possible reason for the low response rate may relate to the issue of baseline selection. With treatment response partly defined as a 20% reduction in the BPRS-A total score, the initial baseline calibration of symptom severity is critical. This baseline selection strongly influences the determination of treatment efficacy. In our design, two legitimate baselines were available. The first option was the naturalistic baseline, and the second option was the haloperidol baseline; the 1-week washout was not considered a valid baseline. Examination of
figure 1 and
table 5 shows that symptom severity at these two potential baselines was different. Group BPRS-A total scores worsened when patients were placed on 4 weeks of haloperidol treatment as compared to the naturalistic baseline. When an exploratory ANOVA was performed by using BPRS-A total change from haloperidol baseline to last observation carried forward, it was interesting that the basic results did not change although the magnitude of BPRS-A changes scores was greater. On close inspection, the absence of greater statistical significance resulted from a twofold increase in the level of variance between the naturalistic baseline and the haloperidol baseline. The switch to haloperidol as a standardized lead-in to the clozapine treatment significantly increased the level of “noise” in the data. We compared the efficacy of clozapine against the real-life level of clinical care within this state hospital. It may be the case that research designs requiring patients to be switched to some standardized neuroleptic (such as haloperidol) may artificially worsen the clinical condition of the study group at baseline and thus inflate the actual effects of the treatment. Akathisia was present in fewer than 20% of the subjects during the natural baseline but in over 40% of the subjects after 4 weeks of haloperidol treatment, 10 mg/day.
A fourth possible reason for the low response rate may relate to our raters being highly conservative in their ratings of symptoms across time. Admittedly, the study was done within the context of a large state hospital, where the expectations for clinical improvement are often modest. While acknowledging that our raters may have adopted modest expectations for clinical improvement, it is worth noting that all ratings were on the basis of defined anchor points. Moreover, weekly group ratings were performed and scores were discussed by all raters by using a heterogeneous selection of inpatients, outpatients, and training tapes in an effort to guard against various kinds of rater drift.
On the basis of the negative findings of treatment response by using the Kane et al. criteria, it is tempting to assume that the results shed little light on the question of clozapine dose response. However, this was not the case. When we used the Kane et al. criteria, no patient responded to 100 mg/day of clozapine. Indeed, almost 50% of the patients at this dose had a worsening of their psychosis and had to be terminated from this phase of the study. In contrast, only 9% of the patients were terminated from the 300-mg/day dose, whereas 4% were terminated from the 600 mg/day dose for a worsening of their psychosis. On the basis of the ANOVA results of both the 16- and 48-week data sets, we were able to conclude that there was a statistically significant dose response. In the case of the 16-week data, there was a significant difference between 600-mg/day response and response from the other two doses; however, in the 48-week data set, the 300-mg/day and 600-mg/day responses were equivalent; both were better than the 100-mg/day response. The small group size precluded the establishment of any relationships between clozapine blood levels and clozapine response. However, all patients who did respond had blood levels in the range of 350 ng/ml of clozapine. Notably, one subject required 900 mg/day to reach this value.
It is interesting that the cross-sectional 16-week analysis yielded more statistically powerful findings of a dose effect than was found in the 48-week crossover analysis. In general, when patients are used as their own control subjects in a repeated-measures design, the dose response effect is stronger. However, with this data set, the crossover design introduced uncontrolled sources of variance, such as carryover effects from the previous dose and time, and these could not be systematically partitioned in our analysis. Nonetheless, we are not aware of any other multiple-dose, double-blind studies. A recent 12-week, double-blind clozapine study investigated patients with low (50–150 ng/ml), medium (200–300 ng/ml), or high (350–450 ng/ml) blood levels of clozapine that were achieved with mean doses of 165, 373, and 511 mg/day, respectively
(19). This study suggests that therapeutic blood levels are higher than 250 ng/ml (mean dose=373 mg/day) when using equally divided doses and blood drawn approximately 12 hours after the last dose. Prior studies using an evening dose or blood drawn in less than 12 hours after clozapine administration recommended higher blood levels. Hasegawa et al.
(20) recommended 370 ng/ml (mean dose=385 ng/ml), Perry et al.
(21) recommended 350 ng/ml (mean dose=384 mg/day), Potkin et al.
(22) recommended 420 mg/ml (mean dose=400 mg/day), and Kronig et al.
(23) recommended 350 ng/ml (mean dose=484 mg/day). Therefore, it appears that the average patient needs to be treated with at least 300–600 mg/day of clozapine to achieve a therapeutic response. It is also clear that some patients need higher doses to obtain this response.