Cognitive functions such as memory, executive function, and attention are impaired in patients with schizophrenia
(1) and are predictive of vocational and social outcome
(2). Among the domains of cognitive function, secondary verbal memory and executive function have been suggested to be major predictors of functional outcomes in patients with schizophrenia
(2,
3).
Although typical antipsychotic drugs such as haloperidol have minimal influence on cognitive function, recent studies
(3,
4) have shown that atypical antipsychotic drugs such as clozapine, risperidone, and olanzapine enhance cognitive performance in patients with schizophrenia. Facilitation of cortical dopaminergic and cholinergic output may contribute to the ability of these agents to improve cognitive function (3, 5).
Serotonin-1A (5-HT
1A) receptor agonists have been shown to enhance dopaminergic and cholinergic neurotransmission in the cortex and/or hippocampus
(6,
7). There is also evidence from postmortem studies indicating abnormalities in 5-HT
1A-receptor-mediated transmission in the cortical region in patients with schizophrenia
(8). We have previously reported
(9) that the azapirone derivative tandospirone, a selective 5-HT
1A agonist that is used as an anxiolytic
(10), improved logical memory and verbal paired associates in patients with schizophrenia in an open clinical trial.
The aim of this study, therefore, was to determine whether the addition of tandospirone to treatment with typical antipsychotic drugs has a beneficial influence on executive function and secondary verbal memory in patients with schizophrenia.
Method
Participants for this study were 26 outpatients meeting DSM-IV criteria for schizophrenia treated at Toyama Medical and Pharmaceutical University Hospital. All of the subjects had been treated with small-to-moderate stable doses of typical antipsychotic drugs (haloperidol, N=23; sulpiride, N=2; pimozide, N=1) and biperiden as an antiparkinsonian agent for at least 3 months (
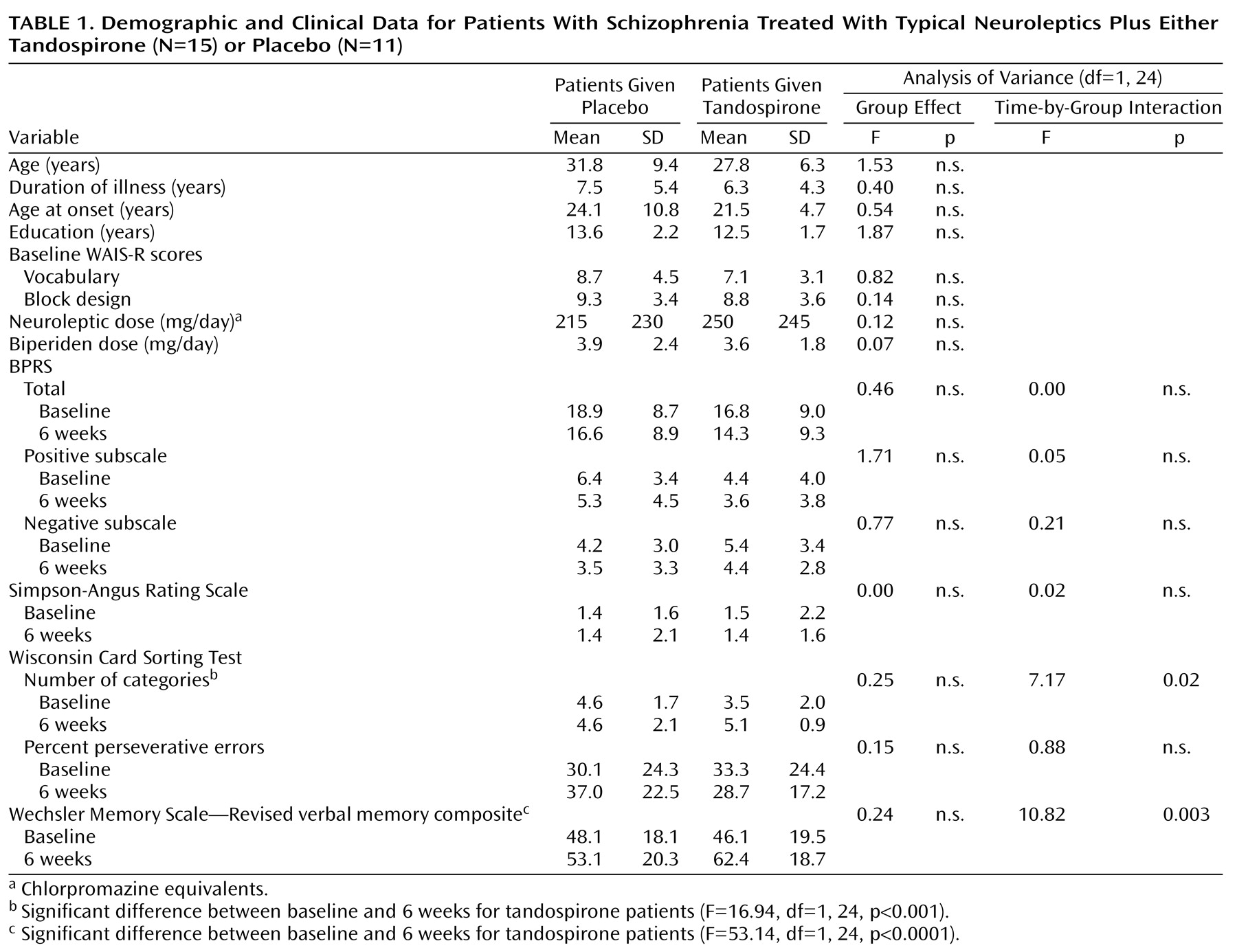
Table 1). Diagnosis was made by two experienced psychiatrists using the Structured Clinical Interview for DSM-IV based on the patient’s current mental status and a review of all medical records. Patients with an axis I diagnosis other than schizophrenia were excluded. Exclusionary criteria also included a history of any alcohol or substance abuse, epilepsy, brain damage, or neurological disorders and the presence of cardiovascular or metabolic disease. After complete description of the study to the subjects, written informed consent was obtained.
At baseline, executive function and verbal memory were evaluated with the Wisconsin Card Sorting Test (the number of categories and the percent of perseverative errors)
(11) and the verbal memory composite score (logical memory I and verbal paired associates I) from the Wechsler Memory Scale—Revised (WMS-R)
(12), respectively, by a well-trained clinical psychologist who was blind to the medication status of the subjects. The 18-item version of the Brief Psychiatric Rating Scale (BPRS) (0–6 scale)
(13) and the Simpson-Angus Rating Scale (14) were completed at the time of the psychological testing by experienced clinical psychiatrists who were blind to the subjects’ medication status and to the results of the neuropsychological assessment. The intraclass correlations for these measures were higher than 0.80. For evaluation of baseline verbal and performance IQ, the WAIS-R Vocabulary and Block Design subtests were performed.
Patients were randomly assigned to receive tandospirone, 30 mg/day
(9,
10) (10 mg t.i.d. in powder form mixed with lactose), or placebo (lactose alone) for 6 weeks. Fifteen patients (nine men and six women) were given tandospirone; 11 patients (six men and five women) were given placebo. All other psychotropic medications were continued unchanged.
Scores on the Wisconsin Card Sorting Test, WMS-R, BPRS (total, positive subscale, and negative subscale) and Simpson-Angus Rating Scale were obtained again after 6 weeks of tandospirone or placebo administration. The changes in the scores of these measures for the two groups were compared by using repeated measures analysis of variance with treatment (tandospirone versus placebo) as a between-subject factor and time (baseline versus week 6) as a within-subject factor. Our main interest was in finding any existing interaction effects of treatment over time; subsequent post hoc tests were also conducted. Significance was considered when the p value was less than 0.05.
Results
All 26 patients completed the 6-week trial; there was no clinically significant side effect attributable to the addition of tandospirone. The mean age, duration of illness, age at onset of illness, education, scores on the WAIS-R subtests, and doses of neuroleptics (in chlorpromazine equivalents) or biperiden did not differ significantly between the two groups (
Table 1). There were no significant group differences in the baseline scores on the BPRS (total, positive subscale, and negative subscale), Simpson-Angus Rating Scale, Wisconsin Card Sorting Test (the number of categories and percent of perseverative errors) or WMS-R verbal memory composite score (
Table 1).
Significant time-by-group interaction effects were noted for the Wisconsin Card Sorting Test categories and WMS-R verbal memory composite score: patients who had received tandospirone performed better at 6 weeks than at baseline for these measures (with effect sizes of 0.63 and 0.70, respectively), but no significant change was found for patients receiving placebo (
Table 1). There was no significant time-by-group interaction effect or main effect of time for the BPRS (total, positive subscale, negative subscale), Simpson-Angus Rating Scale, and Wisconsin Card Sorting Test percent of perseverative errors.
Discussion
The addition of tandospirone to ongoing treatment with typical antipsychotic drugs for 6 weeks was found to improve executive function, as indicated by improvement in the Wisconsin Card Sorting Test categories, and verbal memory without causing significant changes in psychopathology or extrapyramidal symptoms in patients with schizophrenia. The effect sizes of both cognitive measures were in the moderate range. The absence of improvement in the group given placebo rules out the possibility of a practice effect. The lack of change in psychopathology suggests that the improvement in the cognitive measures could result from a primary effect on cognition and was not secondary to decreased positive or negative symptoms. The observed effect of tandospirone on verbal memory confirms the results of our previous 4-week trial
(9).
Pharmacological manipulations of dopamine, norepinephrine, acetylcholine, or glutamate neurotransmitter systems have been suggested to demonstrate cognition-enhancing potential in schizophrenia
(15). The effectiveness of 5-HT
1A agonists for ameliorating cognitive impairment is consistent with the 5-HT
1A partial agonist properties of several atypical antipsychotics, including clozapine, quetiapine, and ziprasidone
(16,
17). The present findings may have implications for improving cognition and possibly social and work function in patients with schizophrenia who continue to receive typical antipsychotic drugs.
Some limitations of the present study need to be considered. First, this is a small study of relatively short duration, raising the possibility of a type II error on some measures examined. A longer trial with a larger number of subjects may have detected significant changes in, for example, psychopathology. Second, it would be worthwhile to determine if improvement persists or even increases with a longer duration of treatment.