Sexual dysfunction is often associated with major depressive disorder
(1). However, antidepressant medications used to treat major depressive disorder can impair sexual function
(2). Problems with ejaculation, orgasm, erection, and sexual desire are frequently reported in patients with major depressive disorder who are receiving treatment with selective serotonin reuptake inhibitors and other antidepressants
(2,
3). Studies
(3,
4) have emphasized the importance of recognizing sexual dysfunction in patients being treated for depression because it may cause noncompliance, dose reduction to subtherapeutic levels, switching to a less effective agent, and relapse into depression. Thus, the management of treatment-related sexual dysfunction is essential.
Sildenafil citrate, the first oral treatment for erectile dysfunction of mixed etiologies
(5), is a selective inhibitor of cGMP-specific phosphodiesterase type 5 that enables an erectile response upon sexual stimulation. We examined whether sildenafil alleviates erectile dysfunction in men taking concomitant serotonin-reuptake-inhibiting antidepressants.
Method
This retrospective subanalysis combined data from 10 phase II/III double-blind, placebo-controlled, fixed- and flexible-dose studies with sildenafil for the treatment of erectile dysfunction of psychogenic, organic, or mixed etiology. Patients received 5 to 200 mg as needed, but not more than once daily, of sildenafil or matching placebo for 12 to 26 weeks. The groups were further subdivided into those who received concomitant serotonergic antidepressants and those who did not. The patients were instructed to take sildenafil approximately 1 hour before anticipated sexual activity but not more than once daily. Adverse events were recorded.
A total of 3,414 male patients aged 18 years or older who had a clinical diagnosis of erectile dysfunction of more than 6 months’ duration were randomly assigned to treatment. The exclusion criteria included major illness or uncontrolled psychiatric disorder, serious cardiovascular disease within the previous 6 months, retinitis pigmentosa, or use of nitrates in any form. After complete description of the study to the subjects, written informed consent was obtained.
Efficacy was assessed at baseline and at the end of treatment by responses to five items from the validated 15-item International Index of Erectile Function
(6). The questions are scored from 1 (“almost never/never”) to 5 (“almost always/always”), with 0 indicating “did not attempt sexual intercourse.” Higher scores indicate better sexual function.
End-of-treatment scores and changes from baseline were analyzed by using two-factor analysis of covariance (ANCOVA). Factor 1 was treatment, sildenafil or placebo, and factor 2 was antidepressant status, taking or not taking a serotonin-reuptake-inhibiting antidepressant. The ANCOVA allowed for the covariates of baseline efficacy measure score, age, smoking status (smoker, ex-smoker, or nonsmoker), duration of disease, and etiology of disease (organic, psychogenic, or mixed). The last-observation-carried-forward algorithm was used for subjects who discontinued treatment. SAS type III sums of squares (Cary, N.C., SAS Institute) were used to assess the significance of the model parameters. A p value of <0.05 with Bonferroni correction was considered statistically significant.
Results
Of 3,414 entered patients (mean age=56.1 years, SD=11.1; mean duration of erectile dysfunction=5.0 years, SD=5.1), 3,019 (88%) completed treatment. Data for efficacy analysis were available for 3,409 patients. Of the available patients, 98 received treatment with serotonin-reuptake-inhibiting antidepressants: 65 were also taking sildenafil (mean age=53.8 years, SD=11.2; mean duration of erectile dysfunction=5.7 years, SD=5.5), and 33 were taking placebo (mean age=51.2 years, SD=12.0; mean duration of erectile dysfunction=4.9 years, SD=5.4). The serotonergic antidepressants included fluoxetine, nefazodone, paroxetine, sertraline, and venlafaxine, although there is some debate regarding the ability of nefazodone to produce sexual dysfunction. The most common adverse events were headache (sildenafil, 22%; placebo, 6%), flushing (sildenafil, 15%; placebo, 2%), and dyspepsia (sildenafil, 11%; placebo, 2%), and they were predominantly mild to moderate in severity.
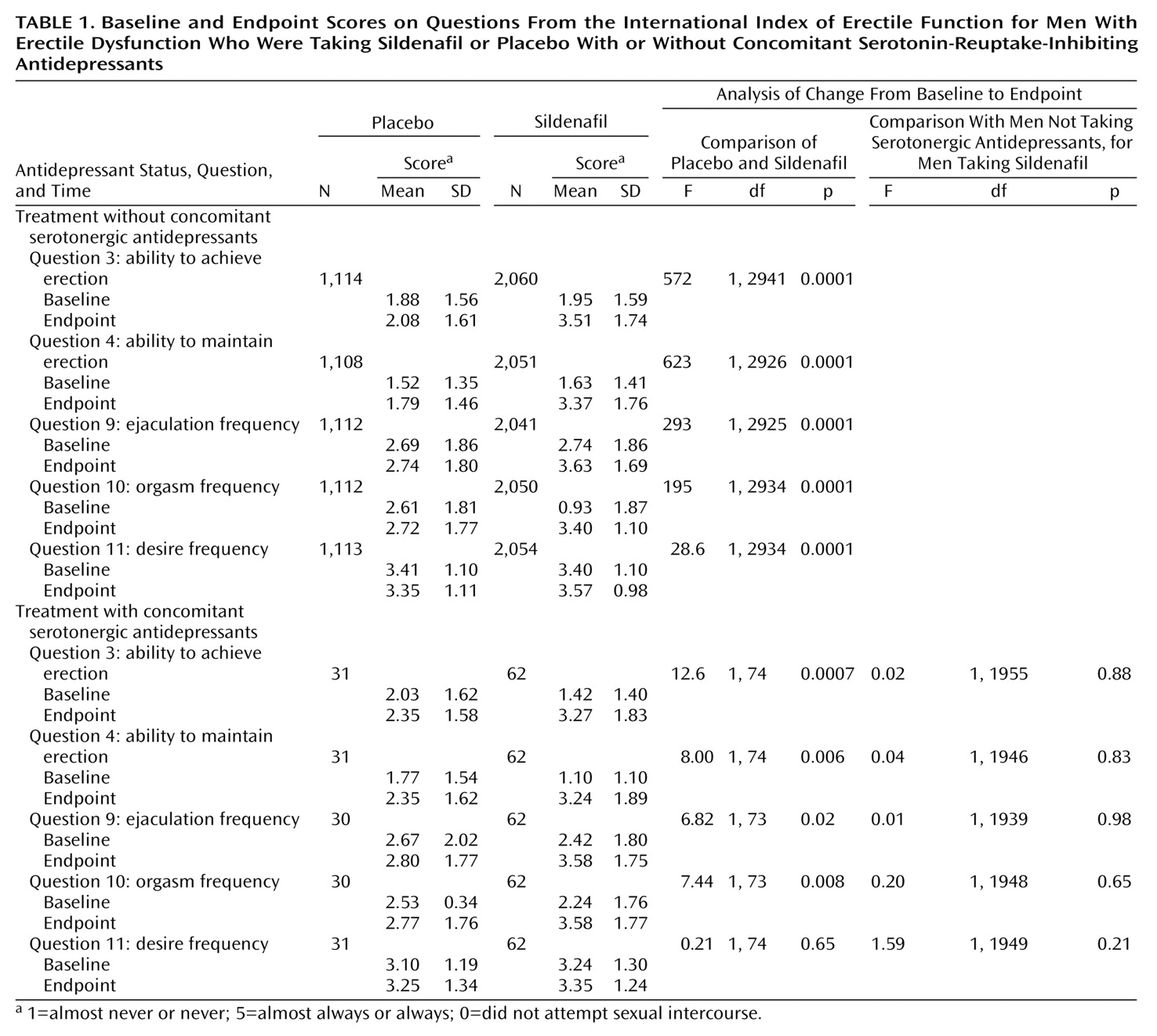
The ANCOVA revealed a significant effect due to sildenafil, factor 1, for each of the five questions from the International Index of Erectile Function, regardless of whether the patients did or did not receive serotonin-reuptake-inhibiting antidepressants (
Table 1). The only exception was “frequency of sexual desire” (Q11), for which the scores for the patients receiving sildenafil and serotonergic antidepressants were not significantly different from the scores for the patients receiving placebo and serotonergic antidepressants.
There were no significant effects due to treatment with serotonin-reuptake-inhibiting antidepressants, factor 2. Indeed, there were no significant end-of-treatment differences in the improvements afforded by sildenafil between the men who were and were not also taking serotonergic antidepressants (
Table 1).
Discussion
The relationship of depression, antidepressant treatment, and erectile dysfunction is dynamic and complex. Patients with major depression initially affecting libido may develop renewed sexual interest with effective treatment but subsequently develop sexual dysfunction (including erectile dysfunction) with antidepressant medication
(1). Our data indicate that sildenafil treatment significantly improved erectile function, frequency of ejaculation, and orgasm in patients with erectile dysfunction whether or not they were receiving concomitant treatment with serotonin-reuptake-inhibiting antidepressants. Consistent with findings from other studies
(5) was our observation of no appreciable increase in the level of sexual desire (Q11) associated with taking sildenafil. The modest observed increase in desire is likely secondary to overall improvement in sexual performance.
Sildenafil has shown significant efficacy in patients with broad-spectrum erectile dysfunction, including those using concomitant medications such as antihypertensives
(5,
7). In case studies and open-label trials
(8–
10) sildenafil treatment for sexual dysfunction associated with serotonergic antidepressants has been effective in alleviating erectile dysfunction and associated symptoms of sexual dysfunction. An easily administered treatment option, such as oral sildenafil, may help keep patients on antidepressant regimens that have been effective.
This retrospective analysis is limited to the finding that treatment with serotonin-reuptake-inhibiting antidepressants did not interfere with the sildenafil treatment of erectile dysfunction. Although the gains in sexual functioning, relative to those for patients receiving placebo and concomitant serotonergic antidepressant treatment, were significant, the number of subjects receiving sildenafil and concomitant serotonergic antidepressants was modest (65 patients). A prospective double-blind, placebo-controlled study of subjects without preexisting sexual dysfunction when they begin taking serotonergic antidepressants for depression would strengthen the evidence for benefits of sildenafil in patients with depression treated with serotonin-reuptake-inhibiting antidepressants. That would support the hypothesis that sildenafil administration may enable patients with major depressive disorder who experience treatment-emergent sexual dysfunction to continue taking the medication that provides effective treatment for their illness.