In middle-aged and elderly men, depression and erectile dysfunction are common and frequently comorbid
(1–
3). The relationship between depression and erectile dysfunction appears to be bidirectional: the presence of or alteration in one of these conditions may be the cause, consequence, or modifier of the other
(2–
4). For example, in depressed men, erectile dysfunction may be a symptom of depression or a treatment-emergent side effect of antidepressant medication
(5). Alternatively, men with erectile dysfunction may develop a “secondary” depression as a reaction to the biopsychosocial stress commonly associated with loss of sexual functioning
(6).
In community samples of adult men, the lifetime prevalence of major depressive disorder is 6%–10%, and the point prevalence is approximately 3%
(7). Accumulating epidemiological and clinical data suggest that, among elderly men, milder depressive disorders such as dysthymia and minor depression are common
(8,
9), frequently misdiagnosed
(8), and associated with high morbidity
(9). Erectile dysfunction, defined as the inability to obtain and maintain an erection sufficient for satisfactory intercourse or other sexual expression
(10), affects up to one-half of men older than 50
(3). It is a para-aging phenomenon associated with poor health, smoking, diabetes, heart disease, hypertension, hyperlipidemia, and substance abuse
(3,
6). Accumulating data support a strong link between erectile dysfunction and depression
(2,
4,
11,
12).
Although erectile dysfunction and depression are highly comorbid, the causal relationship is unclear. There are five models, not mutually exclusive, that may be used to understand the coexistence of both conditions. First, the psychosocial distress that is invariably part of erectile dysfunction might stimulate the development of a “secondary” depressive illness in vulnerable individuals. Second, erectile dysfunction can be a symptom of depression: major depressive disorder is associated with decreased libido, diminished erectile function, and decreased sexual activity
(4). Furthermore, a subgroup of men with major depressive disorder develop a reversible loss of nocturnal penile tumescence
(13–
15), which suggests that depressive illness can interfere with erectile neurophysiology. Third, treatment with antidepressant medications might lead to erectile dysfunction, although loss of libido and delayed ejaculation appear to be more common side effects
(5). Fourth, a common factor (e.g., alcohol
[2], cardiovascular disease
[16], or hypogonadism
[17]) might be etiologically related to both conditions. Finally, depression and erectile dysfunction, both relatively common, might be coincidentally comorbid and etiologically unrelated.
Currently, oral sildenafil citrate is a first-line therapy for the majority of patients with erectile dysfunction. Sildenafil is a competitive inhibitor of cGMP-specific phosphodiesterase type 5, the predominant isozyme causing the breakdown of cGMP in the human corpus cavernosum. After sexual stimulation, neurogenically mediated release of nitric oxide induces the formation of cGMP by guanylate cyclase within the corpus cavernosum smooth muscle. Sildenafil amplifies the effect of sexual stimulation by retarding the degradation of cGMP by phosphodiesterase type 5. Sildenafil has been shown to be safe and effective in men with erectile dysfunction caused by diabetes, spinal cord injury, and treatments for prostate cancer
(18,
19). However, to our knowledge, no prospective studies to date have been conducted in men with erectile dysfunction and concomitant depression.
The efficacy, tolerability, and relative ease of use of sildenafil, coupled with data suggesting that depressed men respond less well to more invasive erectile dysfunction treatments
(12), raise two clinically important questions regarding the treatment of men with both depression and erectile dysfunction. First, does the presence of depression affect erectile dysfunction treatment response to sildenafil? Second, does effective treatment of erectile dysfunction affect comorbid depression and related quality-of-life symptoms? To address these questions, we conducted a double-blind, randomized, multicenter trial of sildenafil versus placebo in men with erectile dysfunction and untreated depressive illness.
Method
Study Participants
The study was conducted at 20 urologic clinics in the United States, each of which had a dual specialty team consisting of a urologist and a psychiatrist. Men who came to a clinic with a chief complaint of erectile dysfunction completed the Center for Epidemiologic Studies Depression (CES-D) Scale, a 20-item depression inventory. Those with CES-D Scale scores ≥16, the standard screening threshold for depressive illness, were offered an appointment with the study psychiatrist for a screening interview. At the psychiatric screening, the interviewer administered an abbreviated Structured Clinical Interview for DSM-IV Axis I Disorders (SCID)
(20) and the 24-item Hamilton Depression Rating Scale
(21). Men who met DSM-IV criteria for depressive disorder not otherwise specified and had a Hamilton depression scale score ≥12 underwent a complete physical examination, provided urine and blood for a baseline laboratory evaluation, and returned 4 weeks later for a second psychiatric interview. Subjects who continued to meet inclusion criteria were randomly assigned to treatment. Institutional review boards at each site reviewed and approved the protocol, and all men gave written informed consent.
Inclusion criteria were as follows: 1) male gender, age 18 years or older; 2) a stable relationship with a female partner for the 6-month period before the screening interview; 3) documented erectile dysfunction for at least the preceding 6 months (established by review of medical records); 4) diagnosis of depressive disorder not otherwise specified (as per DSM-IV criteria); and 5) 24-item Hamilton depression scale score ≥12 at the first and second screening interviews. Exclusion criteria were 1) presence of another current axis I psychiatric disorder, including substance abuse or dependence; 2) current use of nitrates (or nitric oxide donors) or any antidepressant medication; 3) abnormal serum hormone levels (i.e., prolactin, testosterone, or thyroid); or 4) history of major hematologic, renal, or hepatic abnormalities, poorly controlled diabetes, retinitis pigmentosa, spinal cord injury, or serious cardiovascular disease. Subjects with cardiovascular disease were not specifically excluded but were carefully considered for study entry because of the potential impact of resuming sexual activity and the mild and transient vasodilatory effects of sildenafil on blood pressure.
Study Design
This study was a 12-week, randomized, double-blind, placebo-controlled, flexible-dose trial. Subjects were randomly assigned to a 50-mg starting dose of sildenafil or matching placebo and were instructed to take the study drug approximately 1 hour before anticipated sexual activity but not more than once daily. Follow-up clinic visits were scheduled at 2, 4, 8, and 12 weeks. The investigator could adjust the dose of study drug to 100 mg or to 25 mg on the basis of efficacy and tolerability.
At baseline (week 0), week 8, and week 12 (or at the time of discontinuation), subjects were interviewed by the psychiatrist and completed self-report questionnaires. Assessments included the interviewer-rated Hamilton depression scale, the Clinical Global Impression (CGI) improvement scale
(22), and the following self-report instruments: the Beck Depression Inventory
(23), the International Index of Erectile Function
(24), the Life Satisfaction Checklist
(25), and two global efficacy questions: 1) Did treatment improve your erections? 2) Did treatment improve your ability to have sexual intercourse?
The International Index of Erectile Function is a well-validated questionnaire that has been used widely for the clinical assessment of erectile dysfunction and treatment outcomes in clinical trials
(18,
24). In particular, question 3 (“When you attempted intercourse, how often were you able to penetrate your partner?”) and question 4 (“During sexual intercourse, how often were you able to maintain your erection?”) directly address the National Institutes of Health’s consensus definition of erectile dysfunction
(10). Each question of the International Index of Erectile Function is scored on a categorical scale of 1 (“almost never/never”) to 5 (“almost always/always”), with a score of 0 indicating “did not attempt sexual intercourse.” The 15 questions of the International Index of Erectile Function can be partitioned into five sexual function domains: erectile function (six questions, including questions 3 and 4), orgasmic function (two questions), sexual desire (two questions), intercourse satisfaction (three questions), and overall satisfaction (two questions)
(24). The Life Satisfaction Checklist includes questions regarding sexual life and relationships, social relationships with family and friends, and satisfaction with leisure life, vocation, and finances
(25). Categorical responses range from 1 (“very dissatisfying”) to 6 (“very satisfying”).
At the time of this study, there was no standard criterion to define erectile dysfunction treatment response. Therefore, we developed conservative, a priori response criteria: treatment-responsive subjects were defined as study participants who answered “yes” to both global efficacy questions and had a score of ≥21 on the erectile function domain of the International Index of Erectile Function (range=0–30). An erectile function domain score above 21 represents minimal or no erectile dysfunction
(26). All men who completed at least one efficacy assessment were classified as responsive or nonresponsive to treatment, regardless of whether they received sildenafil or placebo.
Men were given a complete physical examination at the screening interview and at the end of the study (or at the time of discontinuation). During the study, all adverse effects observed by the investigator or reported by the study participants were assessed for severity and relationship to study medication.
Statistical Analysis
Intent-to-treat analyses were performed for all variables and included data from all men who received at least one dose of study medication and who had at least one efficacy assessment, regardless of protocol deviations or whether they completed the study. All statistical tests were two-sided, and all hypotheses were evaluated at the 5% significance level. Analyses of depression scale data and responses from the quality-of-life instruments were calculated by using analysis of covariance (ANCOVA) or logistic regression, as appropriate, with age, smoking status, erectile dysfunction duration, erectile dysfunction etiology, and baseline score as covariates. Least squares means were adjusted for the value of covariates. Adjusted means were determined for individual questions and domains of the International Index of Erectile Function with the ANCOVA model. Responses to the global efficacy questions were analyzed by logistic regression.
Results
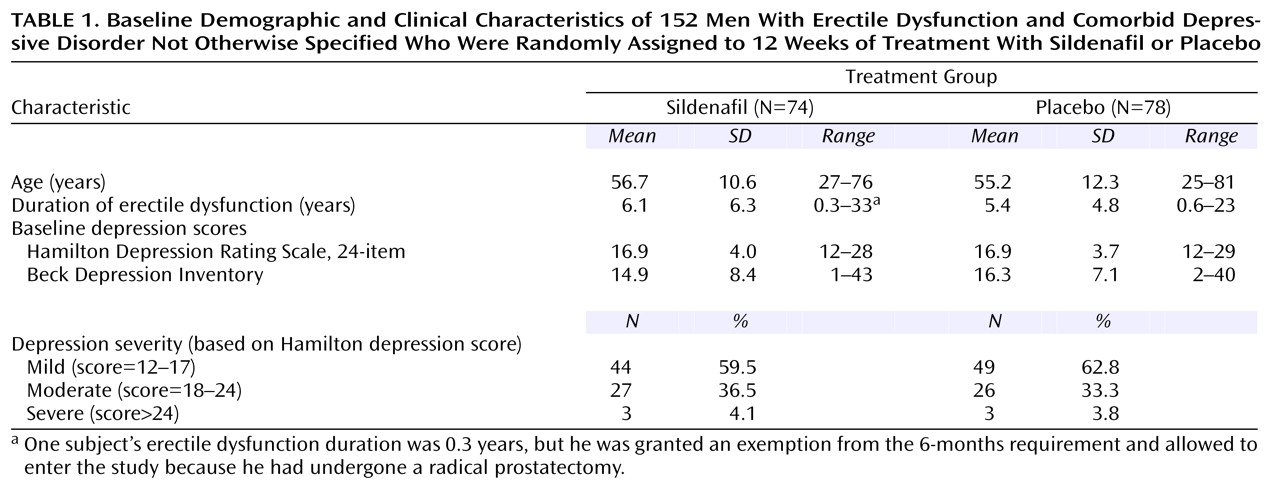
Of the 300 men who met CES-D Scale criteria (i.e., total score ≥16) and were screened for this study, 152 were randomly assigned to treatment (sildenafil: N=74; placebo: N=78) and received at least one dose of study drug. Of the 148 men who did not meet the entrance criteria, 91 did not have a depressive disorder, 11 had major depressive disorder, seven had dysthymia, 10 had hypogonadism (i.e., low testosterone level), seven withdrew consent, one was lost to follow-up, and 21 had other reasons for exclusion. The baseline characteristics of the men participating in the study were similar in the two treatment groups and are presented in
Table 1. The mean age of the study participants was 56 years (SD=11.5), and the mean duration of erectile dysfunction was 5.7 years (SD=5.6). Of the 152 subjects assigned to treatment, 125 (82.2%) completed the study (65 of 74 subjects [87.8%] given sildenafil and 60 of 78 subjects [76.9%] given placebo). Study discontinuation was not associated with baseline demographic or clinical features (data not shown). An analysis of data collected at week 8 produced results that were indistinguishable from the week 12 results (data not shown).
After 12 weeks of treatment or at the time of their last dose, 79.2% (N=57 of 72) of men continuing to receive sildenafil were receiving the 100-mg dose, 19.4% (N=14) were receiving the 50-mg dose, and one man had reduced his dose to 25 mg/day. Of the 75 men continuing to receive placebo, 97.3% (N=73) were receiving the 100-mg equivalent tablet. The median duration of treatment was comparable in both groups (sildenafil: 84 days; placebo: 81 days), as was the average number of doses taken per week (sildenafil: 3.6; placebo: 3.0).
Efficacy
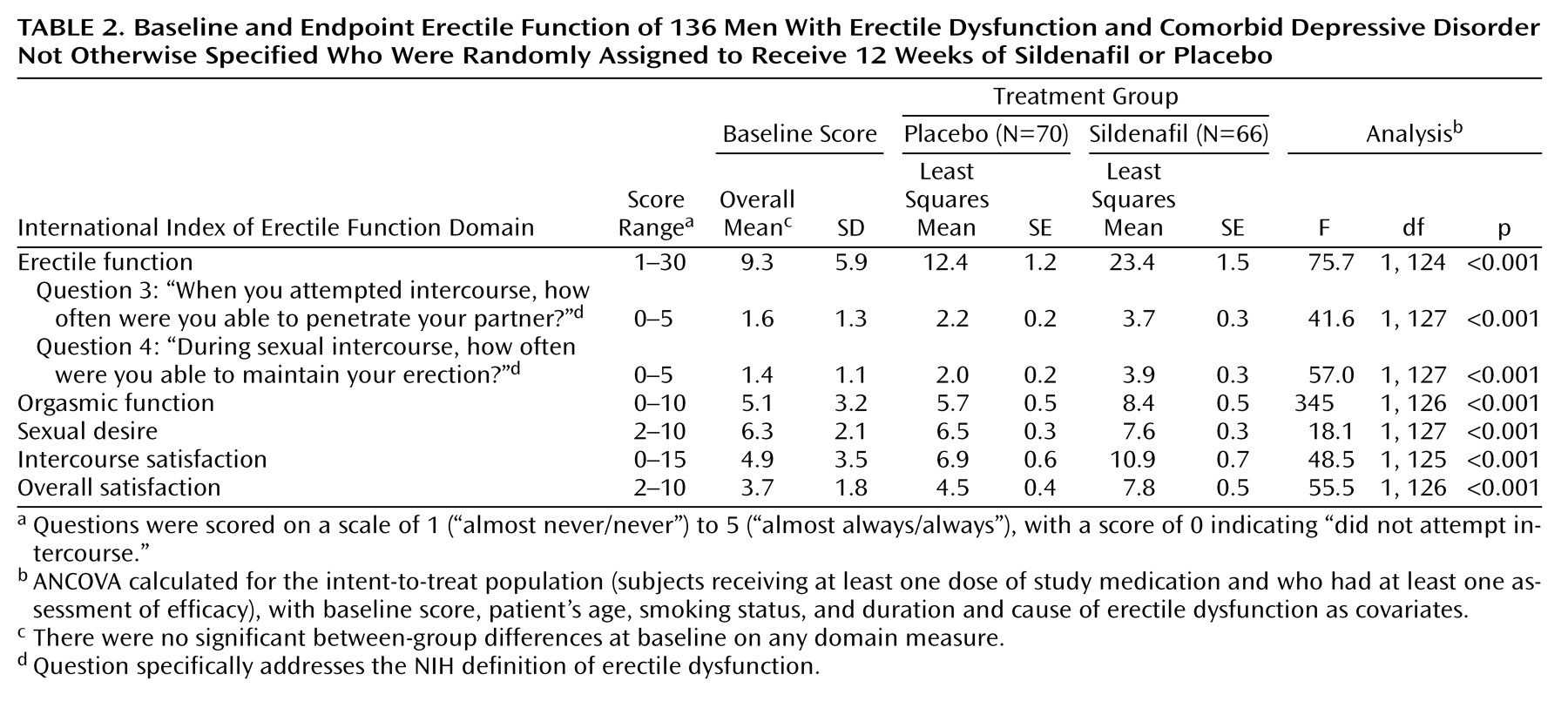
Of the 152 men who received study medication, 136 had at least one postrandomization assessment. Mean scores for International Index of Erectile Function questions concerning the ability to achieve (question 3) and maintain (question 4) erections were significantly higher (indicating better erectile function) in men receiving sildenafil than in men receiving placebo (
Table 2). In response to the two global efficacy questions, 90.9% (N=60 of 66) and 89.4% (N=59) of the men receiving sildenafil reported that treatment had improved their erections and their ability to have sexual intercourse, respectively, compared with 11.4% (N=8 of 70) and 12.9% (N=9) of men receiving placebo. Subjects in the sildenafil group demonstrated significantly greater improvement in each of the five domains of the International Index of Erectile Function than did the placebo group (
Table 2). For example, the least squares mean scores for the erectile function domain increased 152% from baseline among men receiving sildenafil compared with a 33% increase among men receiving placebo. Mean scores for the other sexual function domains had comparable increases, although increases in scores for the sexual desire domain were relatively small.
Of the 136 men assessed for efficacy, 58 (42.6%) were classified as treatment-responsive and 78 (57.4%) as treatment-nonresponsive. There were no between-group differences in baseline demographic or clinical features (data not shown). Of the subjects who received sildenafil, 72.7% (N=48 of 66) were classified as responders; of the subjects who received placebo, 14.3% (N=10 of 70) were classified as responders.
Depressive Symptoms
Change in erectile dysfunction was highly correlated with change in depressive symptoms: there was a significant correlation between changes in International Index of Erectile Function scores reflecting erectile function (i.e., question 3, question 4, and the erectile function domain) and changes in Hamilton depression scale scores, irrespective of treatment received. For example, changes in erectile function domain scores were significantly correlated with changes in Hamilton depression scale scores for all men (r=–0.65, df=138, p<0.0001), among men who received sildenafil (r=–0.46, df=65, p=0.0002), and among men who received placebo (r=–0.58, df=71, p<0.0001).
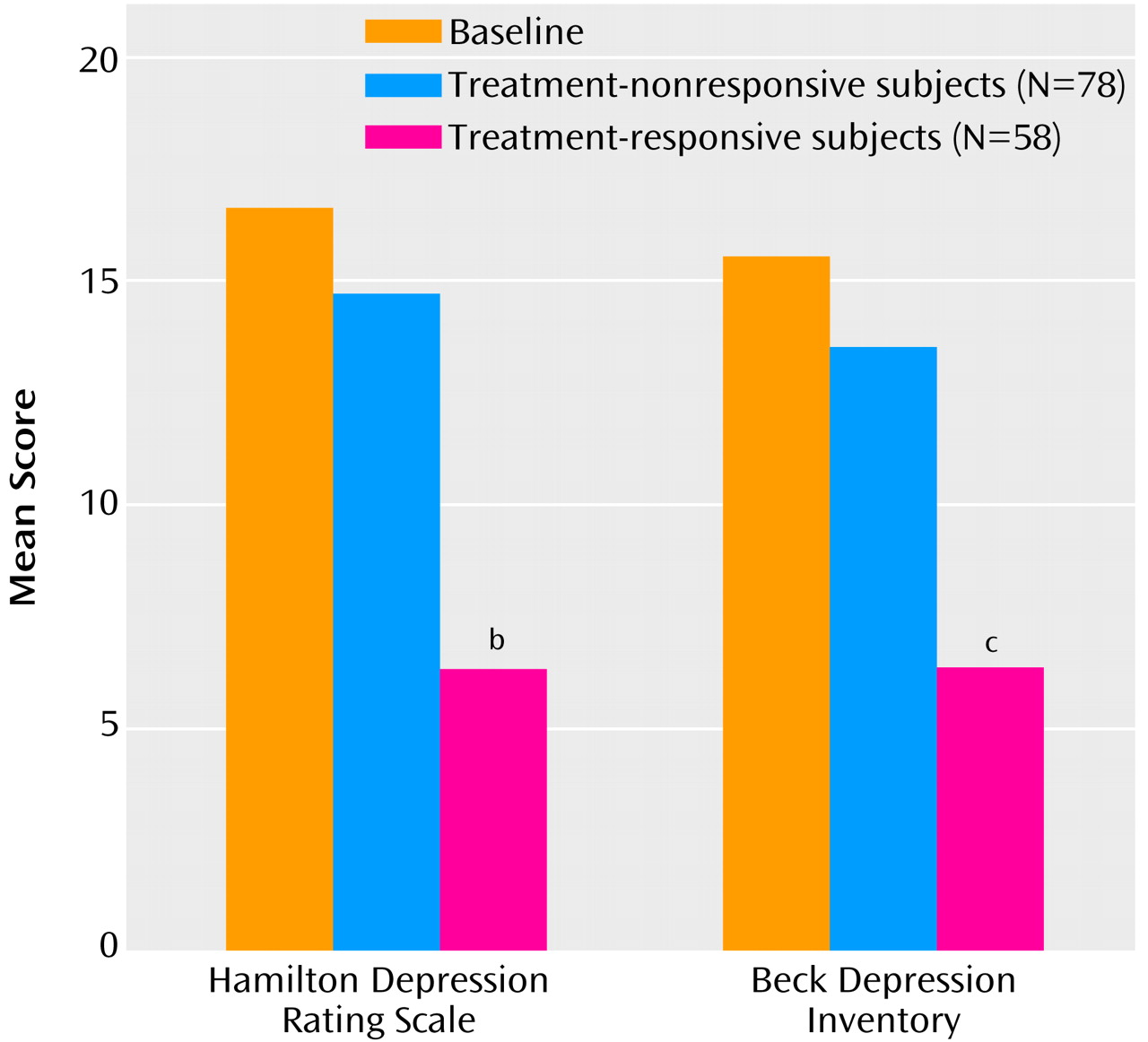
There was a significantly greater decrease in mean Hamilton depression scale score (10.6) and mean Beck Depression Inventory score (10.7) among erectile dysfunction treatment-responsive subjects than in subjects who did not respond (2.3 and 3.7, respectively) (
Figure 1). The CGI response criterion for depression (i.e., 12-week CGI improvement score of 1 or 2, signifying “much” or “very much” global improvement in depression) was met by 82.8% (N=48 of 58) of treatment-responsive subjects and by 7.7% of (N=6 of 78) nonresponsive subjects. Hamilton depression scale depression response criteria (i.e., ≥50% decrease in score from baseline) were met by 75.9% (N=44 of 58) of treatment-responsive subjects and by 14.1% (N=11 of 78) of nonresponsive subjects (p<0.001, Fisher’s exact test).
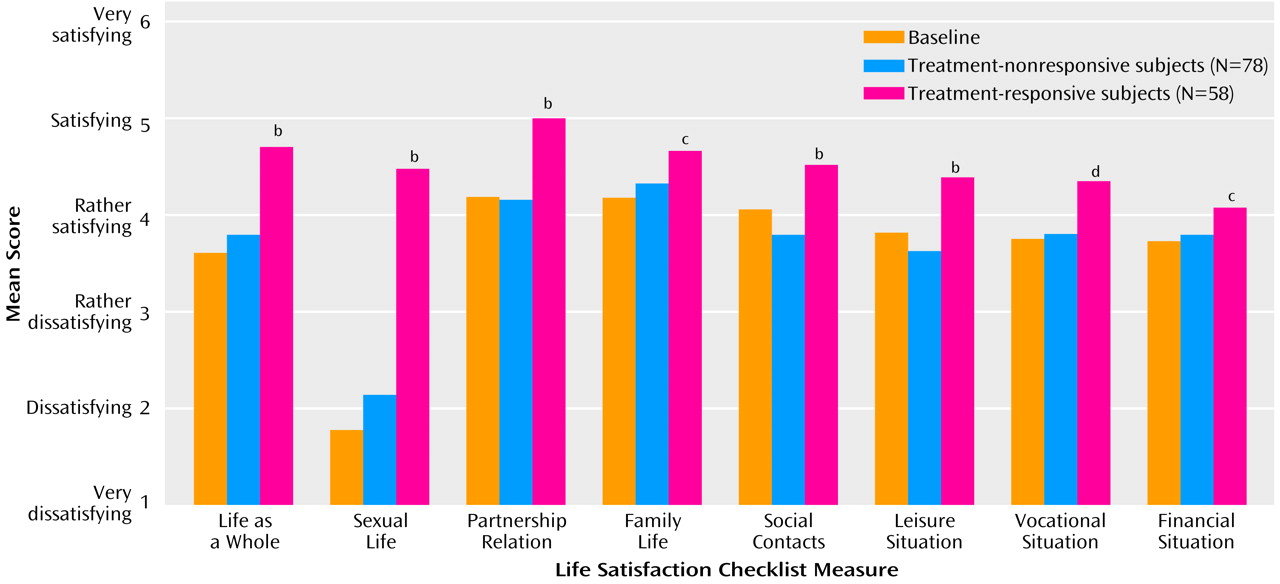
Quality of Life
On the Life Satisfaction Checklist, multiple quality-of-life measures were significantly improved among the treatment-responsive subjects compared to those whose erectile dysfunction did not respond to treatment (
Figure 2).
Adverse Effects of Treatment
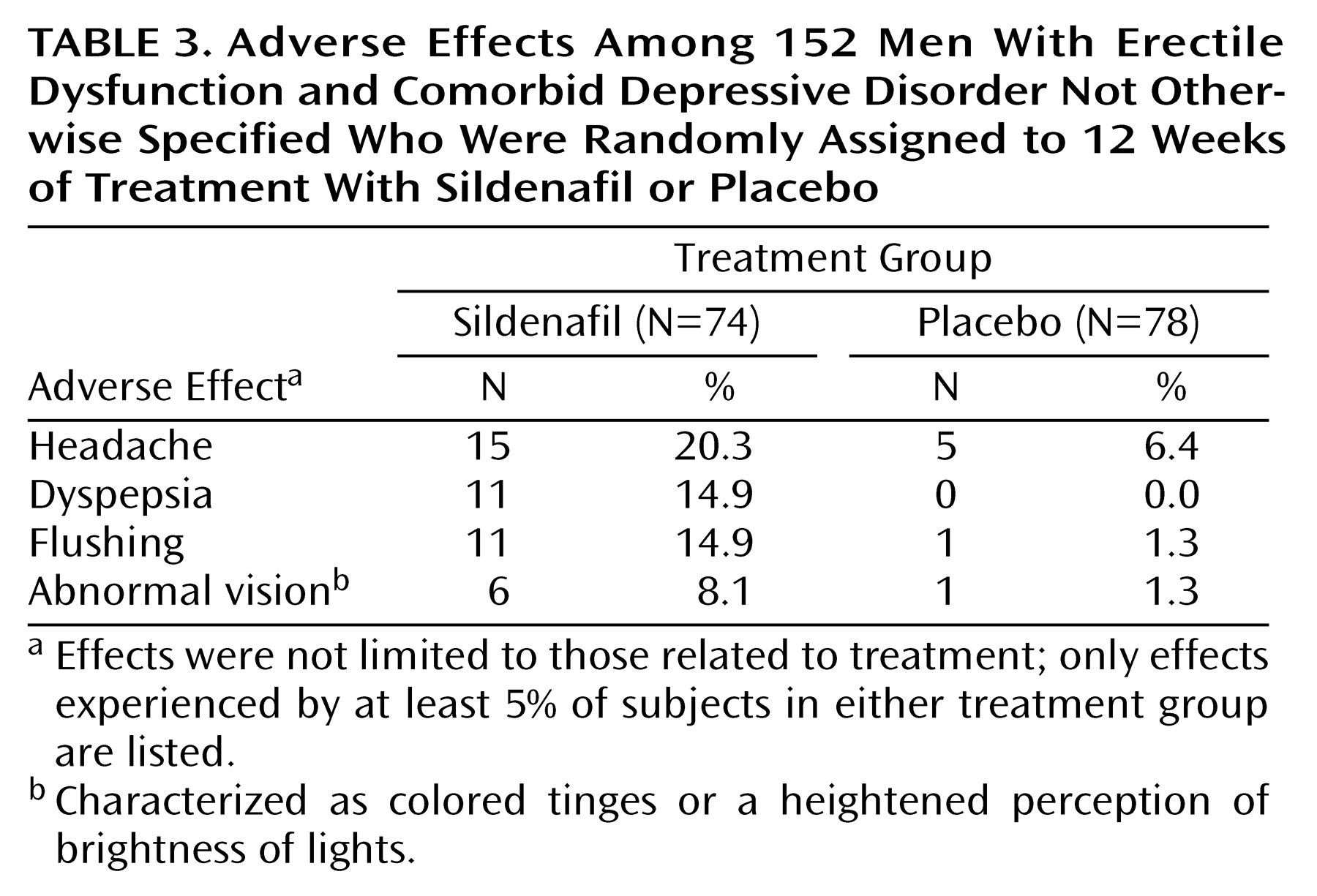
The most commonly reported adverse effects among subjects taking sildenafil compared with subjects given placebo are shown in
Table 3. Adverse effects related to treatment were reported in 35 of 74 subjects (47.3%) taking sildenafil and in 10 of 78 subjects (12.8%) given placebo. Nearly all of these adverse effects were transient and mild to moderate in nature. Of five severe adverse effects (intestinal obstruction, coronary artery disorder, pneumonia, flu syndrome, and headache) only the headache (experienced while the subject was receiving 100 mg of sildenafil) was considered to be treatment related.
Discussion
Depression and erectile dysfunction are highly prevalent and frequently comorbid conditions in middle-aged and elderly men. To our knowledge, this is the first study to specifically evaluate the effects of sildenafil in men with both erectile dysfunction and untreated comorbid depression as well as the effect of improved erectile function on depressive symptoms and quality of life. Overall, treatment with sildenafil in depressed men with erectile dysfunction led to marked improvement in erectile function. Furthermore, subjects classified as treatment responders, regardless of treatment received, showed a clinically significant improvement in depressive symptoms and quality-of-life measures compared with subjects whose erectile dysfunction did not respond to treatment. Indeed, the magnitude of improvement observed in treatment-responsive subjects in this trial was comparable to that commonly observed in clinical trials of either drug or nondrug interventions for major depressive disorder
(4). However, these results should not be interpreted to mean that sildenafil can be used as a primary treatment for depression.
How do the effects of sildenafil in this population compare with other erectile dysfunction subgroups? The efficacy of sildenafil has been demonstrated in large-scale placebo-controlled clinical trials
(18). The magnitude of difference between those receiving sildenafil and those given placebo in the number responding “yes” to global efficacy question 1 (“Did treatment improve your erections?”) ranged from 46% in diabetic men to 72% in men with spinal cord injuries. Results from the present study suggest that sildenafil may be as, or more, effective for the treatment of erectile dysfunction in mildly depressed men: 90.9% of subjects receiving sildenafil answered “yes” to global efficacy question 1 compared to 11.4% of subjects receiving placebo, a difference of 79.5%. Similarly, the magnitude of quality-of-life changes observed in this study was greater than that observed in previous sildenafil trials. Although erectile dysfunction inclusion and exclusion criteria in this study were identical to those of the aforementioned sildenafil efficacy studies, direct comparisons need to be made cautiously in view of potential confounding factors such as different recruitment sites and procedures and presence of other medical comorbidities. Nevertheless, results of this study suggest that men with erectile dysfunction and comorbid depression may be among those most likely to respond positively to treatment with sildenafil.
The major methodological limitation of this study concerns the diagnostic criteria used for defining depression. Two dimensions are generally considered when establishing inclusion criteria for patients with depressive phenomenology: DSM diagnosis and severity. We considered using the standard inclusion criteria for clinical trials of antidepressant treatments, i.e., DSM-IV criteria for major depressive disorder and a minimum Hamilton depression scale score. Although this approach would have better defined the psychiatric status of the clinical cohort, it would not have included the majority of men with erectile dysfunction who have significant comorbid depressive symptoms. We chose depressive disorder not otherwise specified instead, since this is the DSM-IV diagnosis that best captures the range of depressed men with erectile dysfunction generally seen in medical and urologic clinics. We chose also to use a minimum severity score (i.e., Hamilton depression scale ≥12) to ensure inclusion of men with significant depressive symptoms. Although we considered including subjects with erectile dysfunction and major depressive disorder, we decided that it was ethically appropriate in this first trial to exclude men with major depressive disorder, since no antidepressant treatment of established efficacy would be provided for the 16-week study duration. Despite this exclusion, the mean baseline Hamilton depression scale score of 16.9 indicates that subjects enrolled in this study had a level of depressive severity comparable to patients enrolled in clinical trials of mild major depressive disorder and dysthymia. Since this study demonstrated that effective erectile dysfunction treatment has a positive effect on mood and quality of life in depressed men seeking treatment for erectile dysfunction, further studies of this type in men with erectile dysfunction and comorbid major depressive disorder are warranted.
Sildenafil offered a unique opportunity to study “secondary” depression, i.e., depression associated with a specific medical disorder. Although it is generally assumed that resolution of an associated medical condition will lead to remission of the “secondary” depression, this hypothesis has not been amenable to systematic study, since secondary depression usually occurs in the setting of chronic or unremitting medical illnesses. The efficacy of sildenafil in the treatment of erectile dysfunction offered the opportunity to systematically “remove” erectile dysfunction in some men and therefore test the hypothesis of secondary depression. The results from this study demonstrate that successful treatment of this medical condition in depressed men is associated with depressive remission: substantially more treatment-responsive subjects demonstrated a ≥50% reduction in Hamilton depression scale score (76%) or CGI improvement score of 1 or 2 (82.8%) than did nonresponsive subjects (14.1% and 9.0%, respectively).
Several explanations can be offered for the strong association observed between improvement in erectile dysfunction and related improvements in mood and quality of life. First, it is possible that sildenafil itself has direct mood-enhancing properties. This is highly unlikely, since the drug has no known central effects and was administered relatively infrequently. Second, it is possible that erectile dysfunction was a symptom of depression and that spontaneous depressive remission led to a positive erectile dysfunction response in some men. If true, this would only be detectable in the placebo group. However, the 14% placebo response for erectile dysfunction—even smaller than is usual in erectile dysfunction trials—would have been expected to be much higher if this were a common pathway. Finally, it is possible that nonresponse to erectile dysfunction treatment is the “active” agent in these relations as a marker of poor prognosis for depression. Indeed, erectile dysfunction appears to be a sentinel for subclinical cardiovascular disease
(16), and erectile dysfunction nonresponse may be a marker of atherosclerotic severity. In depressed men, atherosclerotic disease may have a negative impact on major depressive disorder prognosis. In this context, it would be informative to evaluate the efficacy of conventional antidepressants in subjects who do not respond to erectile dysfunction treatment.
Results from this study suggest that successful treatment of erectile dysfunction in depressed men can lead to marked improvement in depression. However, this was a single study conducted over 12 weeks in men with erectile dysfunction and mild-to-moderate depression. Whether improvement in depressive symptoms will be maintained over a longer period of time or would occur at all in patients with major depressive disorder is not known. In the present study, subjects came to urologists with a chief complaint of erectile dysfunction; those receiving psychotropic medications were excluded. Notably, a recent placebo-controlled trial that assessed the effects of sildenafil in men with remitted depression who were taking selective serotonin reuptake inhibitors demonstrated that sildenafil is effective for treatment of erectile dysfunction in such men
(27). Further systematic, placebo-controlled studies are needed to evaluate the effects of sildenafil therapy in combination with conventional pharmacologic and nonpharmacologic antidepressant treatments on multiple sexual function domains.
Acknowledgments
This study was conducted at 20 urologic clinics by the following urologist/psychiatrist teams: Ridwan Shabsigh, M.D./Stuart N. Seidman, M.D., (Columbia-Presbyterian Medical Center, New York); Laurence Levine, M.D./John Zajecka, M.D. (Rush-Presbyterian/St. Luke’s Medical Center, Chicago); Arnold Belker, M.D./Barbara Kennedy, M.D. (University of Louisville School of Medicine, Louisville); Robert Weiss, M.D./Matthew Menza, M.D. (Robert Wood Johnson Medical School, New Brunswick, N.J.); Michael O’Leary, M.D./Maurizio Fava, M.D. (Brigham and Women’s Hospital, Boston); Drogo Montague, M.D./George Tesar, M.D. (The Cleveland Clinic, Cleveland); Richard Berger, M.D./David Dunner, M.D. (University of Washington, Seattle); Jacob Rajfer, M.D./Jambur Ananth, M.D. (Harbor/UCLA Medical Center, Torrance, Calif.); Irwin Goldstein, M.D./Isidore Berenbaum, M.D. (Boston Medical Center, Boston); Craig Donatucci, M.D./Leslie Forman, M.D. (Duke University Medical Center, Durham, N.C.); Francois Eid, M.D./James Kocsis, M.D. (Cornell Medical Center, New York); Stanley Bloom, M.D./Hilda B. Templeton, M.D. (Physicians in Urology, P.A., Livingston, N.J.); Harin Padma-Nathan, M.D./Joshua Golden, M.D. (The Male Clinic, Santa Monica, Calif.); Hunter Wessells, M.D./Alan Gelenberg, M.D. (University of Arizona Health Sciences Center, Tucson); Ira Klimberg, M.D./Domingo Cerra, M.D. (The Urology Center of Florida, Ocala); Allen Seftel, M.D./Stephen Levine (University Hospitals of Cleveland, Cleveland); Arnold Melman, M.D./Bruce Schwartz, M.D. (Montefiore Medical Center, Bronx, N.Y.); James McMurray, M.D./Edward R. Green, M.D. (Medical Affiliated Research Center, Huntsville, Ala.); Culley Carson III, M.D./Brad Gaynes, M.D. (University of North Carolina, Chapel Hill, N.C.); Ira Sharlip, M.D./Joe Walker, M.D. (Pan Pacific Research, San Francisco).