Relatively recent reports suggest that hormone-replacement therapy may have a protective effect on the aging brain. Hormone-replacement therapy may lower the risk of Alzheimer’s disease
(1) and prevent normal age-related decline in cognition
(2). Neurobiological studies indicate a number of mechanisms through which estrogen might protect the hippocampus and other brain regions affected by dementia and cognitive aging
(3). In contrast, findings from three recent randomized trials suggest no beneficial effect of hormone-replacement therapy on cognitive function in women with a diagnosis of Alzheimer’s disease
(4–
6). Together, these studies suggest that hormone-replacement therapy may confer cognitive benefits only to women who are free of dementia. In this field of research, studies of cognition in women without dementia have produced the most conflicting results, with some studies showing no beneficial effects of hormone-replacement therapy on cognitive function
(7,
8), others revealing a benefit to memory
(9–
13), and another showing a general cognitive benefit
(14).
Factors contributing to the discrepancy in results across these studies include differences in the age of participants, the focus on current versus any use of hormone-replacement therapy, the cognitive domains studied, the specific tests administered, and the statistical approaches used to control for group differences in confounding variables. However, the most notable limitation of observational studies is the “healthy-user bias,” which is the tendency for women who receive hormone-replacement therapy to be healthier and better educated than nonrecipients
(15,
16). Any cognitive advantage seen among recipients of hormone-replacement therapy in nonrandomized studies may be due to the characteristics of the women who elect to receive hormone-replacement therapy rather than the physiological properties of estrogen. To control for healthy-user bias, researchers commonly adjust cognitive test scores for group differences in education. This statistical correction may not adequately control for such bias or may result in overadjustments that mask a reliable but subtle hormonal effect. One approach to limiting the healthy-user bias in observational studies is to examine recipients and nonrecipients of hormone-replacement therapy in a cohort of women whose health and educational status is uniformly high. Study groups selected from such a cohort are more likely to be naturally matched on education, intelligence, and health status, thus eliminating the need for statistical correction. We followed this approach in the present study.
Our primary aims were 1) to compare cognitive function in nondemented older women receiving hormone-replacement therapy and women who had never received such therapy who were naturally matched on factors that have limited previous studies, 2) to investigate whether the apparent beneficial effect of hormone-replacement therapy on cognition is limited to memory or whether the benefits extend to other cognitive domains, and 3) to determine which aspects of memory might be most affected by hormone-replacement therapy. A better understanding of the effects of hormone-replacement therapy on specific aspects of memory processing—encoding, consolidation, and retrieval—might lend critical insights into the discrepancy in findings across several large-scale observational studies using different memory tests. To this end, we conducted a cross-sectional observational study comparing cognitive outcomes in two groups of women—active recipients of hormone-replacement therapy and women who had never received such therapy—that were naturally matched on cognitive and health status. The prospective nature of the study allowed us to exclude women who developed dementia within 5 years of test administration and thereby focus on the effects of hormone-replacement therapy on normal aging. We report evidence that hormone-replacement therapy is associated with an enhancement in cognitive function in nondemented women that is selective to verbal learning and memory.
Method
Study Population
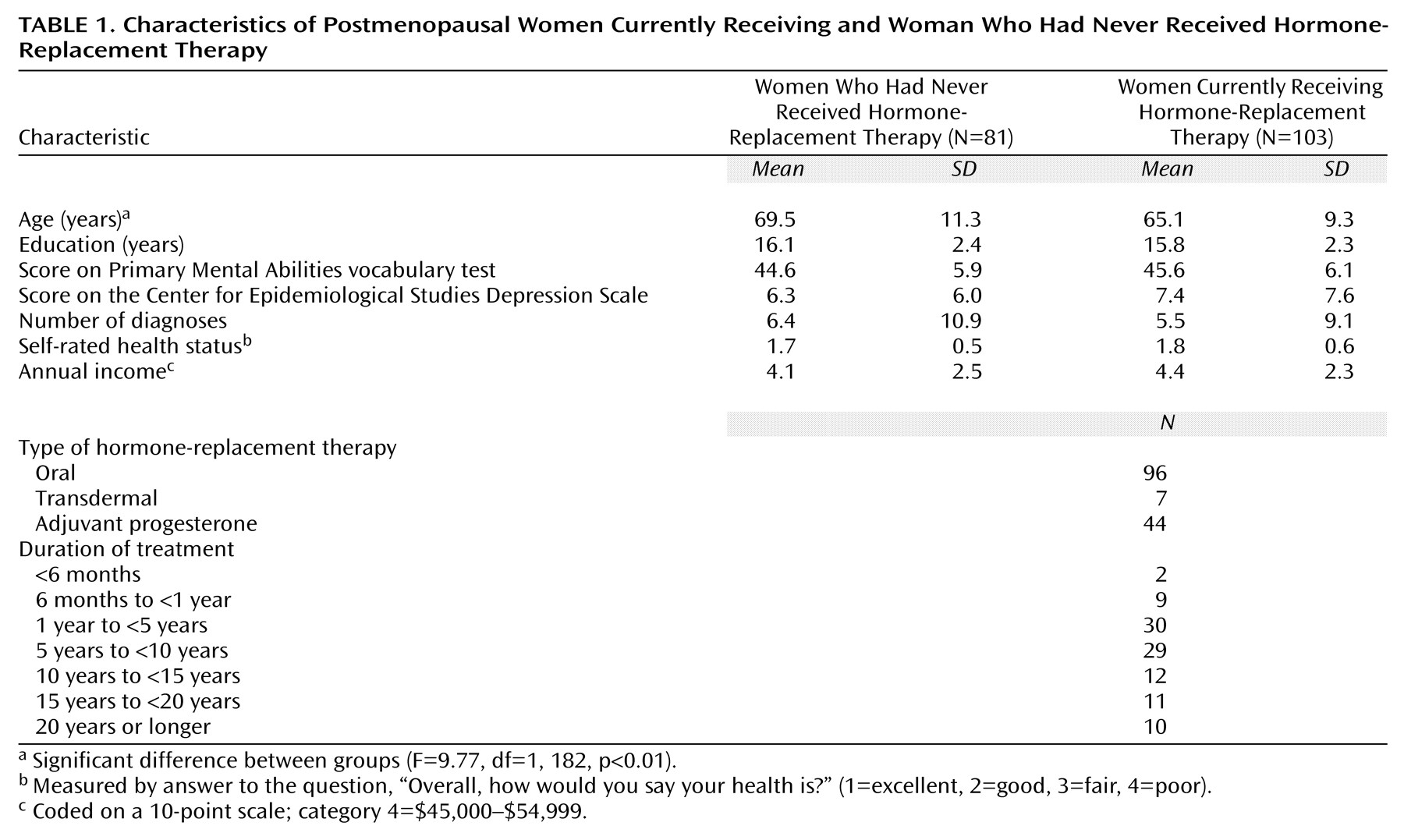
The participants were 184 nondemented postmenopausal women who were enrolled in the Baltimore Longitudinal Study of Aging at the National Institute on Aging
(17). Participants were aged 50–89, had completed the California Verbal Learning Test, and either were receiving oral or transdermal estrogen or had never received hormone-replacement therapy at the time of assessment. From an original sample of 561 women in the Baltimore Longitudinal Study of Aging with California Verbal Learning Test data, we excluded 240 because they were not postmenopausal, 87 because they had received estrogen in the past but were not active recipients at the time of assessment, 28 because they used estrogen creams alone at the time of assessment, 19 because they received a diagnosis of suspected, possible, or probable dementia within 5 years of follow-up (11 who had never received hormone-replacement therapy and eight active recipients), one who had not received hormone-replacement therapy because she had radiation-induced menopause, and two because they demonstrated poor verbal knowledge by scoring three standard deviations below the mean (less than the first percentile) on the Primary Mental Abilities vocabulary test
(18) (one who had never received hormone-replacement therapy and one active recipient). Thus the final study group consisted of 81 women who had never received hormone-replacement therapy and 103 active recipients. After a complete description of the study was given to the subjects, written informed consent was obtained.
Use of Hormone-Replacement Therapy
Current and past estrogen and progesterone use, duration of treatment, and type of treatment (i.e., oral, transdermal, or cream) were documented prospectively at each assessment by the Baltimore Longitudinal Study of Aging by use of a questionnaire designed specifically to assess history of hormone-replacement therapy. The questionnaire data were supplemented at each visit by a complete inventory of all current and past medications. The two study groups of interest were women with no history of transdermal or oral hormone-replacement therapy and women receiving transdermal or oral hormone-replacement therapy at the time of cognitive assessment. To ensure the most reliable estimate of hormone-replacement therapy use, we reviewed each individual’s longitudinal data for self-reported hormone-replacement therapy use and, where necessary, recoded the data. The recoding was based on the assumption that accuracy in the recall of use of hormone-replacement therapy decreases with time since use and with advancing age. Women who at the time of the cognitive assessment reported never receiving estrogen but who indicated either past or current estrogen use at earlier visits (N=17) were coded as past recipients. This coding took advantage of the prospectively collected information and avoided artificially lowering the estimate of memory performance by those who had never received hormone-replacement therapy. The recoding minimized the likelihood of finding significant group differences where none existed. The duration of hormone-replacement therapy was coded on a 7-point scale: 1=less than 6 months, 2=6 months to less than 1 year, 3=1 year to less than 5 years, 4=5 years to less than 10 years, 5=10 years to less than 15 years, 6=15 years to less than 20 years, and 7=20 years or longer. Reliable information on dosage of hormone-replacement therapy was not available.
Tests and Outcome Measures
The California Verbal Learning Test
(19) is a widely used assessment of verbal learning and memory composed of two 16-item shopping lists. Each list contains four items (e.g., grapes) from four semantic categories (e.g., fruits). Two of the categories overlap between the two lists, but the items are unique. On the learning trials, the 16 items are read aloud to the participant, who immediately tries to recall as many as possible. The order of administration is five learning trials with list A, a learning trial with list B, and a series of delayed trials with list A, including short-delay free recall, short-delay cued recall, long-delay free recall, long-delay cued recall, and long-delay recognition. California Verbal Learning Test outcome measures consisted of performance on the learning, recall, and recognition trials and several derived measures (see Results). The Benton Visual Retention Test
(20) measures short-term figural memory. In each of 10 trials, participants briefly view a line drawing and then immediately try to draw it from memory. The outcome measure was the total number of errors. A modified version
(21) of the Card Rotations Test
(22) measured mental rotations and required discrimination between two- and three-dimensional rotations of simple geometric figures. The outcome measure was the number of correct responses minus the number of incorrect responses. The digit span test is a subtest of the WAIS-R
(23) consisting of two tasks, forward and backward digit span tests, that measure attention and working memory. The outcome measure for each task was the number of correct responses. Self-reported depressive symptoms were measured with the Center for Epidemiological Studies Depression Scale
(24), and self-reported health was measured by answer to the question, “Overall, how would you say your health is?” (1=excellent, 2=good, 3=fair, 4=poor).
Data Selection
The California Verbal Learning Test was the primary measure of interest in the present investigation before analysis. Scores for the other tests were obtained for the visit at which the California Verbal Learning Test was first administered (1993 and on) and included scores on the Benton Visual Retention Test for comparison with data from a previous report
(9). Participants had taken the Benton Visual Retention Test on average four times previously. In contrast, the Card Rotations Test and digit span tests were introduced into the Baltimore Longitudinal Study of Aging at the same time as the California Verbal Learning Test; in almost every case, measures from those tests are also from the first test administration. The group sizes for some tests were slightly smaller than those for the California Verbal Learning Test measures.
Data Analysis
Statistical analyses were conducted by using SPSS statistical software (version 4.1 for VAX/VMS, SPSS, Chicago). Analyses were conducted on age-corrected z scores. Because of significant differences in age between those who had never received hormone-replacement therapy and active recipients, we applied an age-banding technique for age correction by creating standard scores, a technique that does not assume linearity. This approach was preferred over an analysis of covariance, because the pattern of age-related changes varied across dependent measures, with some measures showing an accelerated increase after age 70 and others showing a more linear increase. To derive age-corrected scores, we first computed the mean and standard deviation for each test measure for each of the 10-year age bands (i.e., 50–59, 60–69, 70–79, and 80–89). For each test, we derived age-corrected z scores for each participant by computing the difference between her score and the average score of the other women in her age band and then dividing the difference by the standard deviation of her age band. These age-corrected z scores are in standardized units (i.e., a score of 0.25 indicates a score one-quarter standard deviation above the mean). The normative group used to compute the average scores included those who had never received hormone-replacement therapy, past recipients, and active recipients of any form of hormone-replacement therapy who met the study’s other inclusionary criteria (i.e., female, aged 50–89, nondemented, and postmenopausal). For those with multiple assessments by the Baltimore Longitudinal Study of Aging, data used in creating age-corrected z scores were from the same test session at which the California Verbal Learning Test was first administered.
To examine the effects of hormone-replacement therapy (i.e., current versus never received) on cognition while controlling for multiple outcomes and intercorrelations among outcome measures, we conducted a multivariate analysis of variance (MANOVA) on all scores simultaneously. When necessary, we entered the inverse value into the MANOVA so that higher values consistently indicated better performance. If the MANOVA revealed a significant group difference, we then conducted a series of univariate analyses to determine the outcome measures on which the groups differed (p<0.05, two-tailed). The same statistical approach was used to explore the effects of adjuvant progesterone use on cognitive performance in recipients of hormone-replacement therapy, although there was limited statistical power to detect such effects in the group of 103 recipients. Effects of the duration of hormone-replacement therapy on cognitive function were examined with Pearson’s product-moment correlations.
Discussion
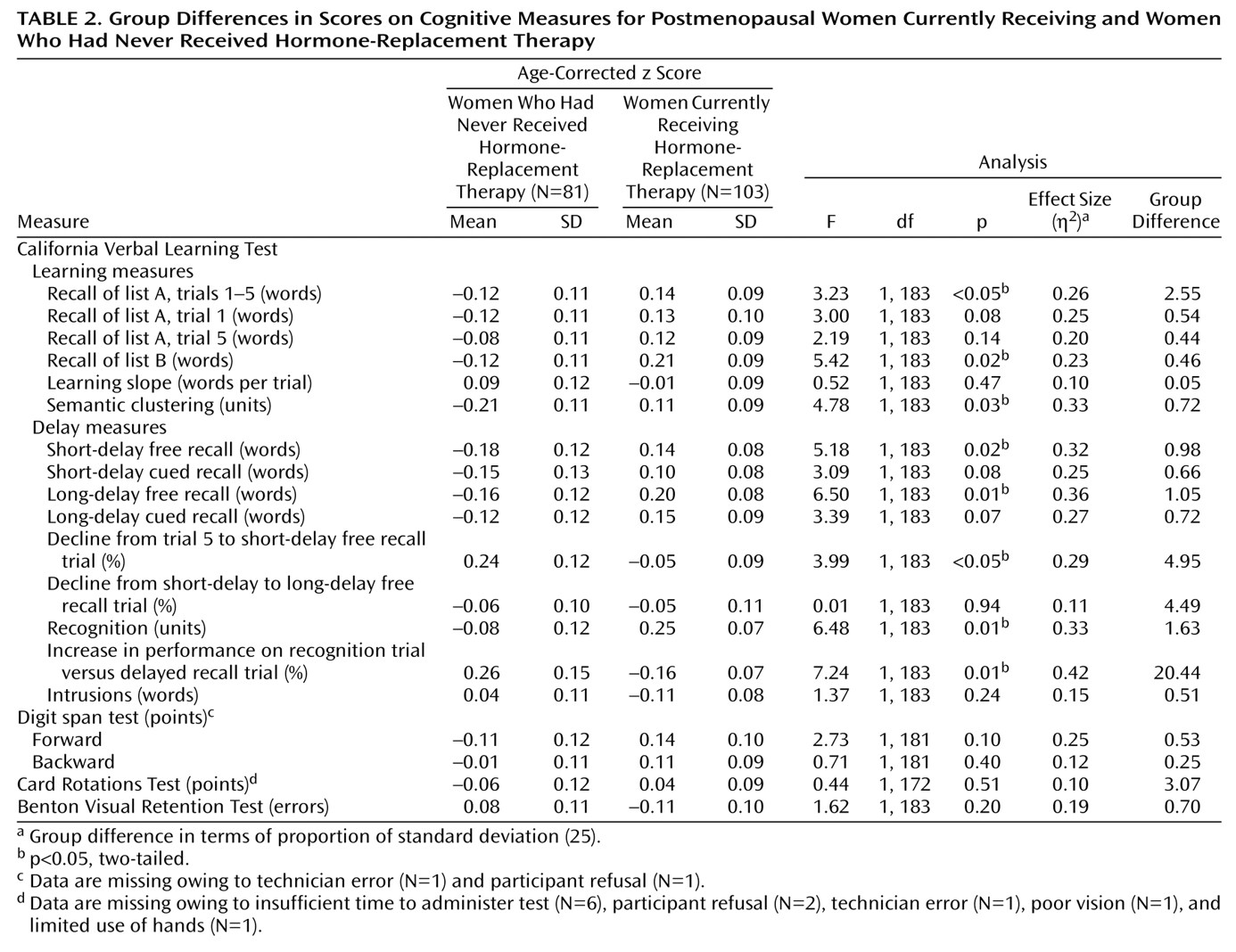
In this study, women receiving hormone-replacement therapy showed significantly better learning and memory on a verbal memory test than women who never received hormone-replacement therapy. Hormone-replacement therapy was associated with better encoding and retrieval of words and better retention of words after an interference task. In contrast, recipients of hormone-replacement therapy and those who had never received such therapy performed similarly on tests of short-term figural memory, mental rotations, attention, and working memory. These data suggest that hormone-replacement therapy is associated with enhanced verbal memory in nondemented postmenopausal women.
Because this was not a randomized trial, it is possible that the observed group differences in verbal memory were not due to the physiological effects of hormone-replacement therapy on the brain but rather to one or more confounding factors distinguishing those receiving hormone-replacement therapy from those who had never received such therapy. However, we observed no differences between groups on any confound affecting cognition except age. Recipients of hormone-replacement therapy and those who had never received such treatment were selected from a sample of highly educated volunteers in the Baltimore Longitudinal Study of Aging who had above-average economic and health status and did not differ in years of education, number of diagnoses, household income, or vocabulary. The availability of naturally matched groups eliminated the need for statistical correction that may have over- or underadjusted cognitive test scores. Scores were adjusted for age, because recipients of hormone-replacement therapy were younger than those who had never received such treatment. That adjustment decreased the likelihood of significant group differences in cognition by lowering the scores of the younger recipients of hormone-replacement therapy and raising the scores of the older women who had never received such therapy. In summary, despite no measurable advantage in health status, education, mood, or economic status, recipients of hormone-replacement therapy showed significantly better verbal learning and memory than the group that had never received such treatment.
Contrary to previous findings in the Baltimore Longitudinal Study of Aging of a small but reliable effect of hormone-replacement therapy on short-term figural memory
(9), we found no significant beneficial effect of hormone-replacement therapy on Benton Visual Retention Test scores in this group. The effect size in the present study was 0.19, which is slightly lower than the 0.25 effect size observed in the previous study in a somewhat larger sample. Follow-up analysis suggests that the difference in effect sizes is attributable to differences in exclusionary criteria used in the two studies. The previous study excluded only women with a diagnosis of dementia at the time of the cognitive assessment because we were interested in the possible links between the effects of hormone-replacement therapy in normal and abnormal cognitive aging, including cognitive decline in preclinical dementia. Analysis of the present data by using the previous study criteria resulted in an effect size of 0.24, comparable to our previous finding. Hormone-replacement therapy may benefit verbal memory more than figural memory; however, the present data cannot unequivocally address that issue because the Benton Visual Retention Test and California Verbal Learning Test do not provide comparable measures of memory. Future studies involving parallel measures of verbal and figural memory are needed to directly address whether hormone-replacement therapy differentially benefits verbal or figural memory. We did not observe significant effects of hormone-replacement therapy on scores on the digit span tests or the Card Rotations Test. Although we had sufficient power to detect an effect size of 0.30, we lacked the power to detect smaller effects that may be associated with working memory or visuospatial ability. Nevertheless, the data indicate greater effects of hormone-replacement therapy on verbal memory than on the other cognitive domains tested.
Most studies of the effects of hormone-replacement therapy on memory have involved measures of immediate and delayed recall that are similar to the measures reported in our study. Such measures speak to whether hormone-replacement therapy affects memory, but they do not specify which aspects of memory are influenced by hormone-replacement therapy. The more comprehensive outcome measures of the California Verbal Learning Test suggest that hormone-replacement therapy enhances encoding, certain aspects of retention, and retrieval. Specifically, recipients of hormone-replacement therapy showed superior encoding of information, as indicated by better learning of lists A and B and better clustering of study items into categories. Recipients also showed better retention of items between trial 5 and the short-delay recall trial than did those who had never received such therapy; this is likely because the former were less susceptible to interference from the intervening list B task. There was no group difference in retention between the short- and long-delay free recall trials, suggesting no effect of hormone-replacement therapy on retention in the absence of a recent interference task. Finally, the memory performance of the group that had never received hormone-replacement therapy improved more from the long-delay free recall trial to the long-delay recognition trial than did that of recipients of hormone-replacement therapy (although recipients showed superior recognition). This indicates that those who had never received such treatment had not forgotten the items initially learned but rather that they had difficulty retrieving them. These findings have important implications for the interpretation of previous studies and the design of future investigations. For example, some studies include memory tests that involve additional rehearsal of unrecalled items before the delay trial
(7,
8). Notably, these tasks may mask the beneficial effect of hormone-replacement therapy on verbal encoding and explain why the largest studies of the effects of estrogen on cognition found no beneficial effect on verbal recall.
Neurobiological studies in animals and humans suggest the biological plausibility of enhanced memory with hormone-replacement therapy
(3), more so than with any other cognitive domain. Studies of the neurobiological effects of estrogen in the hippocampus and other brain regions subserving memory have primarily been conducted in ovariectomized rats given estradiol or in rats across the estrous cycle. Many studies focus on the effects of estrogen on cholinergic transmission. Although the density of estrogen receptors in the hippocampus and basal forebrain is much lower than that in the hypothalamus, changes in estrogen levels are associated with dramatic structural and functional changes in the CA1 region of the hippocampus. For example, administration of estradiol in ovariectomized rats leads to increases in choline acetyltransferase activity
(26), prolongation of long-term potentiation
(27), and recovery from ovariectomy-induced decreases in dendritic spine density
(28). Such changes have been linked to changes in memory
(29–
31). For example, estrogen reverses ovariectomy-induced memory deficits and decreases cholinergic uptake and choline acetyltransferase activity in the hippocampus and frontal cortex
(32). Insights into functional brain changes associated with hormone-replacement therapy in humans have come from relatively recent neuroimaging studies showing changes related to hormone-replacement therapy in brain areas subserving cognitive functions
(33–
36). All together, animal studies have suggested that estrogen may enhance memory by promoting survival and growth of neurons in the hippocampus, and human studies have indicated that hormone-replacement therapy modulates the activity of a number of brain regions that subserve memory processes.
One limitation of the present study was that we lacked sufficient statistical power to compare cognitive function in subgroups using specific types of hormone-replacement therapy (estrogen alone versus estrogen plus progesterone) to those who had never received hormone-replacement therapy. Such a comparison would increase our understanding of the relative effects of estrogen and combined therapy on cognitive aging. Our study also lacked sufficient statistical power to examine the effects of duration and type of hormone-replacement therapy on cognitive function among recipients of hormone-replacement therapy. In this small group of recipients (N=103), there was no correlation between duration of hormone-replacement therapy and any cognitive measure. Concurrent progesterone administration was associated with enhanced performance on a test of mental rotations but did not affect performance on measures of memory. It is unclear whether this was a chance finding or whether adjuvant progesterone therapy actually has a relatively large effect on visuospatial functioning. Studies of larger groups are needed to better address the effects of duration and adjuvant progesterone administration on cognitive function.
In summary, the present data suggest that hormone-replacement therapy may protect against normal age-related changes in memory by enhancing specific aspects of memory processing. Among observational studies, the present one is notable for involving groups of recipients of hormone-replacement therapy and those who had never received such therapy that were naturally matched on cognitive and health variables. Nevertheless, we cannot rule out the possibility that other unidentified confounding factors contributed to enhanced memory among recipients of hormone-replacement therapy. To address the limitations of observational studies while building on their findings, we recently initiated the Women’s Health Initiative Study of Cognitive Aging (N>2,000), a 6-year investigation of cognitive aging in a large subsample of women enrolled in the Randomized Hormone Replacement Trial of the Women’s Health Initiative. These and other ongoing intervention trials will provide critical insights into the effects of sex steroid hormones on the aging brain.