Consistent evidence shows that neuroleptics, administered as a maintenance treatment, reduce relapse rates in schizophrenic patients. A comprehensive review that analyzed 66 studies found a mean cumulative relapse rate of 53% in patients withdrawn from neuroleptic therapy compared to 16% for those maintained on a regimen of neuroleptic therapy over a mean follow-up period of 9.7 months
(1). Despite this conclusion, many questions remain regarding the optimal use of antipsychotics as a maintenance treatment.
A central question is how long to continue maintenance antipsychotic treatment for patients after their first episode of schizophrenia. Patients in the early course of schizophrenia frequently request a decrease or discontinuance of their antipsychotic medications, and many have poor adherence to treatment
(2). After successful antipsychotic treatment, many patients believe that they will no longer need medication. Given that the early course of the disorder is highly variable across patients and is generally not well characterized in the literature, clinicians are hard put to insist on indefinite neuroleptic treatment. Among the 66 studies reviewed in the article by Gilbert et al.
(1), only two examined relapse rates in first-break or recent-onset patients
(3,
4). In the first of the two studies, seven of 17 patients (41%) with remitted first-episode psychosis who received placebo experienced a relapse, whereas none of the 11 patients who continued taking fluphenazine over a 1-year period relapsed
(3). In contrast, Crow et al.
(4) randomly assigned 120 first-break schizophrenia patients to continued treatment with antipsychotic medication (five different agents were used) or placebo following acute treatment. Relapse rates over a 2-year period were 62% for those given placebo versus 46% for those receiving active medication. In these two initial studies, random assignment to possible placebo treatment occurred shortly after resolution of psychotic symptoms. Since the Gilbert et al. review
(1) was published, we know of one other study that has addressed the risk of relapse after medication discontinuation in first-break schizophrenia patients. Robinson et al.
(5) found that patients who discontinued antipsychotic medications (not randomly assigned but self-selected) following at least 1 year of treatment relapsed at a rate almost five times that of those who continued taking medications over a 5-year period. Specific data on relapse rates for patients who discontinued medications were not provided.
In addition to the scarcity of studies involving first-break schizophrenia patients and the discrepant results in these three studies (clear benefit of continued treatment [
3,
5] versus a less robust effect
[4]), many questions remain. For example, the Kane et al. study
(3), with a total of 28 subjects, included patients with diagnoses of mania with schizotypal features (N=1), depression with schizotypal features (N=1), unspecified functional psychosis (N=3), and other psychiatric disorders (N=4), leaving only 19 schizophrenic patients within the study group. Furthermore, as has been described in detail elsewhere
(1,
6,
7), definitions of “relapse” differ markedly across studies. Relapse rates are likely to differ across studies when the definition of the term ranges from return of active medication to a specified change in symptom rating scales or hospitalization. Consistent with this definitional disarray, the rate of relapse during neuroleptic withdrawal across all studies included in the review
(1) ranged from 0% to 100%!
Studies with more sensitive, symptom-based definitions of relapse will likely show higher rates of relapse but lower rates of hospitalization, since reinstitution of treatment will occur more quickly. The Kane et al. study
(3) used the criteria of “substantial clinical deterioration with a potential for marked social impairment,” whereas Crow et al.
(4) defined relapse as a point at which the responsible clinician considered rehospitalization to be necessary or resumption of active antipsychotic medication essential. In the Robinson et al. study
(5), relapse was defined as at least “moderately ill” on the CGI severity scale
(8), “much worse” or “very much worse” on the CGI improvement scale, and a rating of “moderate” for at least 1 week on one or more psychotic symptom items from the Schedule for Affective Disorders and Schizophrenia—Change Version With Psychosis and Disorganization Items
(9).
Current consensus suggests approximately 1 year of antipsychotic treatment for a first episode of schizophrenia followed by consideration of medication discontinuation for stable patients. In 1991, Johnson
(10) recommended continuing medication for 12–24 months depending on the patient’s stability, followed by a gradual discontinuation of medication. Similar recommendations have been made elsewhere in 1997 and 1998
(11,
12). The APA Practice Guideline for the Treatment of Patients With Schizophrenia
(13), published in 1997, states that “a patient who has had only one episode of positive symptoms and has had no symptoms during the following year of maintenance therapy may be considered for a trial period without medication.”
Despite these consistent recommendations, the natural history of clinically stable patients with recent-onset (i.e., nonchronic) schizophrenia who discontinue antipsychotic medication remains relatively unexplored by systematic clinical study, leading to a lack of empirical basis for decisions about continuing maintenance antipsychotic medication after an initial year. Therefore, to help clarify this issue, as one phase of a longitudinal study, we examined the clinical course of a cohort of patients with recent-onset schizophrenia who gave permission to have their antipsychotic medication withdrawn while continuing to receive ongoing psychosocial treatment and prospective clinical management. The design of this study (conducted between 1982 and 1993) parallels fairly closely the schizophrenia treatment recommendations published before, during, and after the current study.
In essence, this study may be viewed as an attempt to apply a targeted medication approach to a recent-onset schizophrenia cohort
(14–
16), in that patients were followed regularly when not receiving medication, and treatment was again recommended as soon as symptom exacerbation occurred. As has been noted elsewhere
(16), targeted medication approaches might be most applicable for clinically stable patients in the early phases of schizophrenia. At this point, patients are frequently unwilling to commit to long-term maintenance medication and voice concerns about avoiding adverse side effects.
Method
The 53 participants in this study were drawn from a larger cohort of patients (N=104) followed as part of a three-phase longitudinal study evaluating the roles of vulnerability factors and stressors in recent-onset schizophrenia patients—the Developmental Processes in Schizophrenic Disorders Project
(17,
18). Entry criteria required all subjects to have had their first onset of a psychotic episode no more than 2 years before study entry. Seventy-nine percent (N=42 of 53) of participants were in their first psychotic episode at the time of study entry. All subjects met Research Diagnostic Criteria
(19) for schizophrenia or schizoaffective disorder, mainly schizophrenic subtype. Full inclusion and exclusion criteria are presented elsewhere
(17,
18). Briefly, subjects were 18–45 years of age (mean=25.0 years, SD=4.3) and had no evidence of a neurological disorder (e.g., epilepsy, encephalitis). Additionally, patients with a history of significant and habitual drug use in the 6 months before study entry and those for whom there was substantive evidence that drug use triggered the psychotic episode were excluded from the study. All subjects were treated every 2 weeks with fluphenazine decanoate administered intramuscularly with an initial target dose of 12.5 mg. Doses were increased to a higher maintenance level during the course of the first outpatient year if the subject’s symptoms were not adequately controlled. Doses were decreased if intolerable side effects could not be adequately controlled by antiparkinsonian medications.
To be considered for inclusion in the neuroleptic discontinuation protocol that is the subject of this report, individuals were required to have their antipsychotic regimen stabilized and to be clinically stable. Specific inclusion criteria were 1) fluphenazine decanoate treatment for at least 1 year with clinical evaluations every 2 weeks, and 2) no notable psychotic symptoms for at least the last 3 months. The mean length of fluphenazine treatment before entry into this protocol was 16.7 months (SD=5.5). An exclusion criterion was the treating psychiatrist’s clinical judgment that the patient was too unstable to be considered for medication withdrawal (even if rating scale criteria for clinical stability were met). With these criteria, 54 of the original cohort of 104 patients were eligible for the study. The 54 subjects did not differ in age, gender, ethnicity, educational background, socioeconomic status, length of illness before study entry, or age at onset from the other 50 individuals who were ineligible for the protocol. Of the 51 subjects who did not enter this study, 28 had dropped out of the clinic, 21 were excluded because of insufficient clinical stability, one subject was excluded per the psychiatrist’s clinical judgment, and one eligible subject declined to enter the study because of fear of a potential return of symptoms.
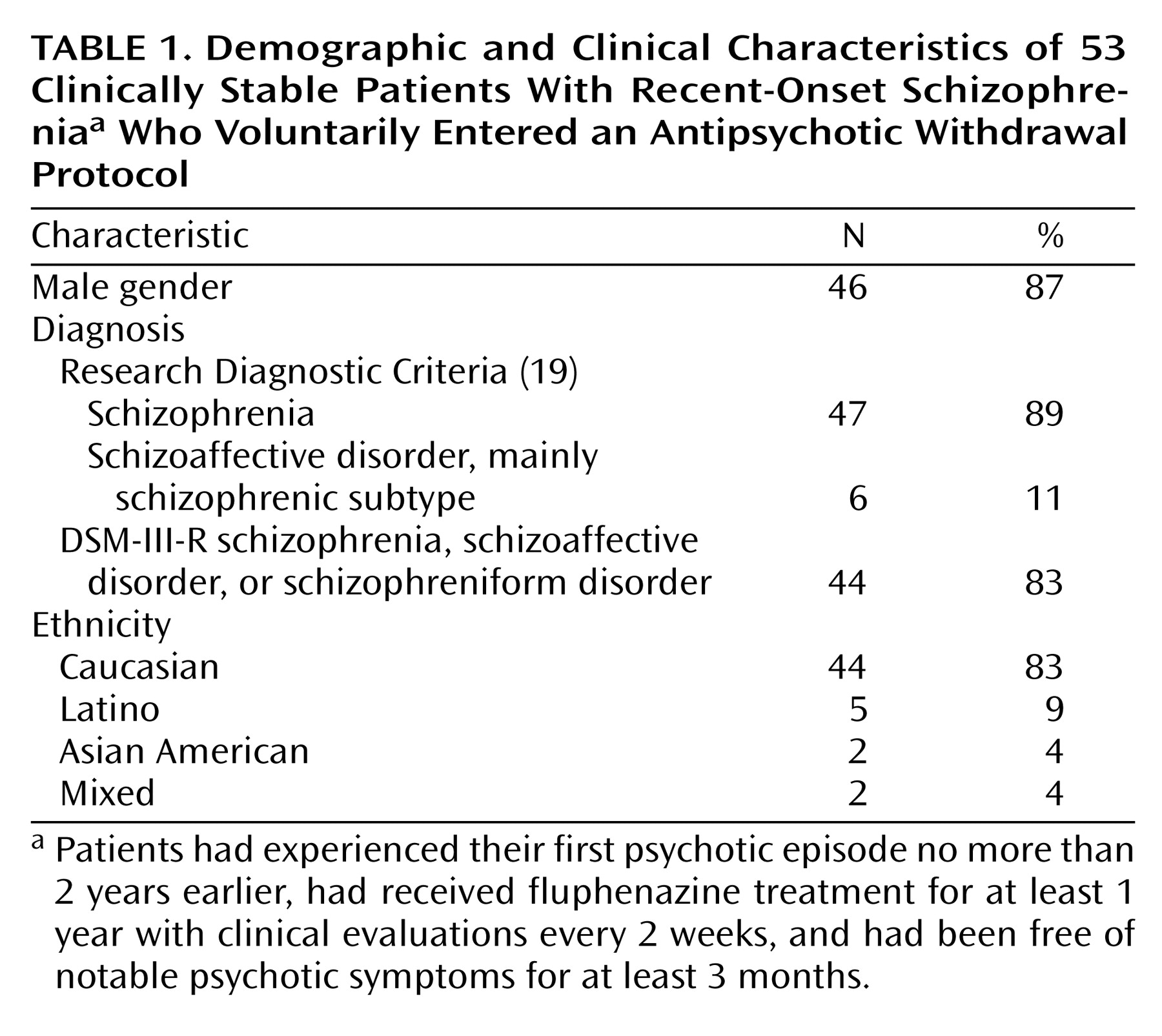
Table 1 presents the demographic data for the 53 individuals included in the study.
Treatment Protocol
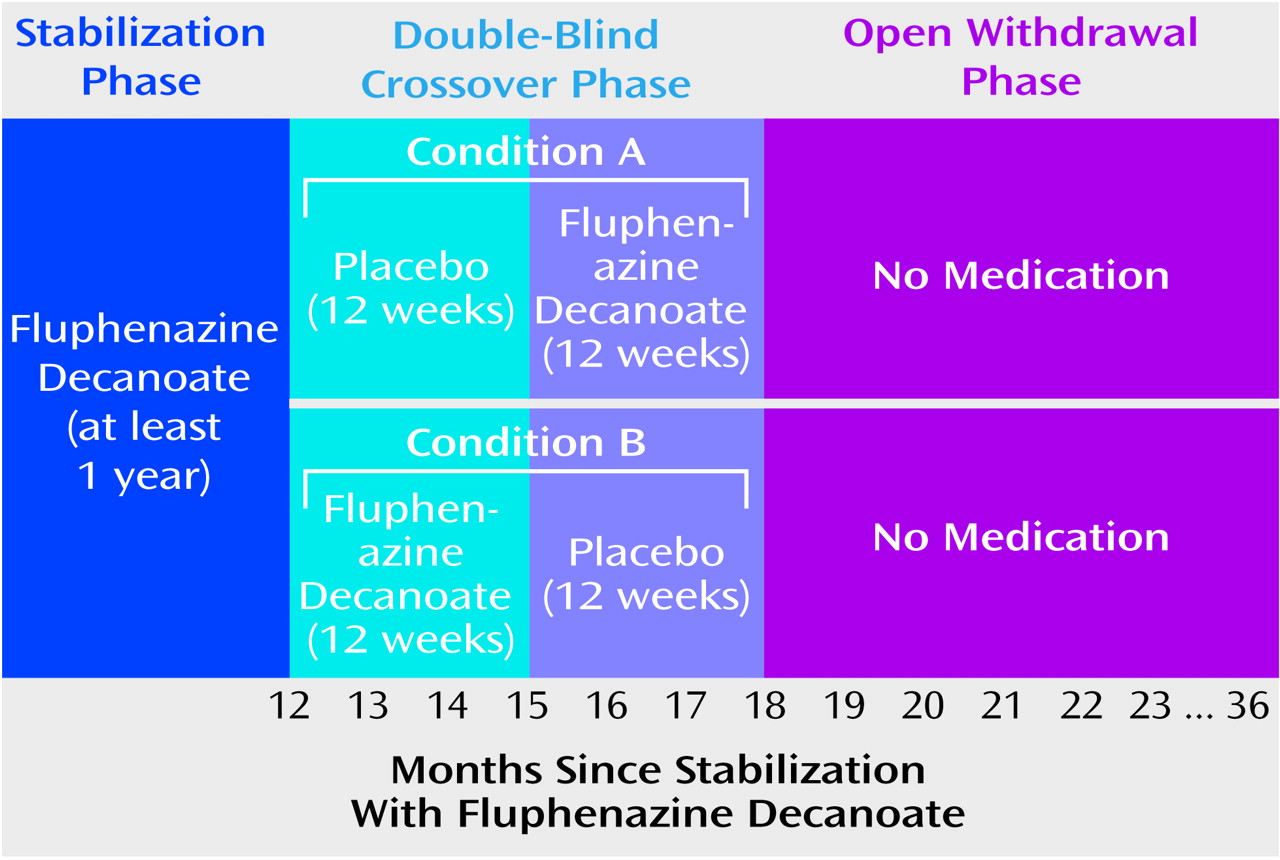
During the first 24 weeks of the study, patients were randomly assigned to treatment in a double-blind fashion to 12 weeks of their usual fluphenazine decanoate regimen or 12 weeks of placebo treatment, administered by a research nurse or physician. As shown in
Figure 1, those assigned to condition A were given placebo followed by active drug, while those in condition B were given their usual dose of fluphenazine decanoate followed by placebo. For patients who did not experience symptom exacerbation or psychotic relapse during these 24 weeks, intramuscular medication shots were openly discontinued, and the patient was followed for up to 18 months. Thus, the entire time of observation in this study was 102 weeks, or just under 2 years.
During the first 24 weeks of the study (the double-blind crossover phase), patients were seen every 2 weeks and were evaluated with the expanded Brief Psychiatric Rating Scale (BPRS)
(20) by their case managers. Each rater achieved a median intraclass correlation coefficient of 0.80 or higher across all BPRS items compared with the criterion ratings
(21). During the open withdrawal phase, patients were evaluated with the BPRS every 2 weeks for the first 6 months and then every 4 weeks thereafter. If dictated by clinical need, the frequency of clinical evaluations was increased. Aside from the scheduled BPRS evaluation, if a patient called with a possible change in symptoms, a BPRS rating was done at that point.
Patients were removed from the medication discontinuation trial if any of the following three conditions were met: 1) An increase in psychotic symptoms assessed with the BPRS met the a priori definition for exacerbation or relapse. The definition of exacerbation was any 2-point change on any of the three BPRS psychotic items (hallucinations, unusual thought content, and conceptual disorganization), excluding changes in which the ratings remained at nonpsychotic levels (i.e., ≤3). Psychotic relapse was defined as a rating of 6 or 7 on any of the three BPRS psychotic symptom items. One patient had a nonpsychotic relapse (depression), which was not included in the current analysis. 2) A worsening of clinical condition occurred that warranted a change in treatment according to the treating psychiatrist, even if the BPRS ratings did not meet criteria for exacerbation or relapse. 3) The patient decided to withdraw from protocol participation. The criteria for removal from the medication discontinuation trial were purposely sensitive to relatively small symptomatic changes. Our goal was to intervene as soon as it became clear that the patient had a clinically significant return of symptoms in order to avoid, as much as possible, a full psychotic episode.
Information on the nature of the double-blind crossover and the open medication withdrawal components of this protocol was described verbally and with a written informed consent form. After complete description of the study to the potential participants, written informed consent (approved by the institutional review board of the University of California, Los Angeles, and the National Institute of Mental Health) was obtained before study entry. These informed consent procedures were separate from the procedures that occurred at entry into the preceding protocol, which involved repeated assessment batteries during an initial year of maintenance treatment with fluphenazine decanoate.
Blood samples for fluphenazine levels were scheduled to be drawn every 2 weeks during the crossover portion of the study and every 4 weeks during the first 6 months of open withdrawal. To accommodate patients’ personal schedules, occasionally patients were seen and had their blood drawn 3 weeks after the prior fluphenazine injection. During the crossover phase of the protocol, blood samples were taken before the administration of the next intramuscular shot.
Plasma levels of fluphenazine were measured by using a specific radioimmunoassay that was validated for specificity by using gas chromatography mass spectroscopy
(22,
23). The sensitivity of the assay was 0.1 ng/ml for most of the samples analyzed. The coefficient of variation was 3%. Some of the initial samples were analyzed by an earlier method with a sensitivity of 0.3 ng/ml.
Data Analysis
In calculating time to return of psychotic symptoms, we used 2 weeks after the last active fluphenazine injection as day 0 of drug withdrawal; for example, a return of psychotic symptoms declared at 14 days occurred 28 days after the last injection. Drug withdrawal was considered to start during the placebo condition for subjects whose psychotic symptoms returned during initial assignment to placebo in the crossover phase (condition A in
Figure 1). Condition A subjects who did not experience a return of psychotic symptoms during the placebo condition and had their medications reinstated after 12 weeks were considered to start drug withdrawal at the time of open withdrawal. Those subjects assigned to condition B (
Figure 1) were considered to start drug withdrawal during the placebo phase of the crossover component of the protocol. Time to relapse was analyzed by using product-limit (Kaplan-Meier) survival analysis
(24). Analysis of the effects of fluphenazine levels on relapse risk were done by using proportional hazard regression, with baseline and most recent fluphenazine level included as time-varying covariates
(25). In addition to the tests of significance for the survival parameters, estimated hazard ratios from the proportional hazard regression are reported with 95% confidence intervals (CIs).
Results
Crossover Phase
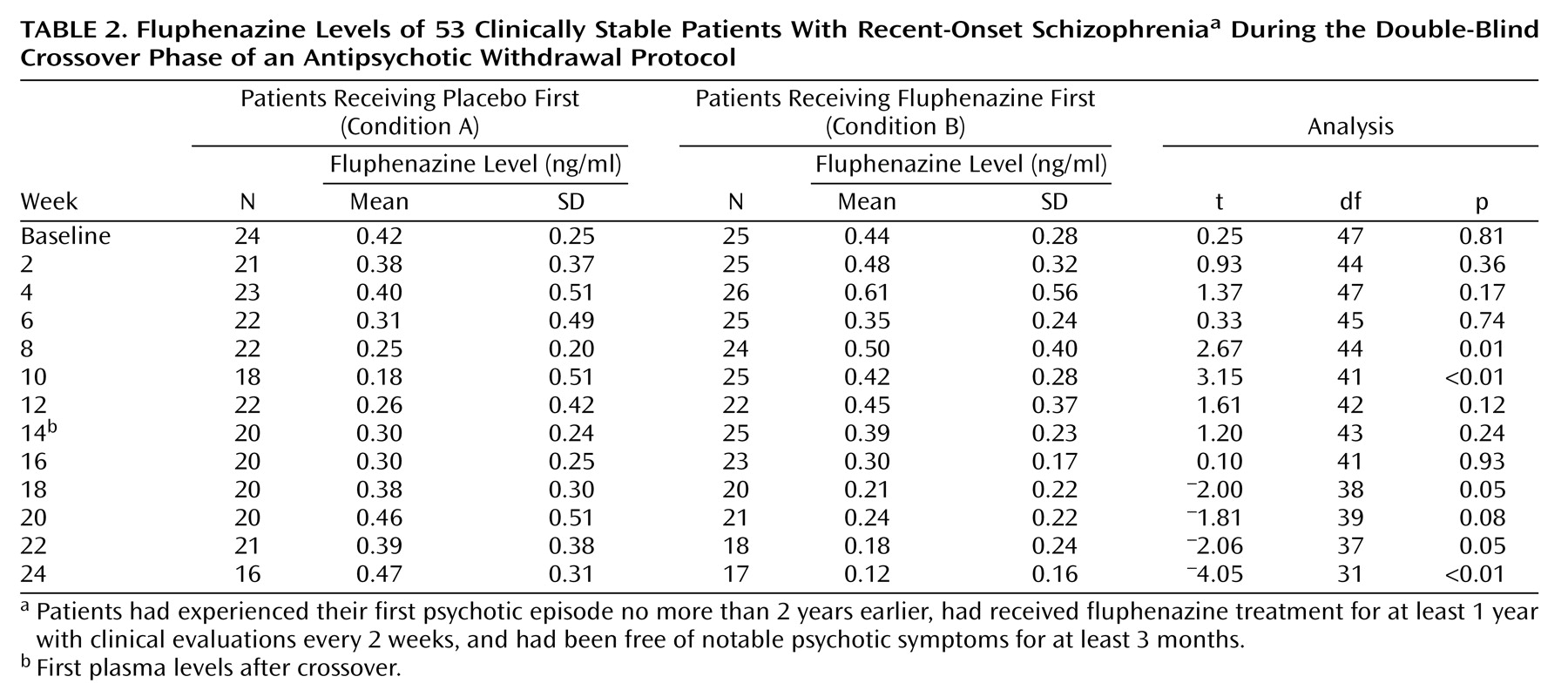
During the 24-week crossover phase of the study, 10 of 53 subjects (19%) experienced an exacerbation or relapse, three during the medication period and seven while receiving placebo. As seen in
Table 2, fluphenazine levels slowly decreased during the placebo period of the crossover protocol. However, even after 12 weeks of placebo, plasma levels of fluphenazine remained substantial in many patients. As an example, at the end of the first 12 weeks, the difference in mean fluphenazine levels between those treated with active medication versus placebo was not significant (
Table 2).
Withdrawal Phase
The three subjects who experienced an exacerbation or relapse during the active medication phase of the crossover protocol were not included in the withdrawal analysis, leaving 50 subjects. The seven subjects who experienced an exacerbation or relapse during the placebo condition of the crossover phase were included in the survival analysis.
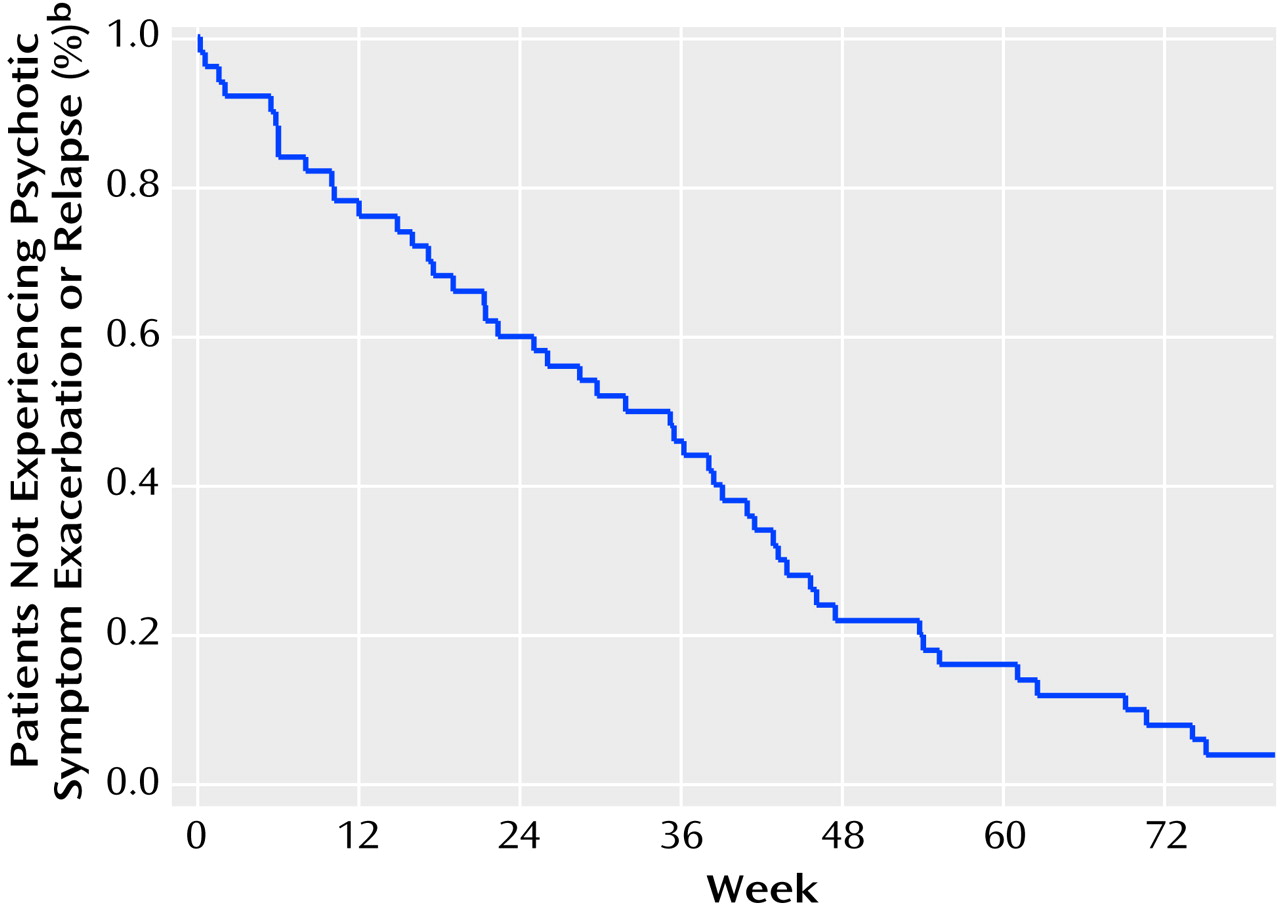
Only 22% of the subjects (N=11 of 50) went 1 year without an exacerbation or relapse following medication discontinuation. By 2 years, 96% (N=48) of the subjects had experienced an exacerbation (N=28) or relapse (N=20).
Figure 2 shows the survival curve for the entire cohort.
The mean time to exacerbation or relapse for the 48 subjects whose psychotic symptoms returned within the time frame of the study was 235 days, or just under 8 months. The median time to exacerbation or relapse was 245 days. The two subjects who did not experience an exacerbation or relapse within the time frame of the study were followed clinically and ultimately developed psychotic symptoms after drug withdrawal: one at 28 months and the other at 93 months (i.e., 7 years and 9 months).
Because of the possibility that subjects in condition A (placebo followed by active drug) had lower fluphenazine levels at the beginning of open withdrawal because the withdrawal period was preceded by the earlier 12-week placebo period, we compared the fluphenazine levels in these subjects after the 12-week drug reinstatement to the levels of subjects in condition B at the point just before drug withdrawal. The difference was clearly not significant (t=0.22, df=43, p=0.83). In order to further ensure that time to exacerbation or relapse was not affected by the order of the double-blind crossover trial, we compared the survival curves for those in condition A versus those in condition B. There was no difference between the two curves (Kaplan-Meier log-rank χ2=0.66, df=1, p=0.42). Additionally, a similar percentage of patients from the two groups experienced an exacerbation or relapse 90 days after medication discontinuation (22% and 26%, respectively).
Because fluphenazine levels during decanoate treatment may remain measurable for months
(26), we evaluated a potential relationship between fluphenazine plasma levels and time to exacerbation or relapse. In the following analysis, we excluded data from one outlier subject whose fluphenazine plasma levels were consistently four standard deviations higher than mean values of all other subjects. We performed a time-dependent covariate analysis with time to exacerbation or relapse as the dependent variable. Three separate survival regression models were evaluated with different fluphenazine variables. Plasma level at the beginning of drug withdrawal was not associated with time to relapse (relative risk=1.28, 95% CI=0.58–2.82; χ
2=0.38, df=1, p=0.54) nor was the last plasma level measured or the last level before exacerbation or relapse (relative risk=1.42, 95% CI=0.29–4.01; χ
2=0.03, df=1, p=0.86). Finally, the slope of the plasma level from the beginning of drug withdrawal to the last fluphenazine level measured or the last one before exacerbation or relapse also was not associated with time to exacerbation or relapse (relative risk=0.67, 95% CI=0.33–4.32; χ
2=1.03, df=1, p=0.31). (This last analysis excluded eight subjects who had only one fluphenazine level measured.) Thus, exacerbation or relapse could not be explained simply by a proximate decrease in fluphenazine levels.
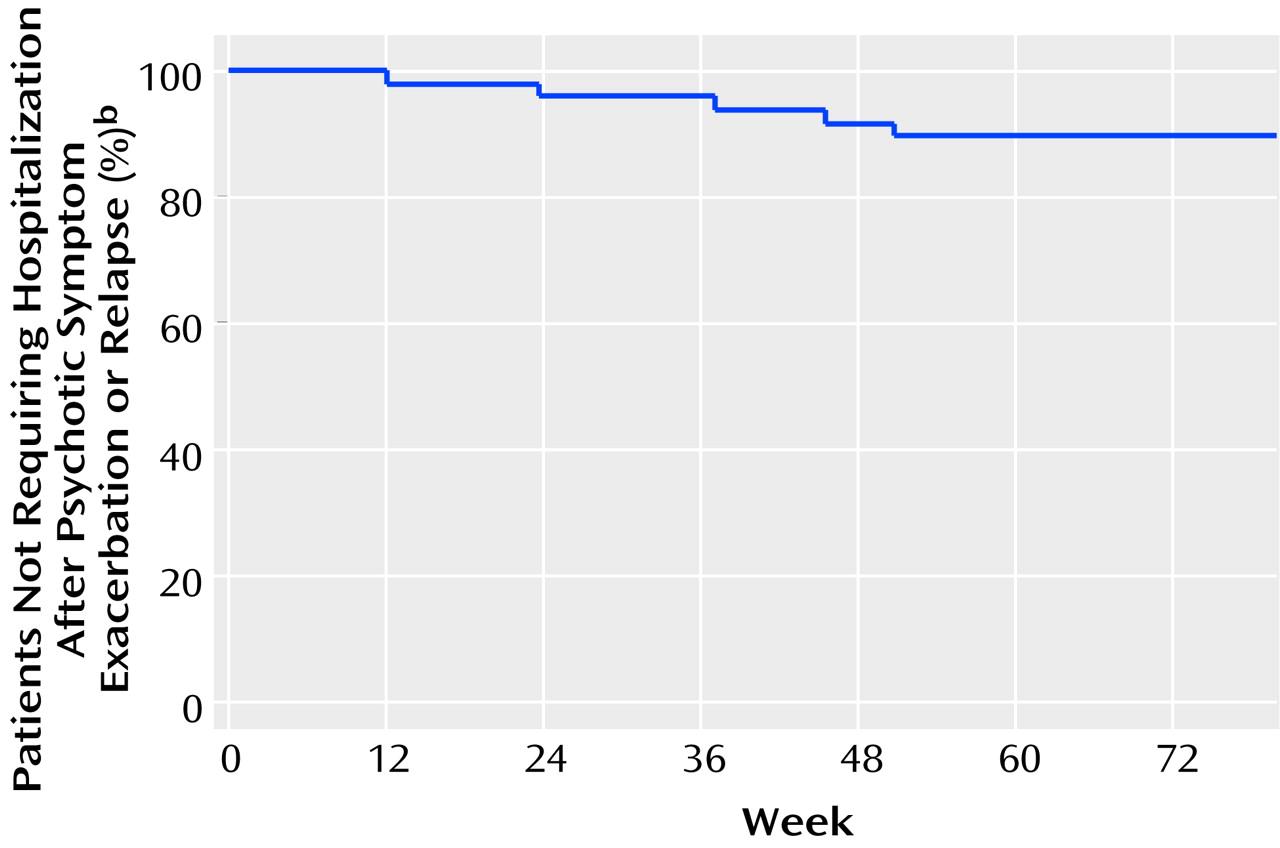
Since our threshold for psychotic exacerbation was so low, we also examined hospitalization rates among those who experienced exacerbation or relapse. Of these 48 patients, 45 continued to be followed regularly in the clinic after medication withdrawal, and only six (13%) were hospitalized within 3 months of exacerbation (N=3) or relapse (N=3). The rest were successfully managed on an outpatient basis in the initial restabilization period. Of the three dropouts, two were hospitalized later, one in the context of methamphetamine abuse.
Figure 3 shows the survival curve for hospitalization over the course of the study, which contrasts strikingly with the exacerbation/relapse curve (
Figure 2). There were no suicides during the course of the study.
Discussion
There were three main findings in this study of clinically stable patients with recent-onset schizophrenia whose antipsychotic medications were gradually withdrawn (as is inherent in decanoate treatment) after at least a year of maintenance treatment. First, exacerbation or relapse was almost universal within 2 years, when low exacerbation threshold criteria were used. Second, these exacerbations or relapses were clinically managed without resorting to hospitalization in the vast majority of cases in which cooperative patients agreed to be clinically monitored. Third, the timing of exacerbation or relapse did not correlate either with overall rate of fluphenazine decline or most recent absolute fluphenazine level.
Our exacerbation/relapse rates were higher than those seen in either the Kane et al.
(3) or Crow et al.
(4) studies, which may be explained by use of different methodologies as well as different methods of analysis. As an example, in the Kane et al. study
(3), dropouts were considered to have completed the risk period without relapse. Examining only patients with Research Diagnostic Criteria schizophrenia in that study, the relapse rate for those randomly assigned to placebo and who continued in the study for the entire year was 86% (N=6 of 7), which is similar to the 1-year rate of exacerbation/relapse in our study (78%). The reasons for the lower relapse rate in the placebo group of the Crow et al. study
(4)—62% at 2 years—are not entirely apparent but may reflect either the sensitivity of our definition of exacerbation or the advantage of a placebo treatment in their study, which differed from the open medication discontinuation used in our study.
Although the results of this and other studies indicate the very high rate of exacerbation or relapse following antipsychotic discontinuation in stable patients with first-episode schizophrenia, we still do not automatically recommend long-term maintenance treatment after a first episode for every patient. Individuals with schizophrenia take into consideration the considerable costs of neuroleptic treatment (e.g., side effects, social stigma), barriers to treatment (e.g., financial, geographical), their assessment of whether they are ill and in need of treatment, and their belief in the efficacy of the treatment
(27). Quite frequently, individuals with recent-onset schizophrenia who respond well to neuroleptic treatment will no longer believe that their need for treatment outweighs the perceived costs of treatment. The prospect of a monitored trial without medication might help the clinician to negotiate a longer period of treatment adherence and prevent covert noncompliance. Many patients included in this study expressed their intention not to continue their antipsychotic medication regimen indefinitely without at least having a chance to be medication free. Medication withdrawal while continuing in ongoing clinical management made it possible to monitor the patients in a controlled setting and to intervene when symptoms reemerged as long as patient treatment adherence was possible. We believe this resulted in the low hospitalization rate that we observed (13%) in contrast with the 2-year rate of exacerbation or relapse (96%). The hospitalization rate would very likely have been higher had more patients dropped out of treatment entirely and then discontinued medication unilaterally and without clinical supervision. The success of early reinstatement of antipsychotic treatment—as evidenced by the low hospitalization rate—is consistent with the results of previous studies
(28,
29).
Some authors have voiced concern about the potential for untreated psychosis to produce lasting harm and a worse long-term prognosis in schizophrenic patients
(4,
30). Both theoretical objections and other interpretations of the data upon which these concerns are based make this concern less plausible
(31,
32). Additionally, a recent study found no evidence that the duration of untreated psychosis was associated with clinical outcome 2 years later
(33). Furthermore, the potential outcome of months of an untreated full psychosis is not relevant to the impact of quick resumption of antipsychotic medications when psychotic symptoms appear, as was done in the current study.
Another concern regarding targeting medications as we did in the current study is whether even more sensitive criteria for medication reinstatement would decrease overall disease morbidity. However, in a study that examined the use of adjunctive antipsychotic treatment at the time of prodromal symptoms, 48% of the prodromes treated with placebo resolved without progression to exacerbation
(34). Thus, we believe that the ability of existing definitions of prodromal (not psychotic) symptoms to herald further clinical worsening is currently poor, limiting the utility of this approach.
We did not find any temporal relationship between decreases in fluphenazine levels after drug discontinuation and timing of exacerbation or relapse. To our knowledge, no previous study has examined this relationship. Of course, since neuroleptic drugs are consistently associated with lower relapse rates
(1), this negative result may reflect limited sensitivity for evaluating changes in fluphenazine levels. Alternatively, this result may reflect slower changes in brain fluphenazine levels, other factors related to intrinsic disease processes, or environmental stressors as proximate causes of schizophrenic exacerbation or relapse. It is clear, however, that the timing of exacerbations and relapses could not be easily explained by a decreasing level of an antipsychotic that had been suppressing a continuous psychosis. The presence of substantial amounts of fluphenazine (and marked interindividual variability in plasma levels) even after 12 weeks of placebo treatment is consistent with other studies, including our own
(26,
35).
Our study has limitations. First, all patients were treated with a decanoate form of an antipsychotic medication. This limits the generalizability of the results to patients treated with oral agents who might become psychotic more quickly after medication discontinuation. Second, there was no parallel group with which to compare exacerbation/relapse rates during medication discontinuation. Third, the lack of relationship between fluphenazine levels and exacerbation or relapse may not apply to oral agents. Fourth, the stringent criteria for entry into our protocol—compliance with injectable medications for greater than 1 year—suggest that our patient group may have been selected for medication adherence and clinical stability before medication discontinuation. The time to exacerbation or relapse might be shorter in less selected populations. The fact that 22 of 50 subjects (44%) initially considered for the study were excluded because of insufficient clinical stability is consistent with this notion. Finally, this study was conceived and executed (1982–1993) before the availability of the novel antipsychotics (other than clozapine). The risk/benefit ratio of ongoing treatment versus drug discontinuation may differ with the newer agents. Since these data were collected, financial considerations affecting hospitalization rates have generally become more prominent. It is possible that hospitalization rates might be even lower now given the current financial climate.
Although the risk/benefit ratio with the novel antipsychotics differs from that of the antipsychotic used in this study, fluphenazine, the results of this study are relevant for today’s clinical practice. Even though rates of extrapyramidal symptoms and tardive dyskinesia are lower with the novel antipsychotics, rates of these symptoms (with the possible exception of clozapine) are not zero
(36). Additionally, the novel antipsychotics confer risks of significant weight gain, sexual dysfunction, and new-onset diabetes mellitus
(37). Given these concerns and the frequent resistance to long-term maintenance antipsychotic treatment early in the course of schizophrenia, at least some recent-onset schizophrenic patients should be considered for a trial of drug discontinuation, but only if careful clinical monitoring is available for rapid resumption of medication should psychotic symptoms emerge. Because no validated predictors of outcome in the early course of schizophrenia currently exist, reasonable clinical recommendations would be to consider a medication discontinuation trial in patients who are clinically stable with very few or no psychotic symptoms for at least many months and who agree to participate in ongoing clinical monitoring. If possible (and with the patient’s permission), the patient’s family members should be involved in the monitoring process. There should be chart documentation of a discussion of the potential for a return of psychotic symptoms and a plan for ongoing monitoring should medication be discontinued.
Despite these caveats, our data suggest persuasively that the vast majority of patients with recent-onset schizophrenia who voluntarily have their antipsychotic treatment withdrawn will experience an exacerbation or relapse within the first 2 years, even after more than 1 year of maintenance treatment. In the context of clinical monitoring, these symptoms can be handled on an outpatient basis in the majority of cases. These findings can be used to help both schizophrenic patients and their families develop informed treatment plans during the first few years of illness.