Postpartum major depression occurs in approximately 10% of childbearing women
(1) and is associated with significant morbidity in mothers and their children
(2).
For many women, the optimum treatment may be an antidepressant alone or in addition to psychotherapy. However, the decision to institute antidepressant therapy is often complicated by the mother’s desire to breast-feed. All selective serotonin reuptake inhibitors (SSRIs) are categorized by the Food and Drug Administration as category C drugs (“benefits must outweigh risks”) for pregnancy. Classification regarding the safety of SSRIs in breast-feeding varies from “safe” to “unknown” in the numerous pharmacology compendia online and in print. Therefore, women and their health care providers are presented with a difficult choice: whether to breast-feed and expose the infant to antidepressant medication or to wean and deprive the infant of the proven benefits of breast milk. On the basis of recent estimates of the percentage of women breast-feeding at the time of discharge from the hospital (59%)
(3), the rate of postpartum depression (10%)
(1), and the birth rate in the United States (approximately 4 million per year)
(4), it is estimated that each year in the United States alone approximately a quarter of a million women may consider nursing while receiving antidepressant medication.
Method
Subjects
The 19 breast-feeding mothers and infants who took part in the study were referred to the Yale Postpartum Mood Disorders Clinic by their psychiatrist, obstetrician-gynecologist, or midwife for the evaluation and treatment of postpartum depression or for consultation regarding the use of antidepressants during lactation. In all cases, the women were counseled regarding the known risks and benefits of receiving antidepressants during lactation; nonpharmacologic treatments for their depression were also discussed. All of the mothers were married or in a stable cohabiting relationship. Each mother’s significant other had knowledge of her decision to breast-feed while taking sertraline, and a concerted effort was made to include each partner in this discussion. The women were instructed to inform their infant’s pediatrician of their decision to take sertraline during lactation. Written informed consent was obtained from the mothers (mean age=32.2 years, SD=4.5) who agreed to participate in this study, which was approved by the Human Investigation Committee of Yale University School of Medicine.
Study Group
Before the mothers began taking sertraline, 19 breast-feeding mothers and their infants had blood drawn for determination of whole blood 5-HT levels. The mothers of four infants decided soon after the preexposure blood sampling to wean their infants or to abstain from taking medication out of concern that even low levels of sertraline might harm their infants. When the mothers decided not to take medication, they were counseled regarding the risks of untreated maternal depression and offered a referral for appropriate nonpharmacologic treatment. A fifth mother started sertraline treatment but was determined to be noncompliant on the basis of an absence of plasma sertraline. All 19 preexposure infant blood samples were included in an analysis of possible age-related changes in whole blood 5-HT levels over the first year of life.
Thirteen of the remaining mothers then began taking sertraline, 50 mg/day, while one began taking 25 mg/day. Each mother was instructed to breast-feed her infant according to their preferred schedule. Postexposure blood sampling occurred 6 to 16 weeks after the mothers had started taking sertraline. The timing of postexposure testing was dictated by whether the mother’s clinical status required an increase in her sertraline dose. All postexposure testing occurred at least 10 days after a change in sertraline dose in order to allow maternal plasma sertraline concentrations to reach a steady state. All blood samples were obtained between 10:00 a.m. and 2:00 p.m., approximately 4–8 hours after the mothers had taken their daily sertraline dose and 2–4 hours after they had last nursed their infants.
At the mothers’ pre- and postexposure blood draws, approximately 5 ml of blood was collected into a tube containing dipotassium EDTA by means of standard venipuncture techniques. At the infants’ preexposure blood draws, approximately 150 μl of blood was obtained with a heel or finger stick by means of a lancet with a 2.2-mm puncture depth. Blood samples were collected into capillary tubes containing dipotassium EDTA and transferred to an associated capped vial. Three ml of blood was obtained for the infants’ postexposure 5-HT and drug level determinations by using a 23-gauge winged infusion set and collected into a tube containing dipotassium EDTA. Blood was kept at room temperature and immediately transported to the research laboratory in which duplicate or triplicate whole blood samples were removed for storage at –70°C; portions were also sent for automated platelet count (Hematology Laboratory, Yale–New Haven Hospital, New Haven, Conn.).
5-HT and Sertraline Concentrations
Whole blood 5-HT levels were determined by means of high-performance liquid chromatography with fluorometric detection
(56). Blood was prepared as previously described: antioxidants and the internal standard
N-methylserotonin were added to the samples while they were thawing, perchloric acid was added after thawing, and the sample was vigorously vortex mixed. After centrifugation, the supernate was directly injected into the high-performance liquid chromatography system. Specimens from given mother-infant pairs were always examined in a single batch. Whole blood 5-HT levels were determined for 250-μl samples obtained by venipuncture with within-day and day-to-day coefficients of variation of 3.0%–4.6% and 3.0%–5.9%, respectively. In replicate heel stick or finger stick blood specimens (of 25 μl), 5-HT levels were determined with within-day coefficients of variation of 1.2%–8.8%. In a comparison of venipuncture and finger stick blood sampling in three adult comparison subjects, similar results were seen (mean absolute difference=3.5%, group mean difference=0.4%). It should be noted that since more than 99% of blood 5-HT is found within the platelets, whole blood 5-HT concentrations (ng/ml) reflect platelet levels and can also be expressed on a per-platelet (ng/10
9 platelets) basis
(56).
Plasma sertraline and desmethylsertraline levels were determined by using reversed-phase, high-performance liquid chromatography employing solvent extraction and micro back-extraction. After addition of the internal standard, an aliquot of plasma was alkalinized and extracted with hexane containing 2% isoamyl alcohol. The solvent was separated and back-extracted with 0.1 ml of 0.1 M hydrochloric acid. After centrifugation, the aqueous phase was submitted to high-performance liquid chromatography on a C18 reversed-phase column and eluted with a pH 4.5 phosphate-buffered mobile phase containing 33% acetonitrile and 2% triethylamine. Primary standards in plasma and comparison samples were carried through the entire procedure. Imipramine was found to be superior to a sertraline analog obtained from the manufacturer as an internal standard. Within-day and day-to-day coefficients of variation were, respectively, 4% and 8% at 25 ng/ml and 7% and 8% at 5 ng/ml for sertraline and 6% and 11% at 25 ng/ml and 10% and 17% at 5 ng/ml for desmethylsertraline. The limit of quantitation for sertraline was defined as the concentration of the lowest standard (2.5 ng/ml); this concentration was at or near the limit of detection in biological fluids. A similarly defined limit of quantitation for desmethylsertraline was 5 ng/ml. Although certain data analyses were performed with the observed metabolite concentrations, drug and metabolite plasma levels were not summed owing to the substantially lower reported activity of desmethylsertraline
(57).
Statistical Analysis
To compare changes in pre- and postexposure 5-HT levels between mothers and infants, a mixed-effects repeated measures analysis with unstructured variance-covariance was performed by using PROC MIXED in SAS
(58). A cluster in that analysis consisted of the pre- and postmeasurements for a mother-infant pair. This design allowed simultaneous tests of mother and infant effects, of pre- and posteffects, and of the interaction between time (pre- and postexposure) and group (mother and infant). A significant interaction meant that the change in pre- versus postexposure 5-HT levels was significantly different in mothers compared to the change in infants. Individual comparisons could then be performed for mothers and for infants to test whether the mean change was significantly different from zero in either group. Pearson’s correlations were performed to explore possible relationships between the variables of interest.
Results
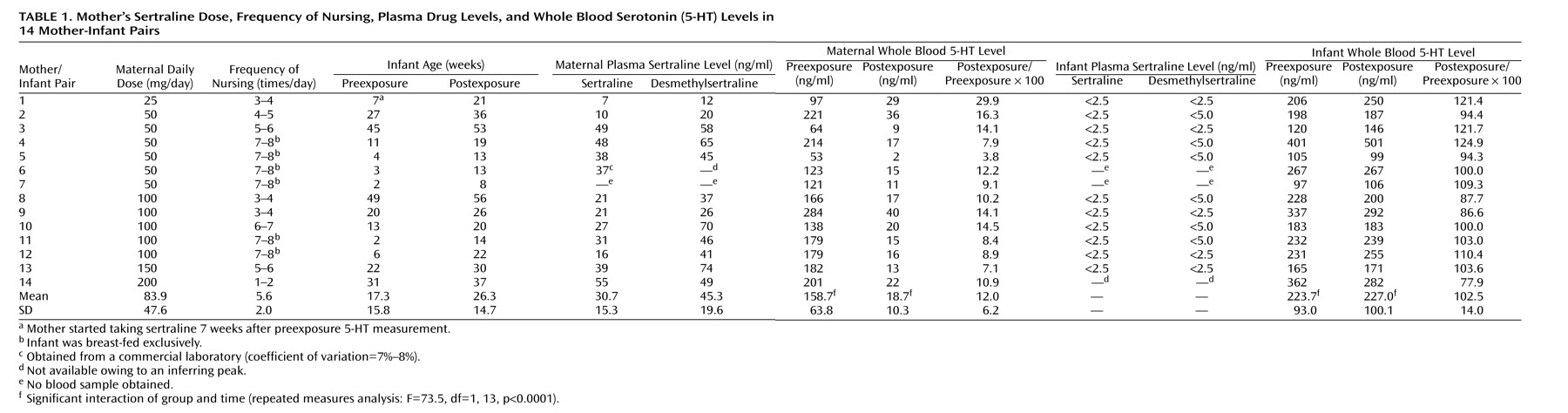
Exposure data for 14 mother-infant pairs, including the mother’s dose of sertraline, the frequency of breast-feeding, the infant’s age at pre- and postexposure sampling, and maternal and infant plasma sertraline and desmethylsertraline levels are given in
Table 1. In all but one case, the mothers began taking sertraline within 24 hours of the preexposure blood sampling. One mother (number 1) did not begin taking sertraline until 7 weeks after the preexposure blood sampling. At the time of postexposure sampling, the mothers were receiving 25 mg (N=1), 50 mg (N=6), 100 mg (N=5), 150 mg (N=1), and 200 mg (N=1) of sertraline per day. The mean ages for the infants at the times of preexposure and postexposure sampling were 17.3 weeks (SD=15.8) and 26.3 weeks (SD=14.7), respectively. Six infants (numbers 4–7, 11, 12) were less than 3 months of age and exclusively breast-fed when their mothers began taking sertraline. These infants remained exclusively breast-fed throughout the course of the study. No infant experienced any adverse effects or behavioral changes that appeared to be related to sertraline exposure.
Plasma sertraline and desmethylsertraline levels were obtained for 13 of 14 mothers and 11 of 14 infants. Mean maternal plasma concentrations of sertraline and desmethylsertraline were 30.7 ng/ml (SD=15.3) and 45.3 ng/ml (SD=19.6), respectively. All 11 infants had plasma sertraline and desmethylsertraline levels that were <2.5 ng/ml and <5.0 ng/ml, respectively. Desmethylsertraline and sertraline levels in the mothers were highly intercorrelated (Pearson’s r=0.72, N=12, p=0.008).
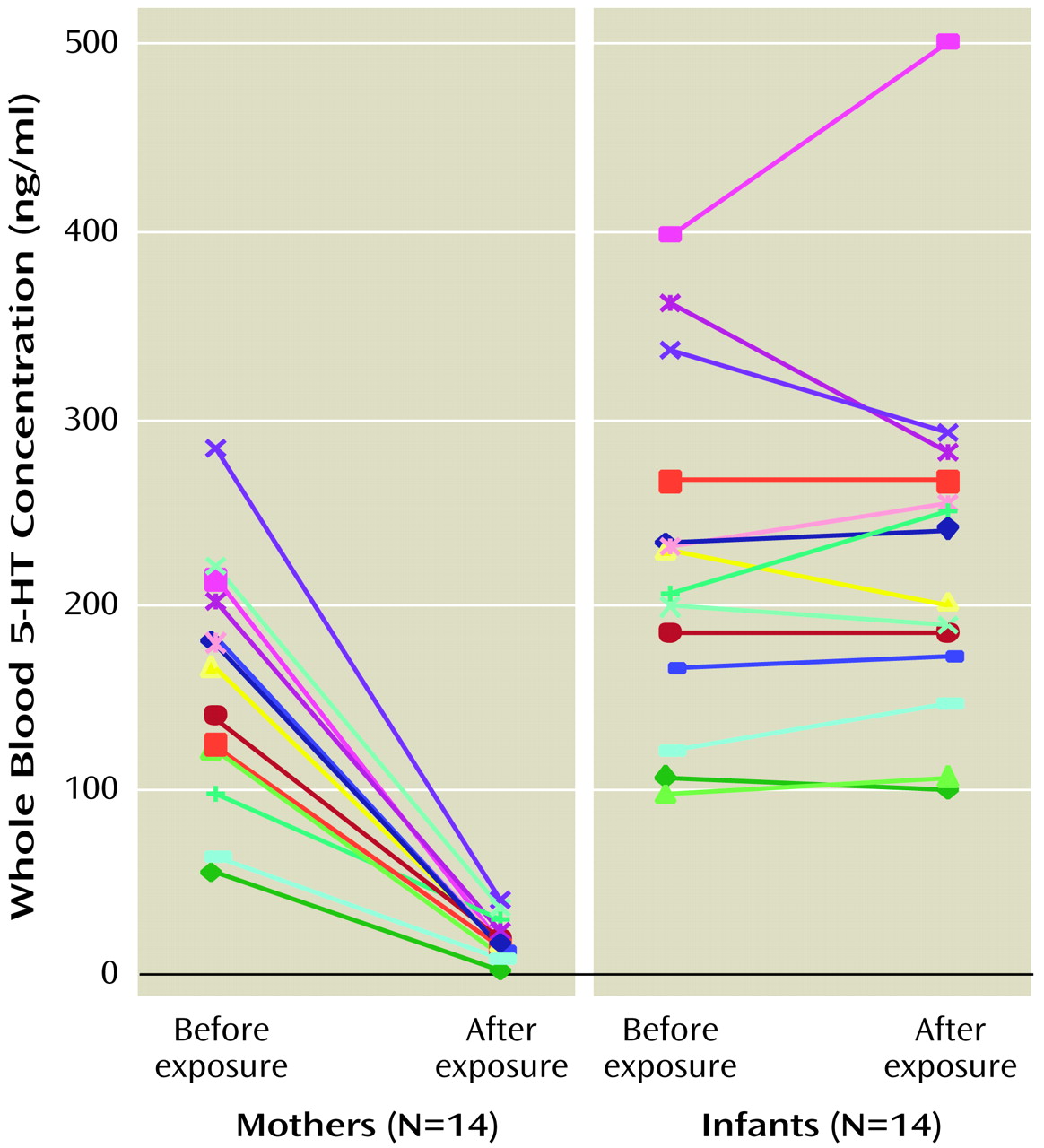
Mean maternal pre- and postexposure 5-HT levels were 158.7 ng/ml (SD=63.8) and 18.7 ng/ml (SD=10.3), respectively (
Table 1), whereas the mean infant postexposure 5-HT concentration was similar to the mean preexposure concentration (mean=227.0 ng/ml, SD=100.1, versus mean=223.7 ng/ml, SD=93.0). Repeated measures analysis using PROC MIXED
(58) with two within-cluster factors of group (mother versus infant) and time (pre- versus postexposure) revealed a significant decrease in maternal whole blood 5-HT levels (F=84.2, df=1, 13, p<0.0001), no significant change in infant whole blood 5-HT levels (F=0.1, df=1, 13, p=0.77), and a significant interaction of group and time (F=73.5, df=1, 13, p<0.0001) (
Figure 1).
Individual declines in maternal 5-HT levels ranged from 70% to 96% (postexposure levels were 30% to 4% of baseline). Intraindividual changes in infant platelet 5-HT levels ranged from –22% to +25%; the absolute pre- to postexposure mean change was 11% (SD=9). Given the coefficient of variation for the analytical determination and the expected biological variability, the mean absolute change in the infant group is reasonable. Similar group mean changes were seen in both maternal and infant groups when the data were expressed as ng/109 platelets. There were no significant changes in platelet counts between pre- and postexposure samples in either the mother (t=0.49, df=13, p=0.63) or infant (t=–0.62, df=13, p=0.55) groups. When the relationship between infant ages and baseline 5-HT levels was examined in the 19 infants who had preexposure 5-HT level determinations, no correlation was seen (Pearson’s r=–0.008, N=19, p=0.83).
Exploratory analysis of the data revealed few substantial correlations between the variables of infant age, frequency of nursing, length of exposure, maternal dose, maternal and infant plasma sertraline and desmethylsertraline levels, and maternal and infant postexposure 5-HT expressed as a percentage of 5-HT (postexposure/preexposure 5-HT). The correlative analyses were not corrected for the number (N=40) of correlations performed. Of the observed correlations, only those between infant age and frequency of nursing (r=–0.73, N=14, p=0.003) and between maternal plasma sertraline and desmethylsertraline concentrations (r=0.72, N=12, p=0.008) were significant with any degree of Bonferroni correction.
To follow up on a possible relationship between maternal sertraline dose and infant postexposure/preexposure 5-HT ratio (Pearson’s r=0.58, N=14, uncorrected p=0.03), the infants were dichotomized (N=7 and N=7) on the basis of maternal sertraline dose (25 or 50 mg/day versus ≥100 mg/day). A biserial Pearson’s correlation revealed a correlation (r=–0.50, N=14, p=0.07) with mean infant postexposure/preexposure 5-HT ratio of 9.4% (SD=13) and –4.4% (SD=12) in the lower dose and higher dose groups, respectively. A Student’s t test found no significant between-group difference in mean postexposure/preexposure 5-HT ratio (t=2.06, df=12, p=0.06). In addition, when the infants were dichotomized on the basis of those who were exclusively breast-fed (N=6), the postexposure/preexposure 5-HT ratio for the exclusively breast-fed infants (mean=107, SD=11) was similar to that of the eight infants who were receiving additional forms of nutrition (mean=100, SD=16).
Discussion
As expected, consistent marked declines in maternal platelet 5-HT concentrations were seen in the mothers treated with the SSRI sertraline. The observed individual reductions of 70%–96% were in the range typically seen
(46–
52). In contrast, mean infant platelet 5-HT levels showed little change (2.5%) after exposure to sertraline through breast milk; changes in individual infants ranged from –22% to +25%. There was some suggestion that the infants of the mothers receiving sertraline doses of 100 mg or more per day may have been more likely to show some decrease in platelet 5-HT levels. However, the absence of a relationship between maternal plasma sertraline levels and infant change in 5-HT with exposure and the small magnitude of the change (–4.4%) seen in the higher dose group indicates that this effect was negligible. In addition, our analysis of the infants who would be considered to have received the greatest sertraline exposure on the basis of the frequency of breast-feeding and their age at the start of the study (those who were exclusively breast-fed and youngest) actually had a slightly higher mean postexposure 5-HT level (expressed as percentage of preexposure level) than the other infants. (All but one of the exclusively breast-fed infants was 6 weeks old or younger at the start of the study; this is a period of relative immaturity of hepatic metabolism
[59] and renal drug excretion
[60].)
Thus, it appears that at typical clinical doses, sertraline administered to mothers usually has a negligible effect on platelet 5-HT transport even in young, exclusively breast-fed infants. The usual absence of a discernible effect on infant platelet 5-HT transport is consistent with the relatively low levels of sertraline observed in infant plasma. However, it should be noted that the maximum decline in platelet 5-HT of –22% seen in one infant (number 14) for whom we could not obtain a drug level is conceivably consistent with the drug effect caused by a 2–3 ng/ml infant plasma sertraline level (compare this 22% reduction in platelet 5-HT to the 70% decline in mother number 1, who had a plasma drug level of 7 ng/ml).
Several aspects of using platelet 5-HT levels as a measure of 5-HT transporter inhibition deserve comment. First, based on the absence of an interindividual correlation between age and 5-HT level, it is doubtful that much age-related intraindividual drift occurred in infant 5-HT levels over the 6–14-week period between pre- and postexposure sampling. Second, although 5-HT is taken up into platelets throughout its life in the plasma, little efflux occurs until the end of the platelet’s lifespan (of ∼10 days). The resulting long half-life of platelet 5-HT (estimated to be ∼5 days)
(61) leads to the measure’s relatively slow response to changes in 5-HT uptake blockade. Thus, steady-state platelet 5-HT levels can be viewed as an integrated measure of transporter activity. The use of platelet 5-HT levels in this manner assumes that the mean platelet exposure to free plasma 5-HT does not change appreciably between conditions. Finally, although the relevant data are relatively sparse, animal studies
(47,
53,
54) strongly suggest that equivalent levels of uptake blockade occur peripherally (in platelets) and centrally (at neuronal and glial uptake sites). Combining neuroimaging techniques for assessing central transporter availability
(62) with platelet 5-HT measures may provide a route for better establishing the relationship between the peripheral and central blockade occurring during SSRI administration.
Although one cannot be certain that the absolute levels of inhibition seen peripherally can be extrapolated to central uptake inhibition, this seems likely. It appears quite probable that the relative amount of platelet blockade seen here in the mother and infant groups is highly related to the relative amounts of transporter inhibition occurring centrally in the two groups
(47,
53,
54). In summary, it appears that mothers receiving sertraline who breast-feed do not typically subject their infants to drug levels that appreciably affect infant 5-HT transport. In most cases, the effects on infant uptake are negligible; the effects that may occur in infants appear substantially smaller than the effects seen on maternal uptake. Additional research is needed to more definitely establish the safety of sertraline during breast-feeding and to determine whether all SSRIs display a similar (usually negligible) effect on infant uptake.