Current nosology separates bipolar affective disorder and schizophrenia into nonoverlapping categories with distinct etiologies (the Kraepelinian dichotomy). This view is supported by the majority of family studies of psychosis. However, the alternative view, that psychosis is a continuum with etiological overlap existing between schizophrenia and bipolar affective disorder, is supported by gradually accumulating evidence from genetic epidemiology, including the results of some twin and family studies and, more recently, linkage and association analysis
(1) . Two examples of such studies include a large population-based study in Denmark that found that patients with bipolar disorder have an elevated family history of both schizophrenia and schizoaffective disorder, albeit to a much lesser extent than bipolar disorder
(2), and a study that reported that the relatives of both patients with bipolar disorder and patients with schizophrenia may be at higher risk of developing unipolar depression and schizoaffective disorder, if not schizophrenia and bipolar disorder themselves
(1) . These studies, taken together with other evidence from genetic epidemiology, suggest that bipolar affective disorder and schizophrenia are partially, but not fully, separable.
Psychotic features may represent a link, since they show familial clustering in bipolar affective disorder
(3), and families with psychotic bipolar disorder show a link to schizophrenia loci on chromosomes 22q and 13q
(4) . Thus, it is important to determine whether genes implicated in the etiology of schizophrenia play a role in bipolar affective disorder and vice versa. Among these genes, there is strong evidence that the dysbindin gene on chromosome 6p is associated with schizophrenia
(5) . There is modest evidence for a linkage between chromosome 6p and bipolar affective disorder, and stronger evidence that loci on chromosomes 6q and 6p interact to increase susceptibility for bipolar disorder (6). Given this and the other evidence suggesting etiological and phenotypic overlap between the two disorders, it is important to explore the potential role of this gene in the susceptibility to bipolar affective disorder. To explore this hypothesis, we analyzed the frequency of genetic variations in the dysbindin gene in a group of patients with bipolar I disorder relative to comparison subjects.
Method
Subjects were recruited through psychiatric hospitals in Scotland, met DSM-IV criteria for bipolar I disorder, and had been receiving lithium therapy for at least 3 years. All subjects were Caucasian with at least three of their four grandparents born in Scotland. Consensus diagnosis was determined by trained psychologists and psychiatrists based on case-note review and clinical interview using semistructured diagnostic questionnaires. The Operational Criteria Checklist for Psychotic Illness program was used to define diagnoses. Patients with schizoaffective disorder were excluded. Final decisions on any diagnostic disagreements ([N=31] for reasons of missing data or discrepancies between DSM-IV and International Classification of Diseases-10 diagnoses [N=31]) were made by one of the authors (D.St.C.) and corroborated by another (R.S.).
The following 10 dysbindin single nucleotide polymorphisms were analyzed from the previous study conducted by Straub and colleagues: 2-dysbindin-P1583 (rs909706); 3-dysbindin-P1578 (rs1018381); 4-dysbindin-P1763 (rs2619522); 5-dysbindin-P1320 (rs760761); 6-dysbindin-P1757 (rs2005976); 7-dysbindin-P1765 (rs2619528); 8-dysbindin-P1325 (rs1011313); 9-dysbindin-P1635 (rs3213207); 10-dysbindin-P1655 (rs2619539); and 11-dysbindin-P1287 (rs760666). An eleventh single nucleotide polymorphism (1-dysbindin-A [rs2619538]; see Williams and colleagues
[7] ) in the promoter region was also genotyped. Genotyping was conducted using KBiosciences (http://www.kbioscience.co.uk), with a competitive allele specific polymerase chain reaction system.
Power analysis was performed using the genetic power calculator (http://statgen.iop.kcl.ac.uk/gpc/). Statistical analysis was performed using both χ
2 and Fisher’s exact test to analyze individual polymorphisms. GENECOUNTING software (version 1.3, March 2003)
(8) was used to estimate the haplotype frequencies (with 1,000 permutations/simulations).
Results
All single nucleotide polymorphisms were in Hardy-Weinberg equilibrium. Our study group had 97% power to detect a heterozygote odds ratio of 2 at the 0.05 level for a risk allele frequency of 0.19. Single nucleotide polymorphisms P1757 and P1320 had p values <0.05 under a recessive genotypic model for the rare allele. Under this recessive genotypic model, P1320 was most significant, with an odds ratio of 0.33 (95% confidence interval=0.12–0.93), (χ
2 =4.39, df=1, p<0.04 [data not shown; available upon request]).
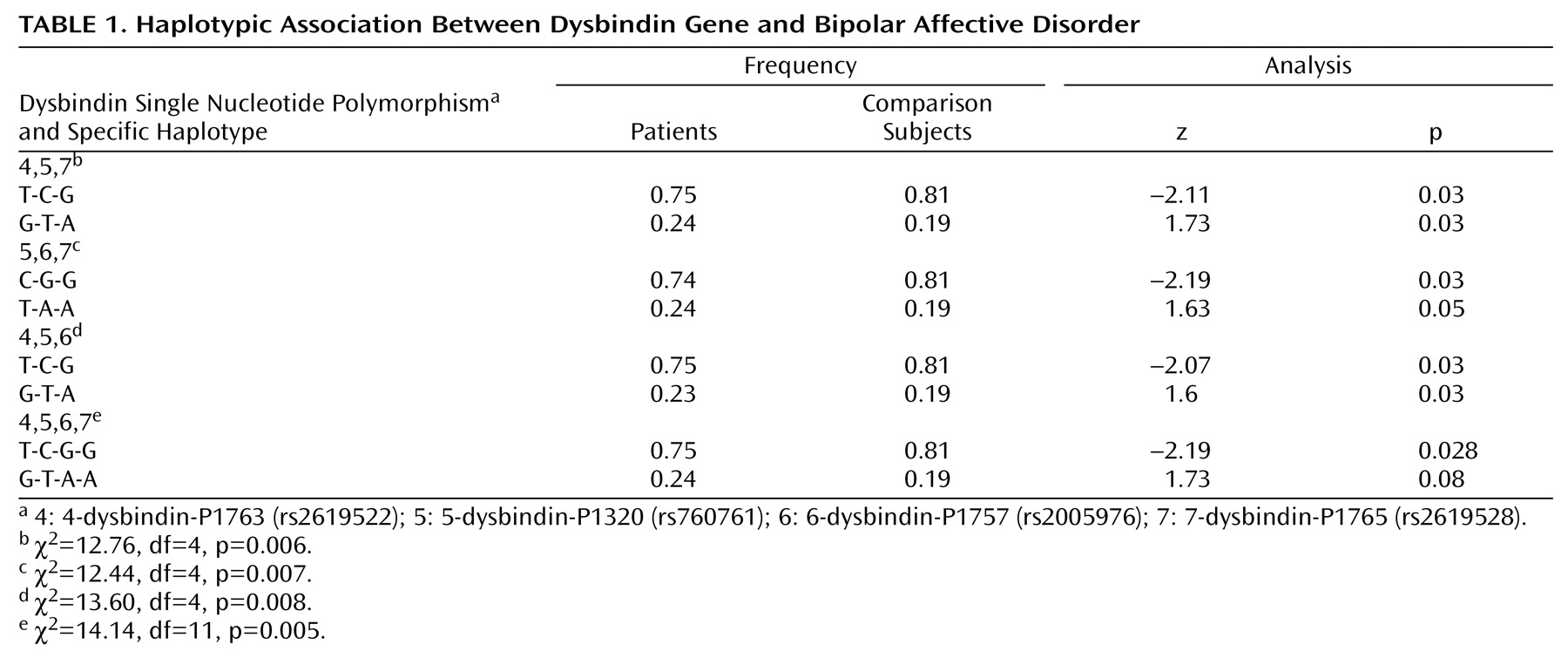
Table 1 shows the most significant 3-marker haplotypes and empirical p values. The 4-5-6-7 haplotype was positive with a significance of p=0.005.
Discussion
We found an individual marker and haplotype-based association between the dysbindin gene and bipolar affective disorder. This finding concurs with the original association reported by Straub and colleagues
(5) and van den Oord and colleagues
(9) in that the less common alleles were greater in patients relative to healthy comparison subjects. Our finding does not, however, concur with the data reported by Schwab and colleagues
(10) or Williams and colleagues
(7), since we found no evidence of a role for the common allele or the single nucleotide polymorphism 1-dysbindin-A (identified by Williams and colleagues) in the promoter region of the dysbindin gene.
It is unlikely that our clinical diagnostic procedures failed to fully separate schizophrenia from bipolar affective disorder, since 1) all patients had been receiving lithium therapy for at least 3 years, and thus subjects who had experienced first-break psychosis, in which the diagnostic boundaries are less certain, were not included, and 2) patients with schizoaffective disorder were specifically excluded.
Our finding provides additional molecular genetic evidence that a portion of the phenotypic overlap between schizophrenia and bipolar affective disorder is caused by the common effects of a shared gene, dysbindin. In addition, this may suggest a real molecular basis of the overlapping symptoms in both schizophrenia and bipolar affective disorder.