Antidepressants have high affinity for a number of receptors, including subtypes of serotonin, norepinephrine, acetylcholine, and dopamine receptors
(1,
2). The majority of antidepressants have high affinity for the serotonin (5-HT) transporter (5-HTT)
(1,
2), and antidepressants selective for 5-HTT are the most common treatments for depression. Binding to the 5-HTT site is a well-proven therapeutic property of antidepressants.
Even though selective serotonin reuptake inhibitors (SSRIs) are often used to treat major depression, the percentage of 5-HTT sites blocked during a typical course of SSRI treatment is unknown. One [
11C]McN5652 positron emission tomography (PET) study found that a single intravenous dose of citalopram (6 mg/kg) or a single oral dose of paroxetine (60–80 mg) reduced the 5-HTT binding potential in baboons and healthy humans, respectively
(3). The binding potential is proportional to receptor density and affinity. One single photon emission computed tomography study using [
123I]2-beta-carbomethoxy-3-beta-(4-iodophenyl)-tropane (β-CIT) reported that, compared to healthy subjects, depressed patients treated with 20–60 mg/day of citalopram had a 50% reduction in the binding potential in the combined thalamus and brainstem regions
(4). This study had two potentially important confounds: 1) a within-subject design was not used and 2) between-group differences unrelated to the effect of citalopram, such as the presence of major depression, could have biased the measure of drug effect. The β-CIT binding potential in the brainstem is lower during major depression
(5), and the β-CIT binding potential in the thalamus is lower during seasonal affective disorder
(6). Because β-CIT has equal affinity for 5-HTT and dopamine transporters
(7), unblocked dopamine transporters in the ventral tegmentum and the substantia nigra within the brainstem
(8) could lead to an underestimate of the drug effect.
[
11C](N,N-Dimethyl-2-(2-amino-4-cyanophenylthio) benzylamine (DASB) is a new PET radiotracer that is highly selective, showing nanomolar affinity for 5-HTT and negligible affinity for other monoamine transporters
(9). Available data suggest that [
11C]DASB is superior to other PET radioligands for 5-HTT. In humans, binding potential values found with [
11C]DASB PET are approximately two- to threefold greater than those found with [
11C](+)McN5652 PET
(9–
12). With [
11C](+)McN5652 PET, 5-HTT binding potential values are not detectable in the frontal cortex and are modestly detectable in only the basal ganglia and the thalamus
(3). With [
11C]DASB PET, 5-HTT binding potential values are detectable in the frontal cortex and are reasonably high in the basal ganglia and the thalamus
(11,
12).
The main purpose of this study was to measure the occupancy of 5-HTT sites the treatment of depressed patients with paroxetine and citalopram by using [11C]DASB PET and a within-subject design. Occupancy is the percent reduction in binding potential after drug administration. A secondary aim was to assess the relationship between serum paroxetine levels and 5-HTT occupancy.
Results
Effect of Major Depression on Striatal 5-HTT Binding Potential
If major depression were to have a considerable effect on striatal 5-HTT binding potential and if such an effect were reversed independently of occupancy, then, in theory, a bias in occupancy determination could occur. To assess this possibility, we compared the striatal 5-HTT binding potential between healthy and depressed subjects. No significant differences in striatal 5-HTT binding potential were found between the healthy and depressed groups at the first [11C]DASB PET scan, although there was a significant decline with age (analysis of covariance [ANCOVA] with age as covariate, effect of age alone, F=4.90, df=1, 18, p<0.04; ANCOVA with age as covariate, effect of diagnosis, F<0.06, df=1, 27, p=0.82). We examined the effect of depression in the striatum because it was chosen as the primary region for analyses of occupancy (see Method).
Effects of Treatment
All patients scanned before and after treatment had detectable serum paroxetine or citalopram levels and were included in the analysis (paroxetine: mean=26 μg/liter, SD=17; citalopram: mean=65 μg/liter, SD=14).
The test-retest data were obtained from healthy subjects. In the test-retest data, among regions, the mean difference, expressed as a percent of the initial binding potential was –3.7% (SD=3.7%) and ranged from –8% to 2%. The mean absolute difference, expressed as a percent of the initial binding potential was 10.9 (SD=2.9) and ranged from 7% to 15%.
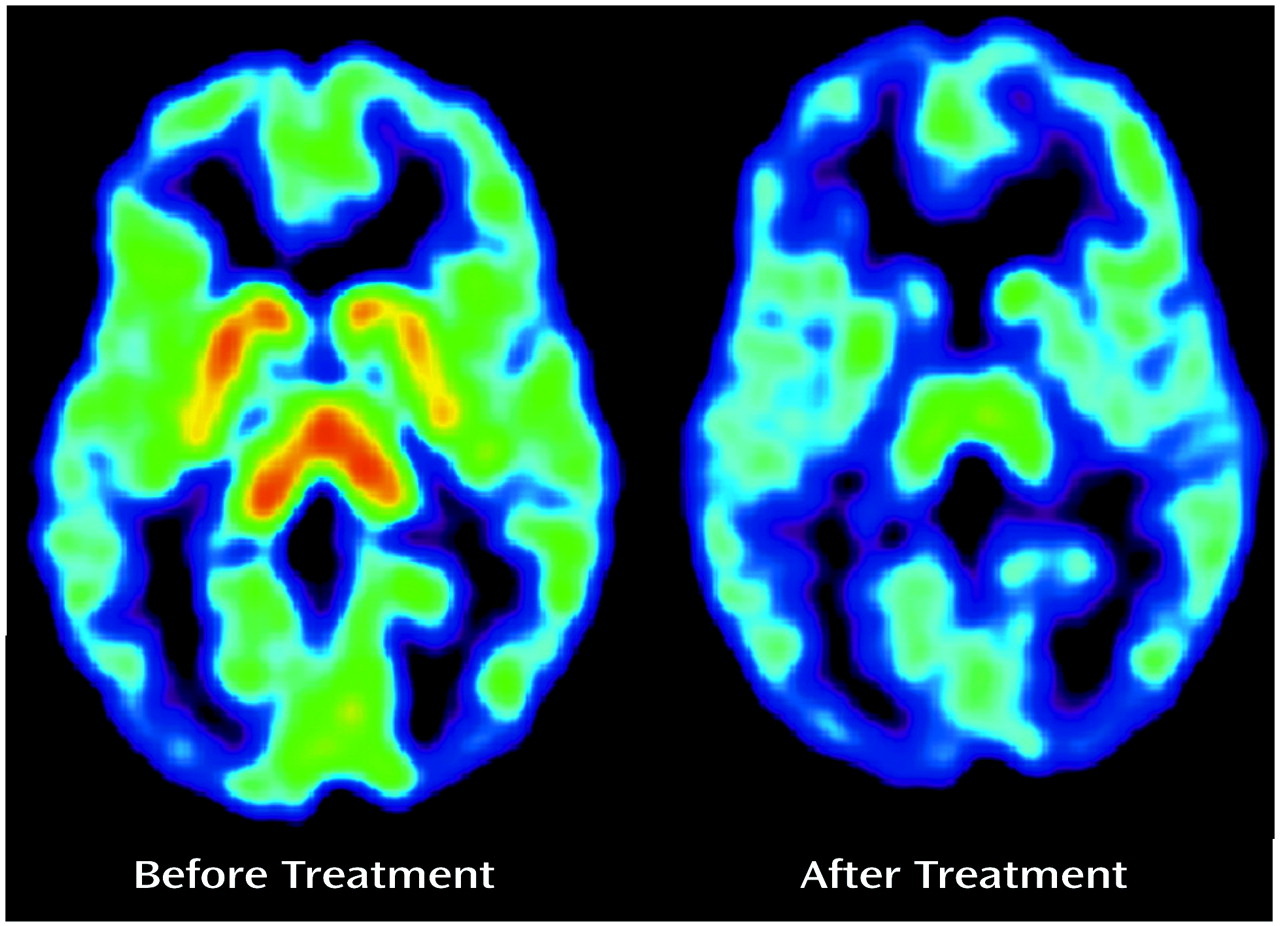
There was an obvious effect of the SSRIs on the summated [
11C]DASB PET images (
Figure 1). The absolute decline in striatal 5-HTT binding potential was significantly greater during paroxetine and citalopram treatment than in the test-retest data for the healthy subjects (repeated measures analysis of variance [ANOVA], effect of paroxetine treatment, F=58.96, df=1, 12, p<0.001; effect of citalopram treatment, F=32.16, df=1, 8, p<0.001). For this repeated measures ANOVA, the 5-HTT binding potential was the repeated measure and effect of the test-retest condition versus drug treatment was compared.
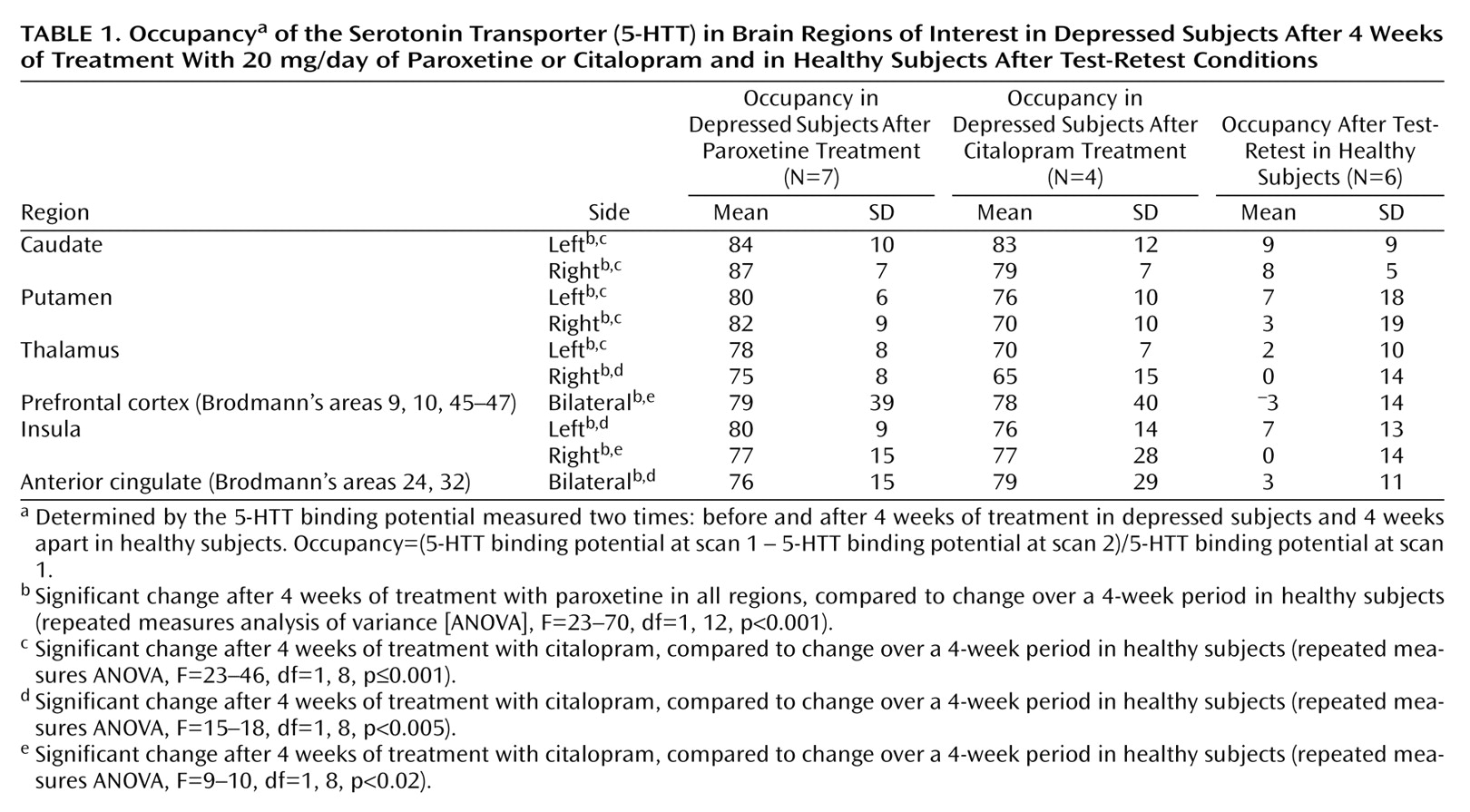
For patients treated with 20 mg/day of paroxetine (N=7), the mean occupancy in the bilateral striatum ([5-HTT binding potential pretreatment – 5-HTT binding potential post treatment]/5-HTT binding potential pretreatment) was 83% (SD=5%). For patients treated with 20 mg/day of citalopram (N=4), the mean occupancy was 77% (SD=10%). See
Table 1 for a comparison of occupancies among test-retest and drug treatments.
There was no association between the final Hamilton depression scale score after 6 weeks and occupancy in the striatum (r=0.07, df=10, p=0.84, N=11). In addition, there was no association between the final Hamilton depression scale score after 6 weeks and occupancy in any other brain region, after correcting for multiple comparisons (r=–0.31 to 0.61, df=10, p=0.047 to 0.88 [before correcting for multiple comparisons], N=11, 10 correlations, regions listed in
Table 1).
Relationship Between Serum Paroxetine and Occupancy
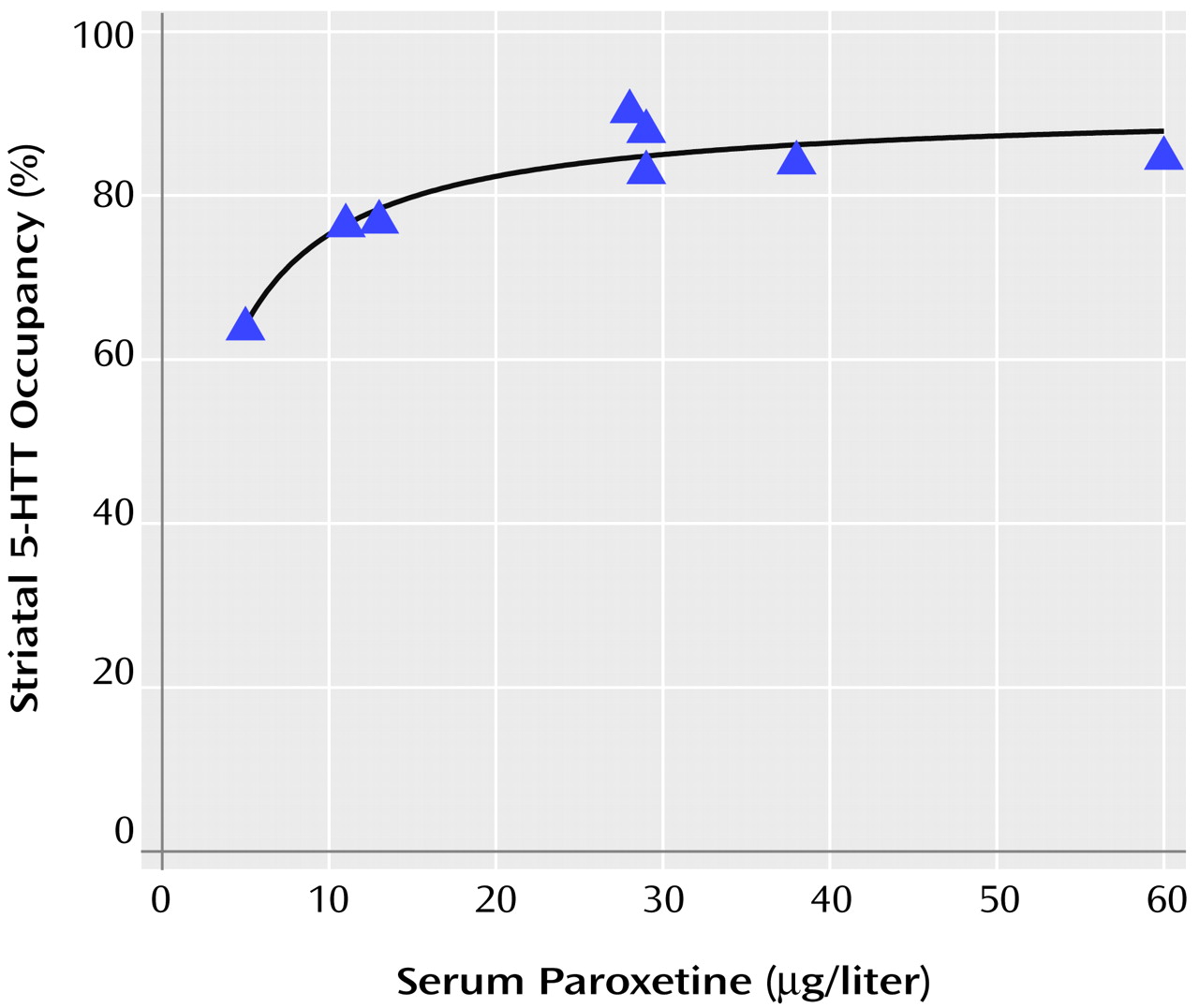
Occupancy increased as serum levels of paroxetine increased. Previous studies of serum level and occupancy typically have described a hyperbolic relationship of the form f(x)=a*x/(b+x)
(32–
34). Thus, we chose this model to describe our data, and the fit was significant (F=41.00, df=1, 6, p=0.0007). Visual examination of the curve revealed that approximately 85% occupancy is reached at serum paroxetine levels of 28 μg/liter and that a considerable increase in serum levels is needed to obtain greater occupancy. No significant change in occupancy occurred for paroxetine levels greater than 28 μg/liter (linear regression, slope=–0.09% μg/liter, F=0.50, df=1, 3, p=0.53).
Figure 2 shows a plot of the relationship between occupancy and paroxetine serum levels.
Discussion
To our knowledge, this is the first study of 5-HTT occupancy during SSRI treatment of depression to use a within-subject design and a PET radioligand that is highly selective for 5-HTT. We found, on average, an 80% decrease in 5-HTT binding potential in the basal ganglia after 4 weeks of treatment. We also found that occupancy increased as the serum levels of paroxetine increased, reaching a plateau for serum concentrations greater than 28 μg/liter.
The 80% decrease in 5-HTT binding potential during treatment is best explained by the high affinity of paroxetine and citalopram for 5-HTT
(1,
2). As a result of drug binding to 5-HTT, fewer 5-HTT sites are available for [
11C]DASB binding, decreasing the detected 5-HTT binding potential. A second mechanism by which 5-HTT binding potential may decrease is by down-regulation of 5-HTT. It is reported that 5-HTT down-regulates and that 5-HTT mRNA is reduced in animals after long-term administration of SSRIs by minipump
(35–
37). Occupancy is a useful measure because it can reflect both blockade and down-regulation, and it is possible that both could reduce 5-HT clearance by means of 5-HTT and raise cortex 5-HT levels
(35). Raising cortex 5-HT levels is thought to be a key step in the therapeutic mechanism of SSRIs
(18,
38,
39).
The 80% decrease in 5-HTT binding potential appears to be lower than what would be predicted by the in vitro affinities of these SSRIs for 5-HTT
(1,
2). If SSRI serum levels and brain levels (near the 5-HTT site) were identical, one would expect greater than 99% occupancy (for a K
d for the 5-HTT of approximately 1 nM and 0.1 nM for citalopram and paroxetine, respectively [
1,
2]). The most plausible explanation for the discrepancy is that serum paroxetine and citalopram concentrations are different from the concentrations of these SSRIs near 5-HTT in the brain. This in not unexpected because other factors such as lipophilicity and degree of nonspecific binding may influence brain drug concentrations. These factors would affect serum concentrations differently.
Eighty percent occupancy of 5-HTT sites reduces the proportion of functioning 5-HTT sites to a lower level than would occur under physiological conditions. In animal studies of long-term serotonin depletion, the largest reductions in 5-HTT binding potential (estimated by changes in receptor density × affinity) are about 30%
(40,
41). It may be that an 80% occupancy is required to increase cortex serotonin levels to the degree that most therapeutic effects can occur. Virtually no investigations have been done to show that doses of citalopram and paroxetine below 20 mg/day effectively treat depression
(42). In fact, only a few clinical trials of low-dose SSRI treatment of depression have been done
(42–
44). Further studies with low-dose SSRIs would be needed to understand how occupancy levels specifically relate to therapeutic effects.
The relationship of increased occupancy with increased serum levels for very low drug serum levels and an occupancy plateau for higher serum levels has also been reported for neuroleptic medications
(32–
34). To our knowledge, the relationship between occupancy and serum levels has not been previously reported for any SSRI.
It has become common clinical practice to raise paroxetine dosing higher than 20 mg/day in the event of nonresponse or partial response. The data of the current study suggest that for the majority of patients, increasing paroxetine beyond 20 mg/day has minimal effects on 5-HTT blockade. However, it is still possible that higher dosing of paroxetine has therapeutic benefit either by inducing minimal increases in 5-HTT blockade or by increasing binding to sites other than 5-HTT.
What level of occupancy is optimal for clinical response is an interesting question. In the treatment of depression, multiple mechanisms of SSRI effect are possible, and it is known that placebo has a significant therapeutic influence. Therefore, it would be unrealistic to expect an association between occupancy and clinical response in our sample. However, findings of strong relationships between drug serum level and occupancy will eventually contribute to answering this question. By using large samples of data from clinical trials in which serum levels of antidepressants and clinical response were measured, occupancy estimates could be derived from serum antidepressant levels. This approach should provide good power to determine the relationship between occupancy and clinical response.
This study had several methodological limitations. The binding potential reflects Bmax/Kd, and we were unable to discern between these two parameters. However, the combined measure may be more relevant to 5-HTT function. With respect to the SSRI treatment, we took many precautions to enhance compliance, such as informing patients that their serum levels were to be assayed at the time of the second PET scan as part of the study. We also took serum measurements and detected paroxetine and citalopram levels in every patient. Even so, it is possible that some patients may have temporarily interrupted their treatment at an earlier point, and this interruption would not be detected in our protocol.
In conclusion, by using a new, more selective PET radioligand for 5-HTT, we found that 80% of 5-HTT receptors were occupied during treatment with clinical doses of paroxetine or citalopram. Although this change in 5-HTT binding potential was greater than what has been observed under physiological conditions, this level of change might be necessary for some therapeutic effects. The plateau found in the relationship between occupancy and serum paroxetine level indicates that raising paroxetine doses beyond 20 mg/day is unlikely to have much effect on 5-HTT.