Despite advances in the pharmacological treatment of depression, the delayed onset of therapeutic response to antidepressants remains a major clinical dilemma. Substantial mood benefits typically do not become evident until 2–3 weeks after the initiation of treatment with any of the currently available antidepressants
(1). Findings in animal models from microdialysis and electrophysiological studies have led to a better understanding of the neurochemical basis for the delayed onset of action of antidepressant medications
(1). Almost all currently used treatments for depression, including the tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors, and lithium, directly or indirectly enhance serotonin (5-HT) neurotransmission in a time course consistent with their delayed therapeutic effect
(2). Such enhancement may be mediated by a range of mechanisms, including postsynaptic sensitization to 5-HT or desensitization of the presynaptic 5-HT
1A autoreceptors in the raphe area
(1).
It has been hypothesized that strategies directly affecting serotonin receptor mechanisms could subsequently lead to an acceleration of the antidepressant response. One such strategy, the concurrent administration of the β-adrenergic/5-HT
1A receptor antagonist pindolol with an SSRI, appears promising
(3), although not all placebo-controlled studies have yielded positive results
(4–
6). Another strategy reported to accelerate the antidepressant response of medications, which might be mediated by an increase in cortical 5-HT neurotransmission
(7,
8), is the combination of thyroid hormones with antidepressants. The immediate addition of thyroid hormone to speed antidepressant treatment response was first reported in a controlled design in the classic study by Prange and associates in the late 1960s
(9). Since then, two types of studies have been reported in the literature: 1) studies of acceleration strategies, which assess whether thyroid hormones prescribed initially with antidepressants speed the time for response to antidepressants in depressed subjects, and 2) studies of augmentation strategies, which assess whether thyroid supplementation added to treatment after a partial or minimal antidepressant response can potentiate the therapeutic response. The usefulness of thyroid augmentation for the treatment of a partial response or nonresponse to antidepressants has been assessed repeatedly over the past two decades
(10–
15), culminating in the positive meta-analysis by Aronson et al.
(16). In contrast, the potential usefulness of thyroid hormones as a primary treatment to accelerate antidepressant response seems to have been lost in the literature.
Discussion
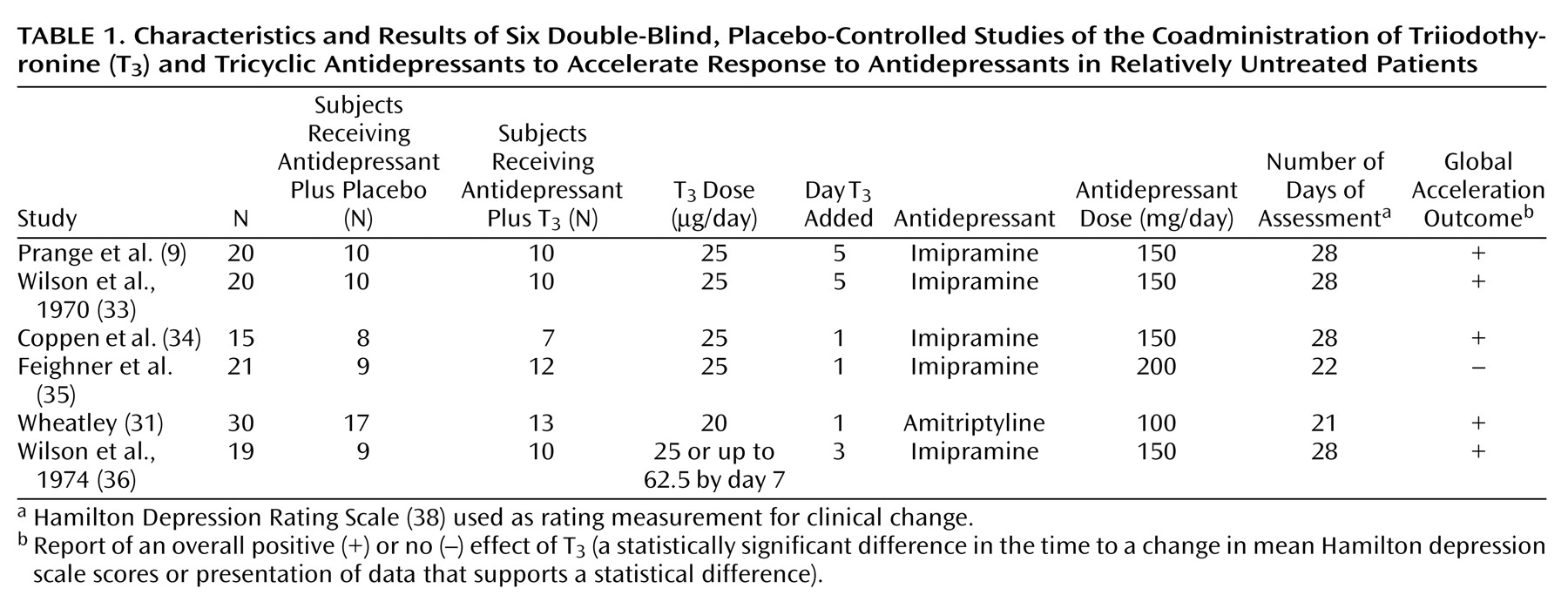
The results of this meta-analysis support an acceleration of antidepressant response when adjunctive T3 is included early in antidepressant treatment. Five of six double-blind, placebo-controlled studies, conducted between 1969 and 1974, found that the addition of T3 had a statistically significant effect on the time to treatment response, compared with the effects of placebo. Treatment with a dose of 20–25 μg/day of T3 was well tolerated by patients, thus suggesting that the addition of T3 is a viable treatment option to accelerate antidepressant response.
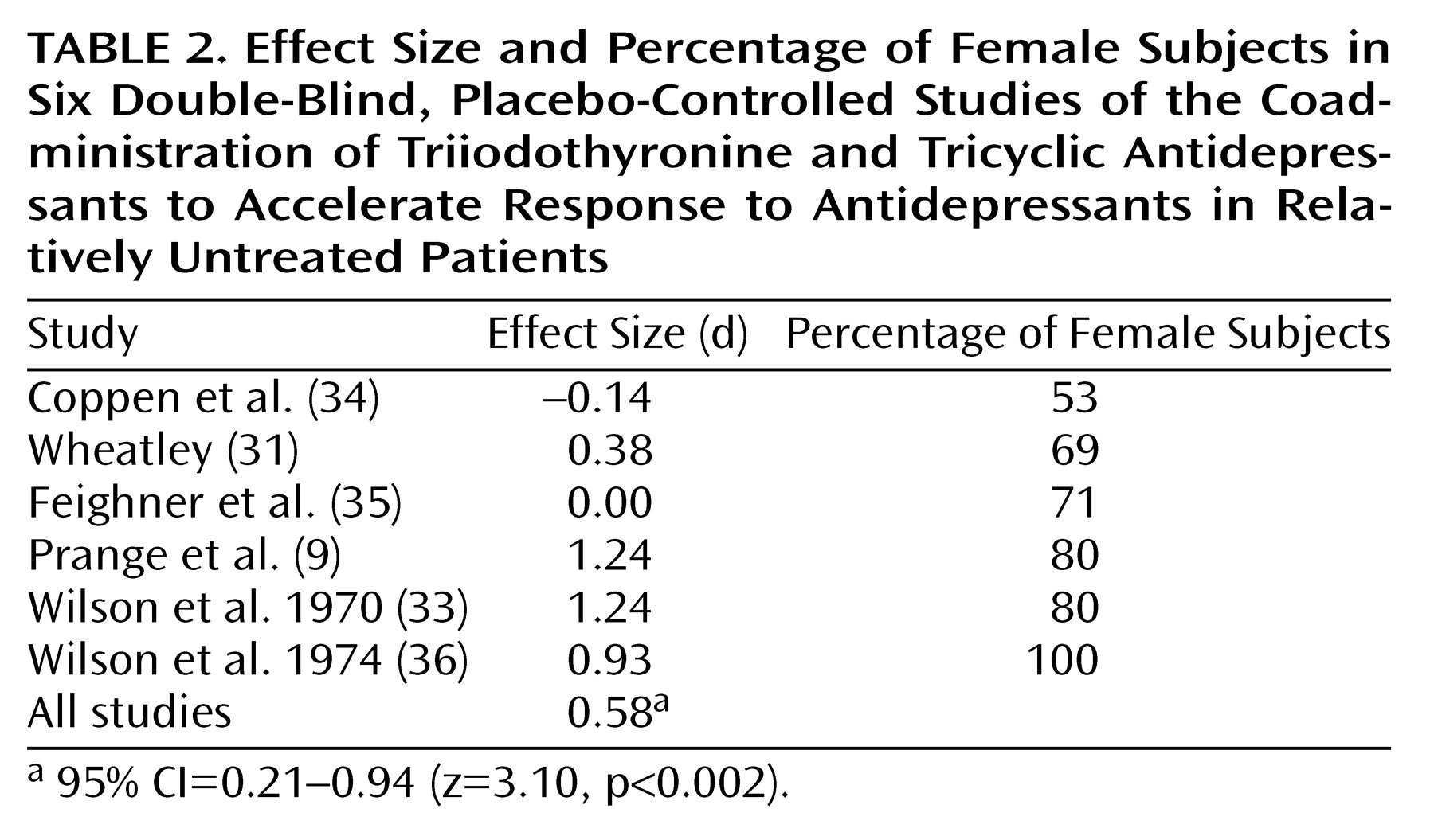
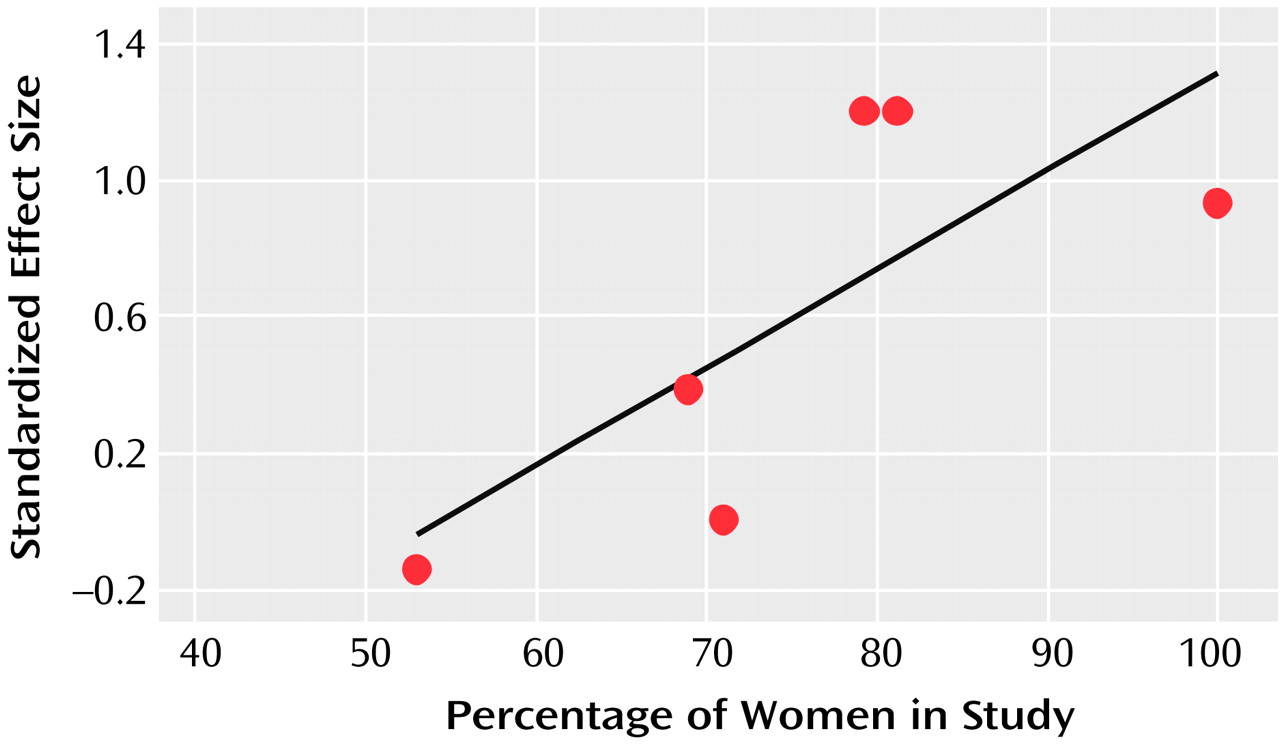
This meta-analysis also revealed that women may be more likely than men to benefit from the addition of T
3. Women have a greater prevalence of both clinical and subclinical thyroid dysfunction than men
(40). Treatment response to antidepressants may be impaired among patients with overt and subclinical hypothyroidism
(41). This might explain why women responded more favorably than men to the addition of T
3, particularly because thyroid status was not thoroughly examined in some of the studies included in this meta-analysis. However, a more recent study involving ECT suggested that T
3 has beneficial effects on rates of improvement in men as well
(42). In that study, male patients with major depression undergoing ECT were randomly assigned under double-blind conditions to receive either T
3 (50 μg/day) or placebo the night before each ECT treatment. The patients who received T
3 and ECT required only eight treatments for improvement, while those who received placebo and ECT required 12 (p<0.001).
How can the acceleration effects of thyroid hormones be explained? Studies examining the relationship between thyroid hormones and neurotransmitter systems have focused on the noradrenergic and serotonin systems. In studies assessing the thyroid-serotonin systems, the effects of thyroid hormone application to experimentally induced hypothyroid or euthyroid animals include an increase in cortical 5-HT concentrations and a desensitization of autoinhibitory 5-HT
1A receptors in the raphe area, resulting in disinhibition of cortical and hippocampal 5-HT release
(43,
44). A recent in vivo microdialysis study by Gur et al.
(45) indicated a loss of autoinhibitory 5-HT
1A receptor sensitivity mediated by T
3. In the latter study, the decrease in hippocampal and cortical serotonin release, which should follow the application of a 5-HT
1A agonist, was significantly reduced in euthyroid rats by administration of T
3 or combined T
3 and clomipramine
(45). These results indicate that thyroid hormone application may reduce autoinhibitory 5-HT
1A receptor activity and thus increase cortical serotonin release. In this way, it may have actions similar to those of pindolol, a 5-HT
1A receptor antagonist that when added to SSRI treatment facilitates serotonin release
(3). Further evidence for an interaction between thyroid status and the serotonin system derives from studies that have demonstrated a significantly blunted cortisol and prolactin response to the 5-HT agonist
d-fenfluramine in hypothyroid patients
(46,
47). This blunted response to
d-fenfluramine stimulation normalized with thyroid hormone replacement therapy, suggesting reduced central 5-HT functioning in hypothyroidism
(47). Thyroid hormones also appear to play an important role in regulating central noradrenergic function, and its impact on this system may also contribute to the acceleration effect. Recent findings indicate that T
3 may function as a cotransmitter with norepinephrine in the adrenergic nervous system
(48).
Due to its faster onset of action, T
3 is probably the prime thyroid candidate for accelerating antidepressant response. The efficacy of thyroxine (T
4) in patients with acute depression has been studied only as an augmentation strategy. The pharmacokinetic properties of T
4, e.g., long half-life and action as a prohormone, suggest that it is not a very promising agent for the acceleration paradigm. In one study, the onset of remission in depressed patients receiving T
4 as an augmentation strategy did not occur until between week 5 and 8
(15).
There are no published studies on the optimal length of time to continue T
3 in persons who have had a successful T
3 acceleration. In three of the studies described here, T
3 was discontinued after 12
(36) or 14
(34) days of treatment or between 19 and 28 days of treatment
(9). In all three studies, the majority of the patients who had an initial positive response remained well after T
3 was discontinued. The investigators concluded that T
3 may exert its greatest therapeutic effect within the first week of use
(36). It seems wise to continue T
3 at least until stable remission is achieved.
T
3 is generally well tolerated
(19). A possible connection between the use of thyroid hormones and the risk of developing osteoporosis has been a concern. To our knowledge, no data address whether long-term use of T
3 affects bone mineral density in patients with mood disorders. However, treatment with supraphysiological doses of thyroxine (T
4) for 1 year or longer does not appear to significantly influence bone mineral density in pre- and postmenopausal women with mood disorders
(49,
50).
The studies included in this meta-analysis were all performed more than 25 years ago and thus have some methodological limitations that could confound interpretation of the data. First, the studies included relatively small numbers of patients, evidently without matching groups by gender, age, and other clinical variables (e.g., duration or severity of the depressed episode). Second, the studies did not consistently screen for thyroid dysfunction before treatment, and ultrasensitive assays for determining the level of thyroid stimulating hormone, presumably the most accurate marker for thyroid dysfunction, were not available when the studies were conducted. Third, these trials included different types of depressed patients, and modern operationalized diagnostic criteria for depression were also not available; thus the samples may have been nonhomogeneous. On the other hand, it should be emphasized that these studies were homogeneous with respect to the type of antidepressant (all studies used tricyclic antidepressants, five of the six studies used imipramine), the dose of T3, and the rating scale used to measure clinical outcome (Hamilton depression scale). Fourth, the overall dose of antidepressants was low (100–200 mg/day), perhaps lower than what would currently be used as monotherapy for a tricyclic antidepressant.
Given the limitations of the quality of the studies included in this meta-analysis, definite conclusions about the efficacy of T3 as an accelerator of antidepressant response cannot be drawn. However, the results of this meta-analysis are strikingly positive and point to a need for further studies assessing the efficacy of T3 acceleration with the newer, more selective antidepressant agents. The studies conducted on T3 acceleration used tricyclic antidepressants. To our knowledge, no double-blind study has assessed the role of thyroid hormone in accelerating treatment response to SSRIs or other newer selective antidepressants. Thus, the generalizability of these findings to the ability of T3 to accelerate SSRI or other nontricyclic antidepressant response remains to be tested.
Studies to specifically assess the role of T3 in accelerating SSRI response are needed. Further studies may also shed light on whether there are gender-specific effects of T3 on accelerating antidepressant response. Any agent that has a low side effect profile and reduces the delay in onset of antidepressant response (thus reducing the duration of patients’ functional impairment) would be a welcome addition to the pharmacotherapeutic armamentarium.