Bipolar disorder is a neuropsychiatric disorder characterized by cyclic variations in mood, from depressed to manic states, and a typical onset in the late teens and 20s
(1,
2). Its prevalence in the general population is approximately 1%, with equal proportions of affected men and women
(3). Bipolar disorder has a high degree of inheritability. Bipolar I disorder (characterized by full manic and major depressive episodes) shows approximately 80% concordance in monozygotic twins
(4). While it usually follows a relapsing, chronic course, with variable quiescent intervals between episodes, both social and cognitive functioning appear to be affected even during asymptomatic phases
(5). Recently, deficits in verbal memory and executive functioning have been confirmed during asymptomatic, “euthymic” states
(6).
Structural neuroimaging studies have pointed to the possibility of either neurodevelopmental or neurodegenerative regional brain changes in patients with bipolar disorder. Low volume and metabolism of the left subgenual prefrontal cortex have been noted in patients with familial unipolar and bipolar depression
(11). Large ventricles and both high and low temporal lobe volumes have been reported as well, albeit not replicated by all groups
(12). More recently, higher than normal volumes of the amygdala have been observed, together with high left ventricular volumes
(13). Data obtained with magnetic resonance spectroscopy point toward low phosphocreatine concentrations in the frontal lobes and high choline- and inositol-to-phosphocreatine ratios in the frontal cortex and basal ganglia. These anomalies have been observed during both symptomatic and asymptomatic states
(14–
16) and may reflect anomalies in cell membrane integrity.
While studies with functional and neuroanatomical imaging techniques have shown anomalies in the function or structure of brain regions possibly implicated in the pathophysiology of bipolar disorder, data addressing specific neurochemical systems are lacking. Abnormalities in monoaminergic (e.g., serotonin, norepinephrine, dopamine) function have been hypothesized to underlie some of the clinical manifestations of bipolar disorder for a quarter of a century. However, the supporting data have often been confounded by the use of peripheral or indirect measures, a paucity of postmortem studies, and the effects of medication treatment and clinical state on the markers examined
(17). In the present study we focused on the possibility that abnormalities in more stable markers of monoaminergic synaptic concentrations may exist in bipolar disorder, perhaps associated with a vulnerability to this illness or reflecting trait characteristics. We examined the integrity of monoaminergic terminals with (+)[
11C]dihydrotetrabenazine (DTBZ) and positron emission tomography (PET). This radiotracer labels the central vesicular monoamine transporter (VMAT2) and allows its quantification in living human subjects
(18,
19). The VMAT2 binding site is exclusively located in the membranes of presynaptic vesicles of monoaminergic neurons
(20) and mediates the transport of monoaminergic neurotransmitters from the cytoplasm to their storage vesicles. Unlike levels of the synaptic membrane dopamine transporters, VMAT2 concentrations do not appear to be modulated by short- or long-term administration of a variety of drugs affecting monoaminergic function or metabolism
(21–
24). Furthermore, striatal VMAT2 binding is reduced after nigral 6-OH-dopamine lesions in rats, and it linearly reflects the concentration of monoaminergic synaptic projections in this region (primarily dopaminergic in the striatum)
(25). Striatal binding also decreases with advancing age (approximately 0.5%–0.8% per year) and is low in neurodegenerative disorders affecting dopaminergic innervation
(26,
27). These properties (e.g., insensitivity to medication effects, binding reflective of monoaminergic synaptic density) are desirable for the examination of possible trait-related abnormalities in bipolar disorder.
In the present study, [
11C]DTBZ binding to VAMT2 sites was measured in three brain regions rich in monoaminergic terminals, but with differing concentrations of each monoamine, to test whether generalized or more neurochemically specific anomalies are present in bipolar disorder. These regions were the caudate nuclei, reflecting primarily dopaminergic projections, the thalamus, rich in serotonin and noradrenaline but with minimal dopaminergic content in humans, and the brainstem, containing monoaminergic cell bodies and local terminal projections. In addition, we were interested in examining whether abnormalities in VMAT2 binding would be associated with clinical features of the illness, such as age at onset or course, or with trait cognitive dysfunctions we have found (unpublished data) or those recently described by others
(6). In this regard, monoaminergic input at the level of the caudate nuclei and the thalamus has been implicated in the regulation of frontal cortical networks, affecting memory consolidation, attention, and executive functions
(28–
31).
Results
Subjects
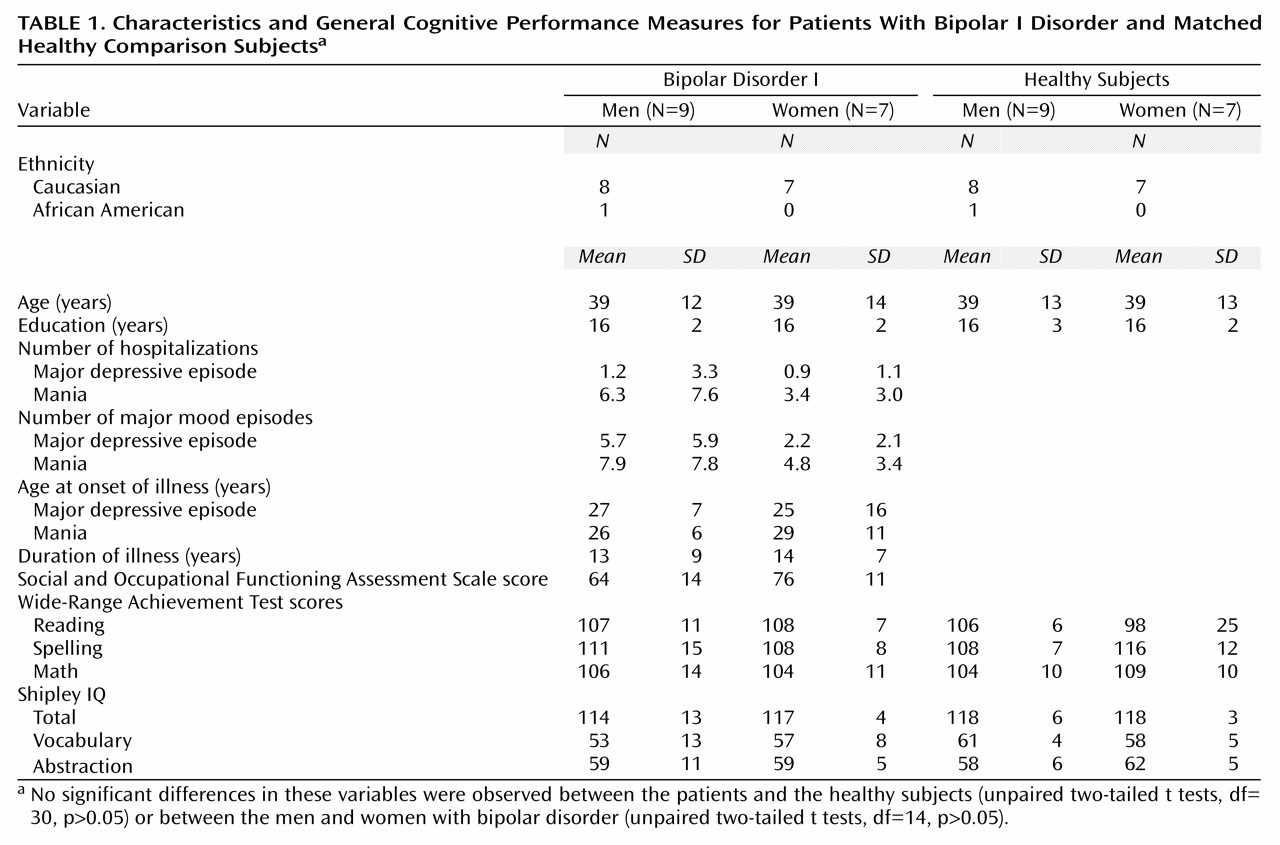
There were no significant differences in age, educational level, or measures of scholastic aptitude or IQ between the patients with bipolar I disorder and the healthy comparison group (
Table 1). Similarly, no significant differences in demographic characteristics, general measures of cognitive function, or clinical characteristics between the men and women with bipolar I disorder were noted (
Table 1). The women showed a tendency toward higher social functioning (score on the Social and Occupational Functioning Assessment Scale) and a slightly earlier onset of depressive episodes and later onset of mania; however, these differences did not reach statistical significance (
Table 1).
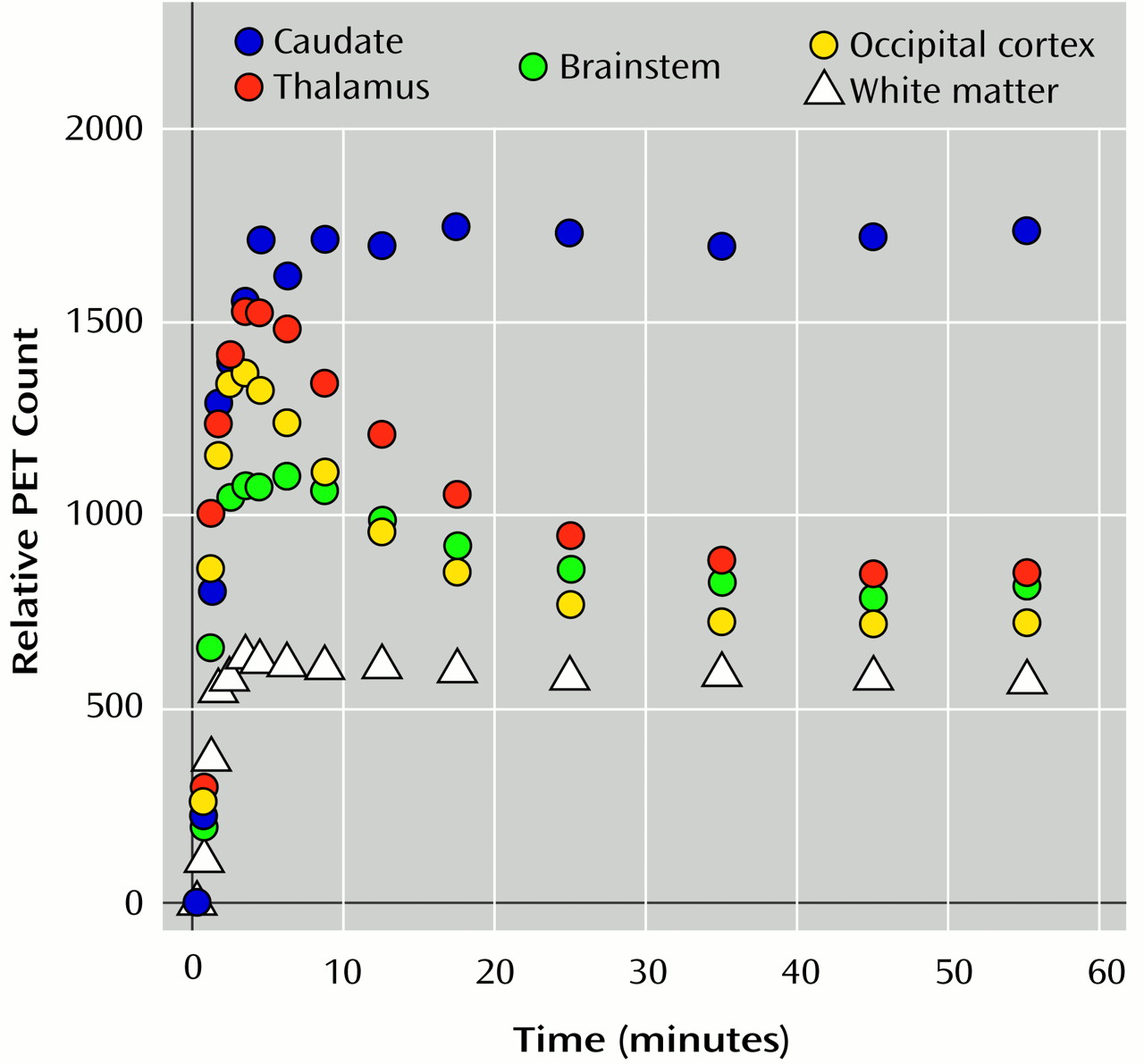
Tracer Transport and Binding
The proportion of authentic (unmetabolized) [11C]DTBZ in the plasma sampled over the scanning period did not significantly differ between the patients and the comparison group at any time, according to a repeated measures analysis of variance (ANOVA) of the group-by-time interaction (F=0.86, df=8, 232, p=0.55).
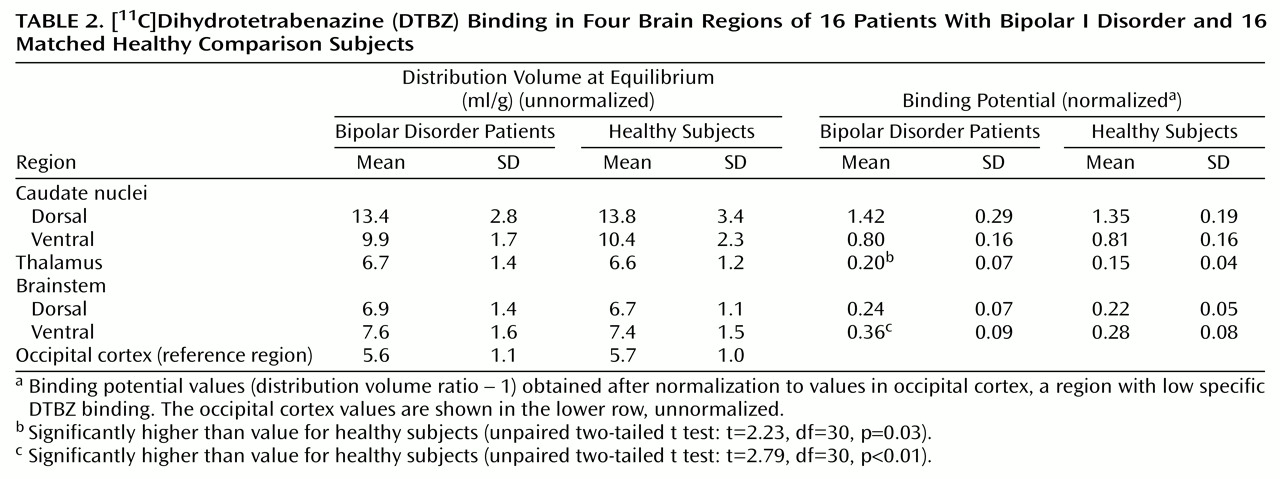
The K1 values did not significantly differ between diagnostic groups for any the brain regions examined: caudate (t=0.15, df=30, p=0.98), thalamus (t=0.49, df=30, p=0.63), ventral midbrain (t=0.32, df=30, p=0.75), or dorsal midbrain (t=0.75, df=30, p=0.46).
Differences in binding potential between diagnostic groups, with higher binding in the bipolar disorder group, were detected in the thalamus (mean difference between groups, 31%) and ventral midbrain (mean difference between groups, 28%) (
Table 2).
Relation of Thalamic and Ventral Brainstem Binding to Clinical Variables
Possible relationships between regional binding potentials and clinical variables were examined for the thalamus and brainstem. We found no significant correlations between binding potentials in these regions and any of the clinical variables examined, which included the Hamilton Depression Rating Scale score and Young Mania Rating Scale score immediately before imaging, numbers of hospitalizations for depression and mania, numbers of episodes of depression and mania, years of illness, Social and Occupational Functioning Assessment Scale ratings, years of exposure to mood stabilizers, antidepressants, and antipsychotic drugs, and blood levels of mood stabilizers before imaging.
Relation of Thalamic and Ventral Brainstem Binding to Neuropsychological Measures
Thalamic binding potential correlated positively with Wisconsin Card Sorting Test perseverative responses (r=0.43, df=29, p=0.01) and perseverative errors (r=0.39, df=29, p=0.03). There was a slightly lower correlation, which did not reach statistical significance, between thalamic binding and the score on the Wechsler Memory Scale paired associates subtest of immediate recall (r=–0.34, df=28, p=0.06). Ventral brainstem binding correlated negatively with the test of immediate recall (r=–0.38, df=28, p=0.04) and with the score on the Stroop Color and Word Test (r=–0.38, df=29, p=0.03). There was a lower, nonsignificant correlation between binding in this region and the Wisconsin Card Sorting Test categories score (r=–0.32, df=29, p=0.07).
Sex Differences
A secondary exploratory analysis was conducted to determine whether sex effects would have influenced the preceding group differences in regional binding potentials. A two-way (diagnosis, sex) factorial ANOVA was used for the thalamus and ventral brainstem to determine whether sex effects and sex-by-diagnosis interactions were present in the data.
ANOVAs showed significant diagnosis (F=4.99, df=1, 28, p=0.03) and sex (F=5.28, df=1, 28, p=0.02) effects in the thalamus but no diagnosis-by-sex interaction (F=1.27, df=1, 28, p=0.27). In the ventral midbrain, a significant diagnosis effect was noted (F=7.00, df=1, 28, p=0.01), as expected, but not a sex effect (F=1.13, df=1, 28, p=0.29) or sex-by-diagnosis interaction (F=1.39, df=1, 28, p=0.25).
In the thalamus, the region where a sex effect was detected, the healthy men and women and the women with bipolar I disorder showed similar mean values and distributions for binding potentials. The men with bipolar I disorder had higher binding potentials (mean difference of 42%) than the women in the same diagnostic group.
Discussion
In this study we examined selected regional concentrations of a stable presynaptic monoaminergic protein, VMAT2, in euthymic patients with bipolar I disorder and in matched healthy volunteers, using [
11C]DTBZ and PET. The purpose of this study was to determine whether trait-related abnormalities in the concentrations of VMAT2 sites, a marker of monoaminergic synaptic density, could be detected. While dysfunctions of monoaminergic networks have been postulated in the mood disorders
(17), to our knowledge the integrity of these neurochemical systems has not been directly examined in patients with bipolar disorder.
The main finding of this study was the observation that the concentrations of VMAT2 binding sites were higher in the thalamus and ventral brainstem of the patients with bipolar I disorder than in the healthy subjects, with mean differences between groups of 31% and 28%, respectively.
In addition, the VMAT2 concentrations in the thalamus and ventral brainstem correlated negatively with performance on tests of frontal, executive function and with a test of verbal learning. Lower than normal performance on these and similar tests have been reported in patients with bipolar disorder, even after prolonged euthymic states
(6).
A secondary finding was the sex difference in the concentration of VMAT2 binding sites in the bipolar disorder patients. ANOVA showed an effect of sex on the concentration of VMAT2 binding sites in the thalamus of the bipolar disorder patients but not the healthy comparison group. The women with bipolar I disorder showed thalamic VMAT2 concentrations in the same range as that of the comparison men and women, while the male patients with bipolar disorder demonstrated greater binding in this region than the female patients (mean difference, 42%).
We found no consistent associations between thalamic or ventral brainstem VMAT2 concentration and clinical variables, blood levels of mood stabilizers, or prior exposure to psychotropic medications.
The greater thalamic and ventral brainstem binding values were not coupled with similarly high levels of regional tracer transport, a measure proportional to blood flow. This indicates that the observed differences in binding values were not likely to be secondary to nonspecific differences in variables such as regional brain volume or anomalies in initial tracer uptake. The presence of anomalies in regional brain volumes in bipolar disorder still remains a controversial issue, with both high and low volumes having been reported
(12,
13). However, the fact that the abnormalities in volume so far observed are relatively small in comparison to the effects noted in the present paper indicates that they are not likely to entirely account for the findings reported. The sizes of the regions of interest applied to the image data (approximately one full width at half maximum of their resolution) and the fact that they were centered over relatively large anatomical regions also made the measures obtained less susceptible to nonspecific volumetric differences between groups
(47).
[
11C]DTBZ is a selective marker for VMAT2
(18,
19). This protein, located in the membrane of the synaptic vesicles
(20), mediates the transport of the monoamines from the cytoplasm into presynaptic storage vesicles. It reflects the concentration of synaptic vesicles in monoaminergic cells, which in turn is thought to provide an estimate of the density of synaptic contacts, since reductions in their concentration are typically associated with degenerative changes in monoaminergic neurons
(48–
50).
We measured VMAT2 binding sites as a possible trait marker in bipolar I disorder because of their apparent insensitivity to medication effects and because their binding reflects synaptic terminal concentrations
(25). The high-affinity synaptic membrane transporters, while also located presynaptically, are regulated by neuronal function and by a variety of drugs affecting the release or metabolism of the monoamines. This does not appear to be the case for the VMAT2 sites, at least for commonly used compounds, such as antipsychotics and various antidepressants
(21–
24), albeit their possible regulation by mood stabilizers has not been specifically examined. However, the disadvantage of using the VMAT2 site as a marker of monoaminergic synaptic concentration is its nonselectivity for the various monoamines
(51), which is conferred to the specific neurons by the synaptic membrane transporters (the serotonin transporter, dopamine transporter, and noradrenergic transporter). However, some degree of neurochemical specificity may be inferred from the regional distribution of VMAT2 binding sites.
The brainstem area contains the cell bodies and local projections of serotonergic (raphe nuclei) and dopaminergic (retrorubral, substantia nigra, and ventral tegmental area) neurons. In an attempt to relate possible findings to more selective neuronal populations, the brainstem was subdivided into ventral and dorsal regions, as previously described by other authors
(52). The former is rich in dopaminergic cells (regions A8, A9, and A10); however, it receives dense input from serotonergic cells located in the raphe nuclei. The dorsal area contains the serotonergic cells of the nucleus raphe dorsalis
(53). It should be noted, however, that because of the relatively poor resolution of PET cameras, the values obtained from regions in close proximity are likely to be contaminated by spillover of activity from one to the other. This applies to the subdivision of the brainstem into dorsal and ventral portions or the subdivision of the caudate into dorsal and ventral portions (the latter region including the nucleus accumbens). Therefore, the separation between ventral and dorsal brainstem and between ventral and dorsal caudate should be taken with caution, and mostly as an approximation to binding values in those specific brain regions.
Prior studies with the nonselective serotonin transporter and dopamine transporter ligand [
123I]β-CIT and single photon emission computed tomography have shown that brainstem [
123I]β-CIT labeling in humans and nonhuman primates is displaceable with serotonin transporter blockers but not dopamine transporter blockers
(54,
55). This suggests that the majority of monoaminergic presynaptic terminals in this brain area are serotonergic, a possibility that agrees with literature on animal research describing dense serotonergic projections from the dorsal raphe to midbrain dopaminergic cells
(56). This is supported by the finding in our study that the higher levels in the ventral brainstem were not coupled with parallel abnormalities in dopaminergic-terminal-rich areas (caudate nucleus/nucleus accumbens). On the other hand, differences of similar magnitude were noted in the thalamus, a region with a high density of serotonergic and noradrenergic terminals but negligible dopaminergic innervation in humans
(57). Another, perhaps less likely possibility is that the higher levels noted in the thalamus are based on binding to noradrenergic terminals. In this regard, abnormally high indexes of noradrenergic turnover in the thalamus and cortical regions have been observed in a postmortem study of bipolar disorder patients
(58). However, resolving the specific neurochemical network or networks involved in the higher levels observed would require the development of specific radioligands labeling other transporters or receptors involved in serotonergic or noradrenergic responses.
As already noted, [
123I]β-CIT binding in the brainstem has been used as an indicator of serotonin transporter concentrations in this brain region in various studies. Serotonin transporter concentrations have been found to be low in patients with unipolar major depression
(59) and in male abstinent alcohol-dependent volunteers
(52). In the study by Mallison et al.
(59), [
123I]β-CIT binding was approximately 20% lower in the brainstem of patients with major depression than in healthy subjects. In the alcohol-dependent volunteers
(52), [
123I]β-CIT binding was a mean of 30% lower in dorsal but not ventral brainstem. In this study group, only subjects with an early onset of alcohol dependence showed low binding, perhaps suggesting the presence of vulnerability factors related to monoaminergic function in alcohol dependence.
We observed negative correlations of performance on measures of verbal learning and frontal, executive functions with thalamus and ventral brainstem [
11C]DTBZ binding. Although these correlations do not imply a causal relationship, the data obtained suggest that higher VMAT2 binding in these regions may bear a relationship with the poorer performance of patients with bipolar I disorder on specific cognitive measures. Lower scores on measures of verbal learning, executive functions, and motor coordination in the absence of more global cognitive deficits have been observed by our group and by other authors
(6). Monoaminergic function at the level of the thalamus and brainstem is thought to regulate the activity of the basal ganglia and thalamocortical networks
(28,
30,
60–
62). These in turn influence cortical processes such as selective attention, learning of associative tasks, and adaptive responses, as well as the processing of emotion-related stimuli
(29,
63–
67). In the case of serotonin, acute tryptophan depletion has been associated with improvements in Stroop test performance
(68), suggesting that serotonin may regulate some cognitive functions by an inhibitory mechanism.
This study opens several lines of questioning regarding the pathophysiology of bipolar I disorder. Higher than normal levels of VMAT2 binding in the thalamus and ventral brainstem during euthymic states, correlating with poorer performance on tests of verbal learning and executive functions, may represent trait abnormalities in the concentration of monoaminergic synaptic terminals or synaptic vesicles in bipolar disorder. In addition, we found sex differences in VMAT2 binding in the thalamus of the patients with bipolar disorder but not in the healthy subjects. To determine the clinical implications of this finding, such as its possible relationship with reproductive status or sex differences in episode frequency or pattern
(69,
70), would require the examination of a larger patient group. Further studies are warranted to increase the power of the data presented, to ascribe the aforementioned anomalies to specific monoaminergic networks, and to examine possible sex differences in the distribution of these markers. Also, such work is needed to confirm that the differences in the thalamus and ventral brainstem indeed represent a trait abnormality and not the result of either medication exposure or illness progression, although these possibilities do not appear to be supported by the data obtained. For that purpose, it would be of interest to examine both newly diagnosed patients and subjects who are at high risk for developing this illness (e.g., those with a prominent family history of bipolar disorder). This should be followed by a prospective examination of illness progression and the possible changes in monoaminergic innervation associated with it or with the long-term use of mood-stabilizing drugs.