Functional disability exacts a heavy toll on the aged population and on the informal and formal social resources they utilize. Functional disability in later life predicts use of medical resources, institutionalization, and mortality in late life
(1). Physical function declines with age, and the rate of functional decline accelerates as age increases
(2,
3). Unless disability rates are reduced, the rising number of elderly people with high rates of resource use will overwhelm the ability of clinicians, reimbursement systems, and families to provide needed assistance
(4). A potentially promising strategy for reduction of overall disability rates is the development of interventions targeting modifiable risk factors in the causal web of disability
(5).
One such risk factor is depression, which has been consistently associated with functional impairment in cross-sectional and longitudinal epidemiologic and clinical studies
(1,
5–
7). Randomized clinical trials have demonstrated that effective treatment of depression can improve functional outcomes
(6).
Community and clinical studies have also implicated the lack of social support as a modifiable risk factor for disability
(1,
7–
9). Synchrony of change in the severity of depression and social support has been observed in the disablement pathway
(9,
10). The interactive effects of affect and social support may lead to disability. For example, in a large population, subjective social support was a more effective buffer against functional decline among elderly patients with clinically significant depressive symptoms than among asymptomatic elderly people
(11). In contrast, the effects of subjective support on stroke patients were generally salutary, whether or not the patients met criteria for major depressive disorder
(12). However, to our knowledge, no studies have prospectively examined the protective effects of social support in patients with geriatric major depression or estimated the differential effects of social support across the severity-of-illness continuum.
The purpose of this study was to examine the independent and interactive effects of depressive symptom severity and the availability of social support on the functional disablement pathway of elderly patients with unipolar major depression. Two main effects and an interaction effect were hypothesized:
1. Initial severity of depressive symptoms predicts 1-year functional decline in performance on both basic and instrumental activities of daily living, after control for demographic factors, clinical features of the initial episode, and improvement in depressive symptoms during follow-up.
2. Social support, particularly the availability of instrumental aid and a subjective rating of social support, mitigates the harmful effects of depressive symptom severity and functional decline at 1 year.
3. Social support exerts increasingly robust effects against functional decline as depressive symptom severity increases.
Method
Design and Subjects
This study used a prospective cohort design. All subjects were participants in the National Institute of Mental Health (NIMH) Mental Health Clinical Research Center for the Study of Depression in Later Life at Duke University. All inpatients and outpatients of the Duke University Psychiatric Service aged 60 or older who were observed with clinically significant depressive symptoms or a previous diagnosis of mood disorder were screened with the Center for Epidemiologic Studies Depression Scale (CES-D Scale)
(13). Eligibility for the study was limited to patients with CES-D Scale scores of 16 or above or a diagnosis of unipolar major depression subject to the following exclusion criteria: 1) other major psychiatric illness, 2) alcohol or drug dependence, 3) clinically diagnosed primary neurologic illness, or 4) medical illness or physical disability affecting cognitive function. After a complete explanation of the procedures and the purpose of the study were provided, the patients who provided written informed consent were enrolled in the study. Virtually all (98%) of the subjects were treated within the Mental Health Clinical Research Center for the Study of Depression in Later Life. Treatment was naturalistic and determined by patient clinical status rather than by a fixed protocol.
Procedures
A trained interviewer administered the Duke Depression Evaluation Schedule, a composite diagnostic instrument that includes the depression assessment section of the NIMH Diagnostic Interview Schedule
(14) and measures of functional and cognitive status, medical conditions, and social support. Each patient was administered the 17-item Hamilton Depression Rating Scale
(15) as part of a standardized baseline clinical test battery. Clinical assessments were repeated at 6 and 12 months after the baseline assessments. The Duke Depression Evaluation Schedule was administered again at the 1-year follow-up.
Of the 159 patients who completed their 1-year follow-up assessment before August 1, 1999, 46 were excluded from our analyses owing to missing data. The excluded patients did not differ from the retained subjects on any demographic, clinical, or social support variable, with the exception of their higher prevalence of a history of cancer and their lower likelihood of treatment with newer antidepressants (e.g., venlafaxine, nefazodone, bupropion, or mirtazapine). The effective study size was N=113.
Dependent Measures
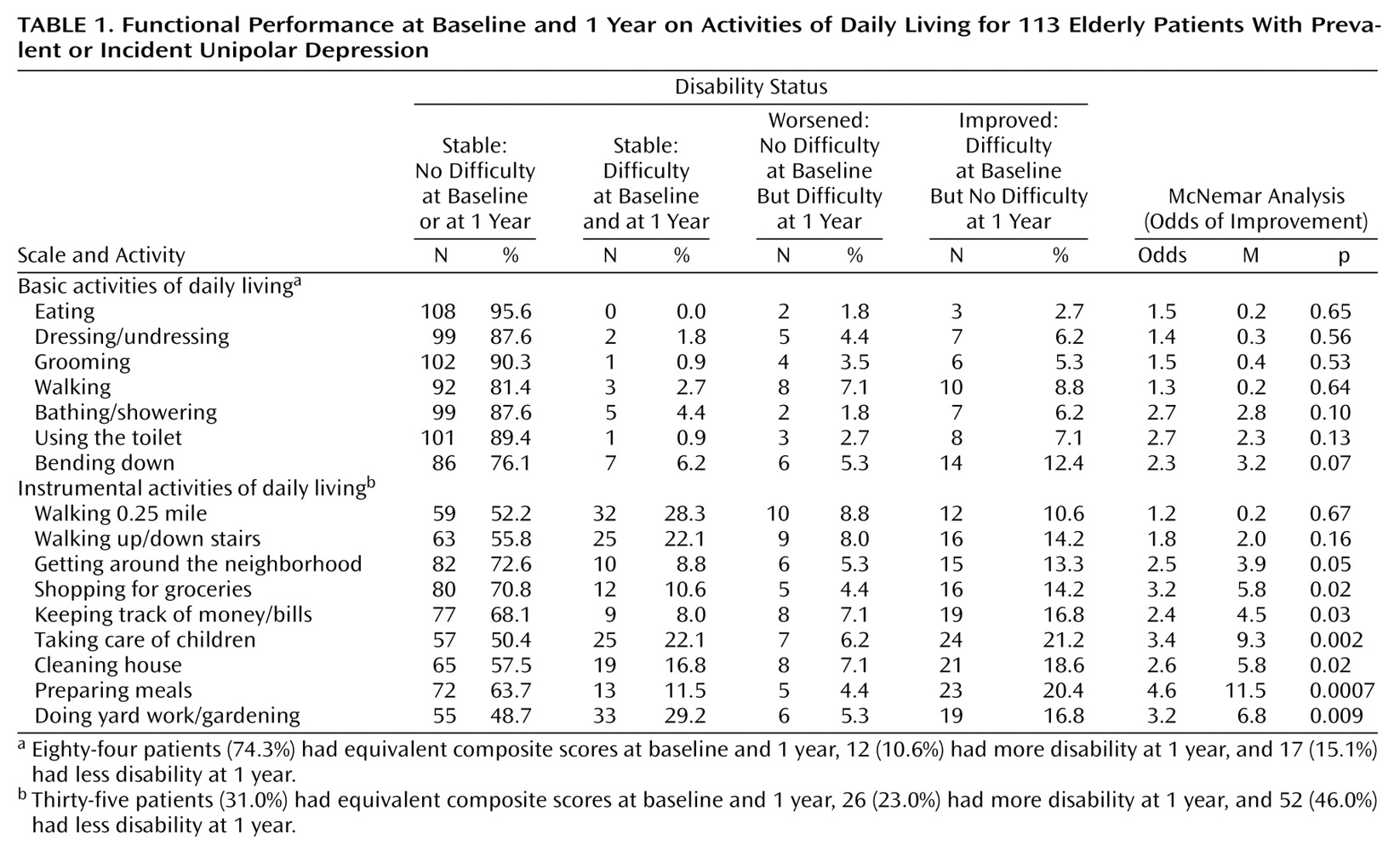
Outcome (functional status at the 1-year follow-up) was measured with 16 items assessing self-reported ability to perform activities of daily living in two domains of functional status (
Table 1). Seven items addressed basic activities of daily living; nine items addressed instrumental activities of daily living
(8). Item wording was standardized (i.e., “Can you…?”) and included a three-option answer format (“yes”=0, “with difficulty”=1, “no”=2). A composite measure was constructed for each domain of physical function by summing the scores for all items within that domain
(8).
Independent Measures
Independent variables included baseline measures of severity of depressive symptoms and availability of social support. Depression severity was assessed with the Hamilton depression scale, an instrument with well-established psychometric properties
(16). Four social support subscales, derived from factor analysis of the 35-item Duke Social Support Index
(17–
19), included size of social network (number of family members, co-workers, and friends and household size), amount of social interaction (family proximity, in-person and telephone contact with friends and family, and group affiliations), availability of instrumental aid (e.g., care during illness and help with errands, chores, finances, transportation), and subjective social support (e.g., feeling useful, listened to, satisfied with relationships). Cronbach’s alpha for the subjective social support subscale was 0.79. Measures of internal consistency were not employed as reliability coefficients for the other scales because there was no reason to expect high interitem correlations (e.g., between the number of family members and the number of co-workers). Social support scales were used in interval-level format for modeling change in disability, but we report descriptive statistics of “impaired social support” on the basis of population norms from the Epidemiologic Catchment Area study
(18).
Covariates
Potentially confounding variables included demographic characteristics (age, gender, education, marital status, living arrangements, and financial sufficiency) and self-reported health status and self-reported history of diabetes, heart trouble, hypertension, stroke, and cancer from the baseline Duke Depression Evaluation Schedule. Clinical psychiatric covariates included age at onset of major depression, number of lifetime depressive episodes, history of ECT, self-reported family history of nervous disorder, and psychotropic medications prescribed during follow-up. Cognitive impairment at baseline was assessed with the Mini-Mental State Examination (MMSE)
(20).
Analysis
For each of the 16 individual activities of daily living, original three-option responses were recoded as a dichotomous variable (“yes”=0, “with difficulty,” and “no”=1). The ability to perform each task at baseline and at the year-1 follow-up was then compared, and the patients/activities were classified as follows: a) stable/not difficult: task not difficult at both baseline and 1 year; b) stable/difficult: task difficult/not performable at both baseline and 1 year; c) worsened disability: task not difficult at baseline, but task difficult/not performable at 1 year; d) improved performance: task difficult/not performable at baseline but not difficult at 1 year. The prevalence of difficulty on any task was the sum of the number of patients in groups b and d; the incidence of difficulty on any task was the number of patients in group c.
If baseline and 1-year function scores had been cross-tabulated by task, scores for groups a and b would have fallen directly on the diagonal slope, and scores for groups c and d would have fallen away from the diagonal slope. When there was a change in function between baseline and 1-year assessments, the McNemar M statistic tested whether the median population change between the paired scores differed from 0. The M statistic is calculated as (d – c)2/(d + c) and is compared to a chi-square distribution; i.e., the critical region for p<0.05 and df=1 is M=3.84. Whenever there was a change in function, the McNemar odds (calculated as d/c) showed the magnitude of change over time, i.e., an improvement when the odds were greater than 1.00 or a worsening when the odds were less than 1.00.
Composite scores for basic and instrumental activities of daily living were also computed for baseline and 1 year as the sum of dichotomized scores on individual tasks
(8). Respondents with stable composite scores across time reported equivalent overall burden of difficulty/inability at both baseline and 1 year. Subjects with stable composite scores may have regained the ability to perform one or more tasks and lost the ability to perform other tasks in that domain, for a net change of 0.
Composite scores only were used for hypothesis testing. Hypotheses were tested with multivariable ordinary least squares regression
(21). First, we regressed scores for performance of composite basic and instrumental activities of daily living at follow-up over baseline depression severity and adjusted each model for both the corresponding composite score for activities of daily living (basic or instrumental) at baseline and for selected covariates. Next, social support scores were added to models containing scores for depression, scores for baseline activities of daily living, and the covariates. To avoid multicollinearity among the dimensions of social support, stepwise procedures (entry level: p<0.10) were used to select the optimal social support scales for prediction of change in domains of functional ability
(22). This strategy maximized the power to detect differences; we had already minimized the possibility of a type II error by using a priori hypotheses. Finally, the models for change in function were tested for significant interactive effects between depressive symptoms and each of the social support measures, with simultaneous control for the main effects of the two constituent variables and the same covariates.
Potential confounders were selected for parsimony, to avoid overfitting the models. Covariates that were related at p≤0.05 in bivariate analyses (not shown) to depression or social support and to one or both outcomes were considered to be confounders
(23) and were included in all models: gender, self-rated health status, and cognitive status. The patients with prevalent and incident unipolar depression differed on baseline composite scores for basic activities of daily living but did not differ on scores for either baseline depression or social support (nor did they differ on scores for baseline composite instrumental activities of daily living) and therefore were not considered to have confounded the observed relationship. Nevertheless, multivariable analyses were performed on pooled data and also on data stratified by patient status (incident or prevalent depression). All models were adjusted for patient age.
For main effects models (hypotheses 1 and 2), unstandardized negative coefficients would represent protective effects against functional decline; positive coefficients would represent risk factors for functional decline. For interactive effects (hypothesis 3), one would expect significant negative coefficients for the interaction terms, indicating that higher-quality social support, in the presence of greater symptom severity, is protective against increasing levels of disability over time.
Results
The mean age of the patients was 69.5 years (SD=7.5); 69% were female, 64% reported college credit, 57% were married, 27% lived alone, and 91% reported that their income covered their needs “fairly” or “very well.” The prevalence of self-reported chronic medical conditions was as follows: diabetes (9%), “heart trouble” (20%), hypertension (38%), stroke (4%), and cancer (11%); just under one-half rated their overall health status as “poor” or “fair.” Their mean MMSE score was 28 (SD=3). At baseline, low availability of instrumental social support was reported by 17 (15%) of the subjects, low frequency of social interactions by 21 (19%), impaired subjective social support by 49 (43%), and small social network size (two or fewer persons) by 75 (66%). Their mean composite baseline score for basic activities of daily living was 0.8 (mode=0, median=0, range=0–9); for instrumental activities of daily living, it was 4.4 (mode=0, median=3, range=0–18).
The clinical characteristics of the subjects at baseline were as follows. Their mean Hamilton depression scale score was 21.9 (SD=8.9). Twenty (18%) of the patients had incident cases of depression. The mean number of lifetime episodes was 7.9 (SD=11.3). Late age at onset (after age 50) was reported by 42 (37%) of the subjects, a history of ECT by 24 (21%), and a family history of nervous disorder by 58 (51%). The prevalences for receiving prescriptions for psychotropic medications during the year between baseline and follow-up were as follows: any anxiolytic: 62 (55%), any benzodiazepine: 48 (42%), any selective serotonin reuptake inhibitor: 57 (50%), any tricyclic: 32 (28%), and any newer antidepressant: 93 (82%). Between baseline and 1 year, Hamilton depression scale scores declined by an average of 11.9 points (interquartile range=5–18). However, 20 (18%) of the participants were moderately depressed (Hamilton depression scale score of 18 or higher), seven (6%) were severely depressed (Hamilton depression scale score of 25 or higher) at follow-up, and 1-year Hamilton depression scale scores were equal to or higher than baseline scores in 11 (10%) of the subjects.
The proportions of subjects who reported stable, worsening, or improving functional ability on each basic and instrumental activities of daily living task are presented in
Table 1. With respect to basic tasks, the proportion of subjects who improved was not significantly different from the proportion who worsened over time. For instrumental tasks, with the exception of walking 0.25 mile and walking up/down stairs, the proportions of patients who improved and worsened were significantly different. Where there was change, the greatest difference was in meal preparation, in which the odds of improvement in ability were 4.6 times the odds of worsening ability.
Composite scores were equivalent at baseline and 1 year for 84 (74%) of the patients on basic activities of daily living and 35 (31%) of the patients on instrumental activities of daily living. In other words, the base rate of change in total scores was 26% (12 + 17/113) for basic activities of daily living and 69% (26 + 52/113) for instrumental activities of daily living.
Multivariate ordinary least squares models provided strong support for hypothesis 1, that depressive symptoms would predict functional declines at 1 year. After control for baseline composite scores for basic activities of daily living, age, sex, self-rated health status, and cognitive impairment, there remained a robust effect of baseline depression severity on composite scores for basic activities of daily living at follow-up (b=0.08, t=3.64, df=1, p=0.0004). The Pearson correlation coefficient for the association of baseline depression score with follow-up score on basic activities of daily living was r=0.38 (df=112, p<0.0001). Depression severity exerted a similar effect on composite scores for instrumental activities of daily living (b=0.13, t=2.94, df=1, p=0.004; Pearson’s r=0.53, df=112, p<0.0001) in models adjusted for baseline functional ability and other risk factors for functional decline. Thus, severity of depression at baseline was highly predictive of deterioration in both domains of functional ability.
There was modest support for hypothesis 2, that social support would mitigate the effect of depression severity on functional declines at 1 year. When change in composite scores on basic activities of daily living was modeled with adjustments for severity of baseline depression and other covariates, only the subscale score for subjective social support met criteria for inclusion as a buffer against increases in functional impairment (b=–0.08, t=–1.69, df=1, p=0.09). A more robust buffering effect was demonstrated for instrumental social support (b=–0.42, t=–2.54, df=1, p=0.01) in the adjusted model of change in composite scores for instrumental activities of daily living. Explanatory variables explained 22% of the variance in the basic activities of daily living model and 42% of the variance in the instrumental activities of daily living model. The discrepancy between these explanatory values is likely due to the lower base rate of change in the composite scores for basic activities of daily living.
There was partial support for hypothesis 3, that the buffering effects of social support against functional decline would be strongest among the most severely depressed patients. The protective effects of improved social support were stronger in the subjects with higher baseline Hamilton depression scale scores for three of the four social support measures in ordinary least squares models of composite scores of basic activities of daily living. The interaction terms between depression severity and three of the measures of social support—larger social networks (b=–0.01, t=–2.14, df=1, p=0.03), more frequent social interactions (b=–0.02, t=–2.17, df=1, p=0.03), and the subjective adequacy of social support (b=–0.01, t=–1.96, df=1, p=0.05)—were significant and negative. These models explained 20%–24% of the variance in declines in composite scores on basic activities of daily living. The availability of instrumental support did not buffer the deleterious effects of depression on composite scores for basic activities of daily living. Furthermore, there was no evidence that high-quality social support in any domain buffered the most depressed elderly patients disproportionately against greater impairment in performance on instrumental activities of daily living.
When multivariable analyses were done with data stratified by patients’ incident (N=20) versus prevalent (N=93) depression status, the results of hypothesis testing among patients with prevalent depression were virtually identical to those of the pooled study group. Among patients with incident depression, the effects were in the same direction as in the total study group, but their magnitude was smaller, and no statistical support was found for any of the hypotheses.
Discussion
Several epidemiologic studies
(24–
26) have indicated that severe depressive symptoms can trigger incident declines in performance of basic activities of daily living and gross motor ability in elderly subjects; however, there is study of very old Canadians with dissenting findings
(27). Our data support the majority of the studies described and extend their findings, showing that symptom severity among elderly patients with recurrent unipolar depression predicts increasing impairment of function in basic activities of daily living and more robustly in instrumental activities of daily living.
Evidence has been more mixed about the role of social support as a buffer between depression and functional ability. In a longitudinal study of community-dwelling elderly subjects
(11), social support was found to buffer the effects of depression on the risk of functional impairment in multiple domains. In contrast, social function directly promoted recovery in a clinical study of stroke patients
(12), but there was no evidence for stronger effects among more depressed patients. Among clinically depressed elderly patients, we found evidence of a stronger compensatory effect for social support among the most depressed patients, with respect to preserving and improving their ability to perform basic activities of daily living.
Several possible mechanisms could account for the interactive effects of social support and depression on functional impairment. Social support may buffer the neuroendocrine effects of depression. Greater social interaction may encourage depressed patients to remain physically active, decreasing the potential severity of their impairment. Patient compliance with treatment may also be greater in the presence of a supportive environment.
All of the conclusions described are predicated on limited power to detect differences. Group size limitations may be relevant to conclusions about decline in function on basic activities of daily living, on which the base rate for deterioration was low. A similar caveat applies to conclusions with respect to patients with incident depression. The observed effects were most robust among patients with recurrent episodes, and those experiencing a first episode were too few to ensure reliable conclusions. A second limitation was the use of self-rated items for the assessment of performance on basic and instrumental activities of daily living as outcome variables. Had these been based on clinician, interviewer, or family observations of performance, more objectivity could be claimed. It is possible, as well, that active depressive symptoms produce artifactual self-ratings of functional ability or social support. However, for the measures of social support used in this study, previous research suggests that such artifactual effects are minimal
(17). A third limitation was the exclusion of the 46 subjects for whom data were missing. These individuals did not differ on any substantive variables in this study, but other underlying factors not assessed may differ between the two groups.
Not only are mood and social support remarkably dynamic states in late life, but so is functional ability. Higher rates of change were characteristic of these depressed elderly patients. This study provides important new information on the natural history of functional ability among clinically depressed elderly patients undergoing treatment and suggests that in collaboration with formal and informal resources, even the most depressed and disabled patients may experience functional improvement over time.