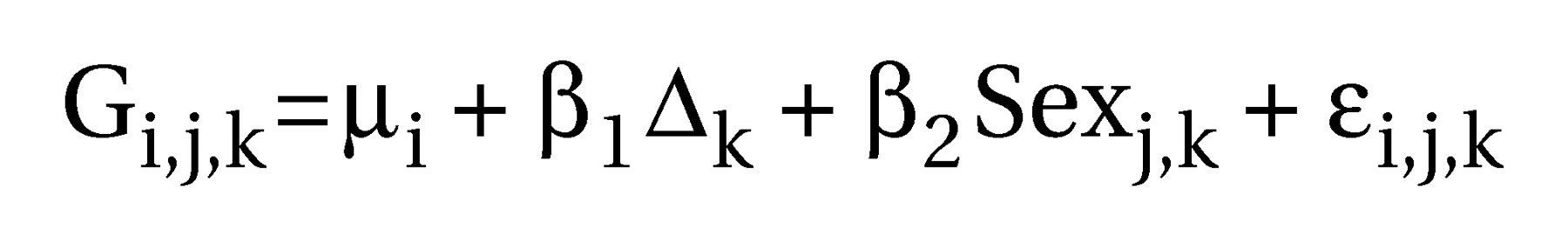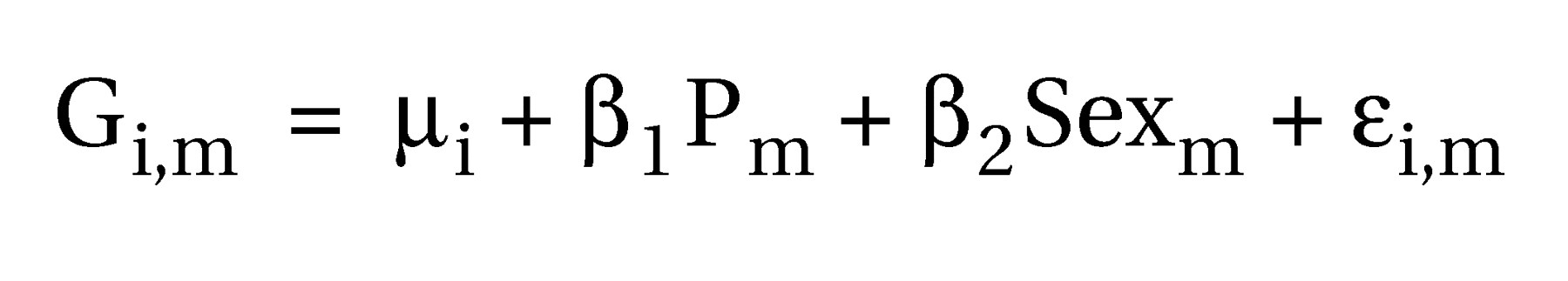In this study, we attempt to address both these issues. First, we studied a clinically homogeneous group of schizophrenic patients with primary and enduring negative symptoms. Second, we used automated morphometric methods that did not restrict attention to previously defined regions of interest but instead estimated gray and white matter deficits that were related to diagnosis or behavioral measures such as clinical symptom scores at each voxel of the images after registration in standard space.
Discussion
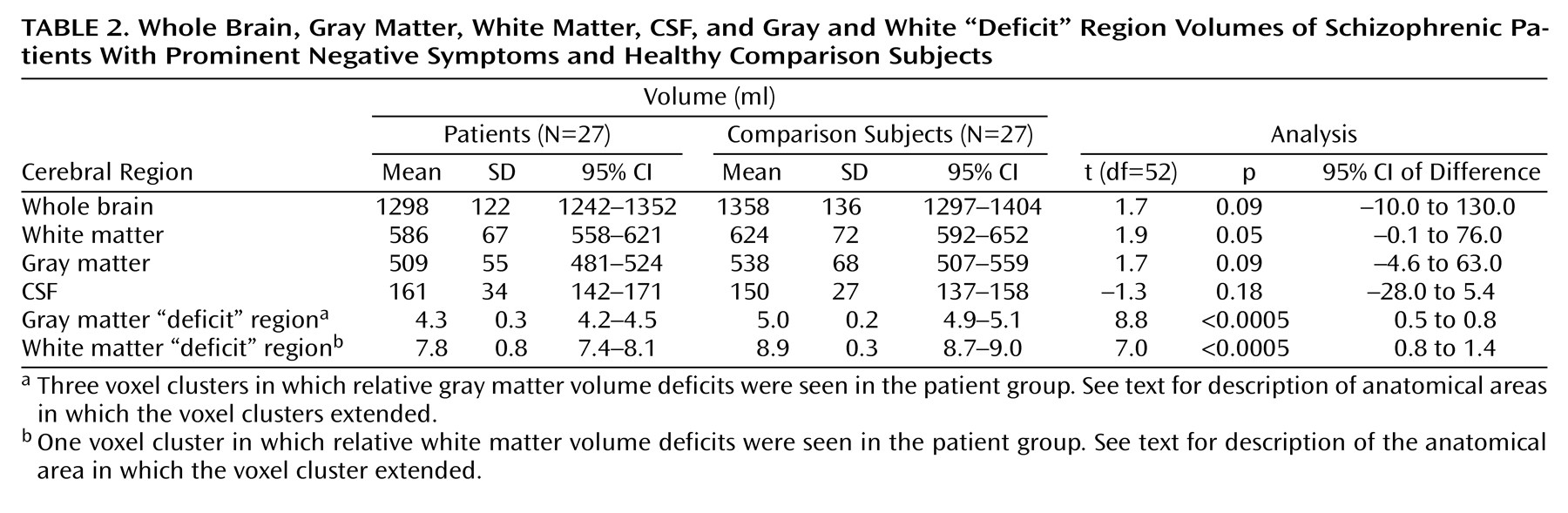
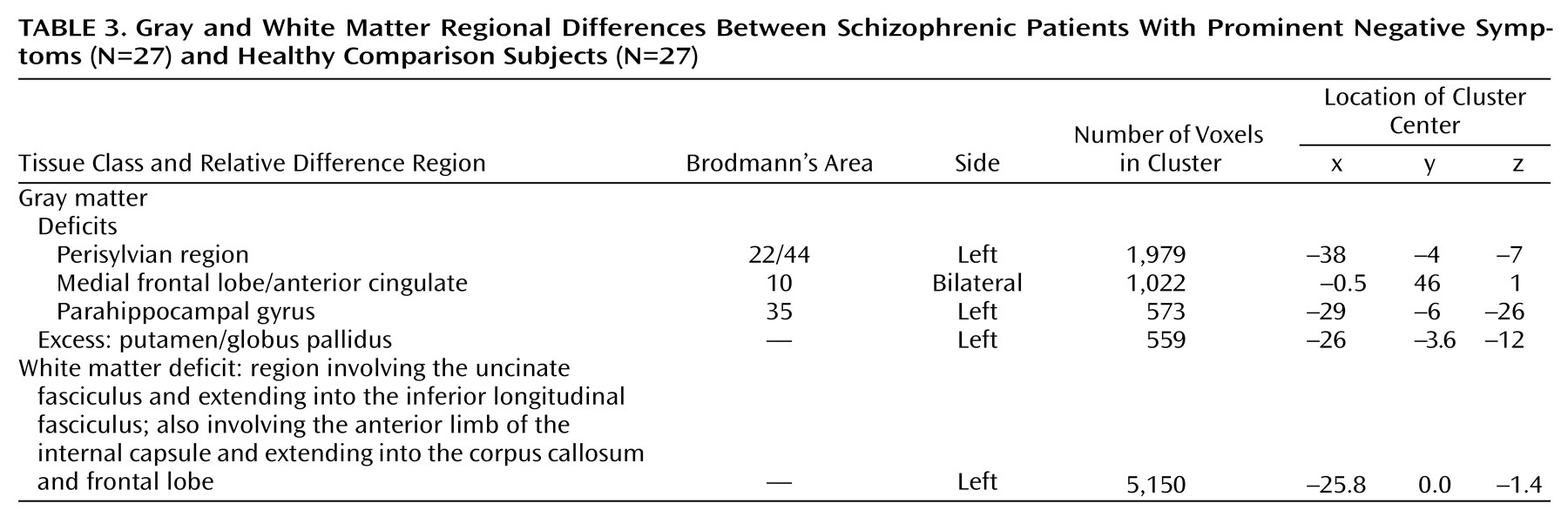
One of the main findings of this study was that significant deficits of gray matter volume in a symptomatically homogeneous group of patients with schizophrenia could be localized to three brain regions by using an almost entirely automated cluster level analysis of dual echo MR images. The regions of deficit included the anterior cingulate and middle frontal cortex, the left medial temporal lobe (including the hippocampus and parahippocampal gyrus), and, most extensively, the left superior temporal gyrus, inferior frontal gyrus, and insular cortex. There was also one area in which there was a relative excess of gray matter volume in the patient group, which was in the region of the globus pallidus and putamen on the left side.
Previous structural MRI studies have frequently adopted the hippocampus and parahippocampal gyrus as regions of interest, and volumetric reduction of both regions has been repeatedly demonstrated (for a review see the meta-analysis by Nelson et al.
[28]). Reduction of the neuronal integrity marker
N-acetylaspartate, suggesting neuronal loss, has also been reported in the medial temporal lobe, and these findings have been more marked on the left
(29–
31). There have also been reports of a reduction in the volume of the superior temporal gyrus in schizophrenia
(32–
36), which is consistent with our findings. Although our analysis found gray matter deficits only in the left temporal lobe, at a less stringent significance level (p=0.005), we also detected deficits in comparable areas in the superior temporal gyrus on the right side (Talairach coordinates of the cluster center: x=49, y=9, z=–14).
There is not as much prior evidence for abnormalities of the insular cortex in schizophrenia. Perhaps because of its location, isolated lesions to the insula are rather uncommon. However, Shuren
(37) reported speech initiation difficulties following left anterior insular infarction, and Dronkers et al.
(38) suggested that lesions in the left insula produce deficits in the articulatory planning of speech. Cancelliere and Kertesz
(39) reported deficits of emotional expression and comprehension in patients with insular lesions. These features of insular damage are also found in patients with schizophrenia, especially in patients with marked negative symptoms of alogia and autism. Neuropathological evidence for insular abnormalities in schizophrenia is limited, but Jacob and Beckmann
(40) have reported cytoarchitectonic abnormalities in the insular cortex. However, the insula has been neglected as a region of interest in previous MRI studies. It is possible that previously reported increases in size of the CSF-filled Sylvian fissure and temporal sulci may reflect some underlying loss of insular cortex volume
(2,
41). But the clearest imaging evidence to date of gray matter loss in the insula has come from studies that have used voxel-based approaches to morphometry similar to our methods
(10).
Similarly, very few previous imaging studies of brain structure in schizophrenia have adopted the medial frontal cortex as a region of interest. However, it has previously been suggested that abnormalities in the anterior cingulate and medial frontal lobe may be responsible for some of the negative or deficit features of schizophrenia. Disruption of a cingulate-striatal circuit causes a behavioral syndrome characterized by apathy, lack of drive, and perseveration
(42). Liddle et al.
(43) found a negative correlation between psychomotor poverty and cerebral blood flow in the anterior cingulate and medial frontal lobe. Dolan et al.
(44) reported abnormal modulation of anterior cingulate activation by apomorphine in patients with schizophrenia. Macroscopic gray matter volume decrements in the medial frontal cortex, such as we have shown, are broadly compatible with these lesion and functional imaging studies as well as with neuropathological evidence for reduced medial frontal cortical thickness in patients with schizophrenia
(45). It is interesting to note that Benes
(45) found increased neuronal density in medial frontal areas, suggesting that reduced cortical thickness was due to impoverished dendritic arborization rather than neuronal depopulation. Since dendritic arborization is a microscopic marker for interneuronal connectivity, it is perhaps not surprising that medial frontal cortical changes in this group were associated with gray matter decrements in several anatomically connected cortical and subcortical regions.
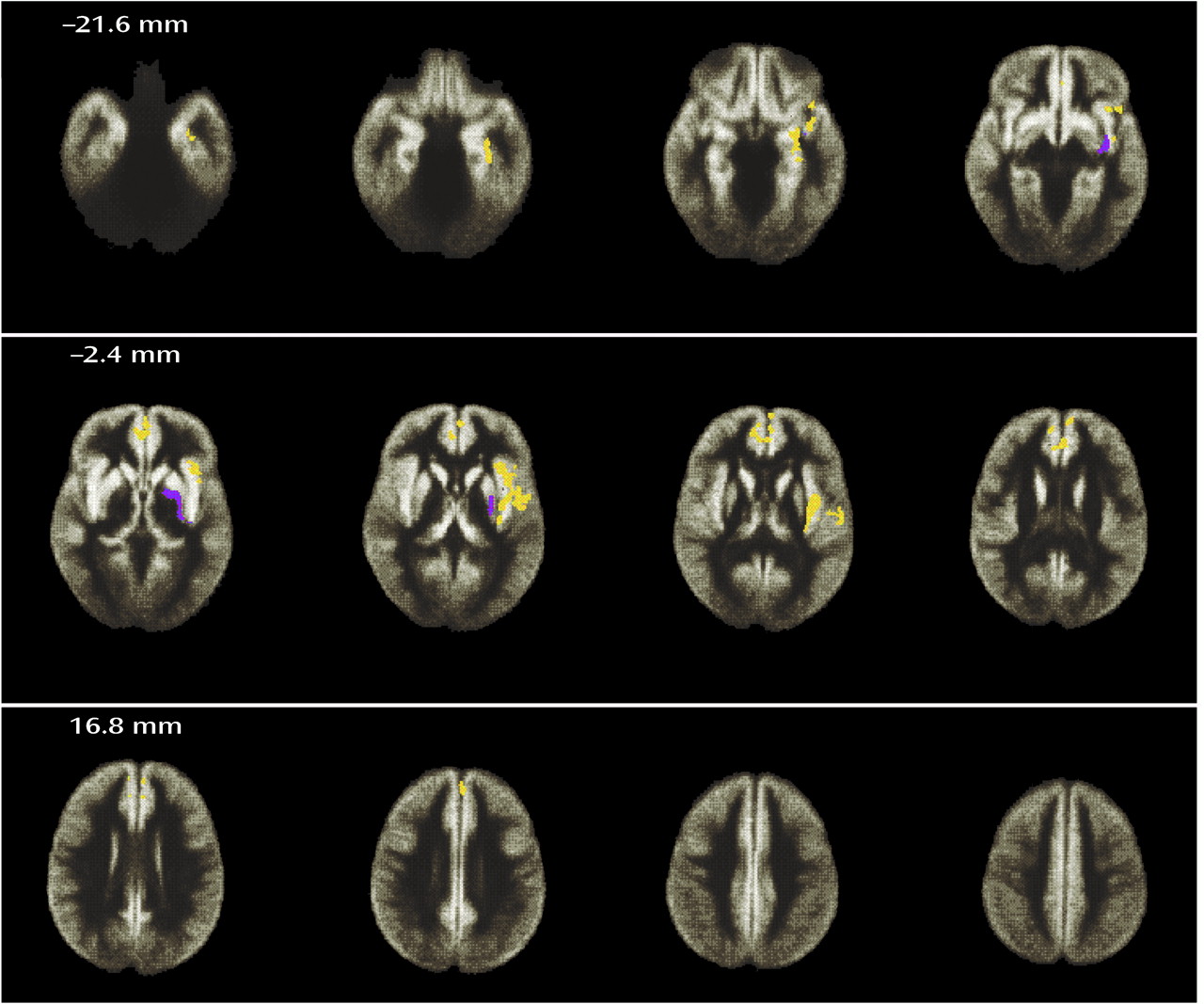
It is known from studies of anatomical connectivity between homologous areas of nonhuman primate cortex that the brain regions we have identified as deficient in schizophrenia are normally linked to each other by dense and reciprocally afferent white matter tracts
(46,
47). This suggests that we should consider pathological change in schizophrenia to be identified at the supraregional level of large-scale neurocognitive networks
(48) rather than at the regional level of analysis preferred by most previous imaging and histological studies. The most salient piece of evidence in favor of such a network model of pathology from our data is the co-occurrence of white matter deficits in left hemispheric tracts that are known to incorporate axonal connections between the regions of gray matter deficit. These areas of white matter deficit included periventricular regions and might, in part, explain the (nonsignificantly) increased volume of CSF spaces in the patient group. In our view it is unlikely that this causal relation is reversed (i.e., that ventricular enlargement should have caused white matter deficit), since 1) that mechanism would imply raised intraventricular pressure—which has not been shown in schizophrenia—and 2) the areas of white matter deficit extended far from the lateral ventricles, reaching the immediately subcortical parts of frontal and temporal lobes.
There have been few previous histological or imaging studies of white matter in schizophrenia, despite the long-standing conjecture that a disorder of the association fibers may be implicated in psychosis
(49). However, white matter deficits in frontal lobe regions of interest have been previously reported
(50,
51), and the recently developed technology of diffusion weighted imaging has shown widespread abnormalities of white matter organization and orientation of fiber tracts in patients with schizophrenia
(52). Neither these findings nor our own data allow us to be conclusive about the relative priority of gray and white matter deficits in schizophrenia. One could argue, within the general framework of the neurodevelopmental hypothesis, that the primary “lesion” is in the neuronal cell bodies of gray matter, and this causes abnormal axonal projection and maturation that leads to secondary changes in adult white matter structure. With almost equal plausibility, however, one could claim that the primary lesion affects axonal growth or synapse formation, and the structure of adult gray matter is abnormal because it has been deprived of the mutually trophic effects normally mediated by active synaptic connections between developing brain regions. These and other questions must await more etiologically or pathogenetically oriented studies.
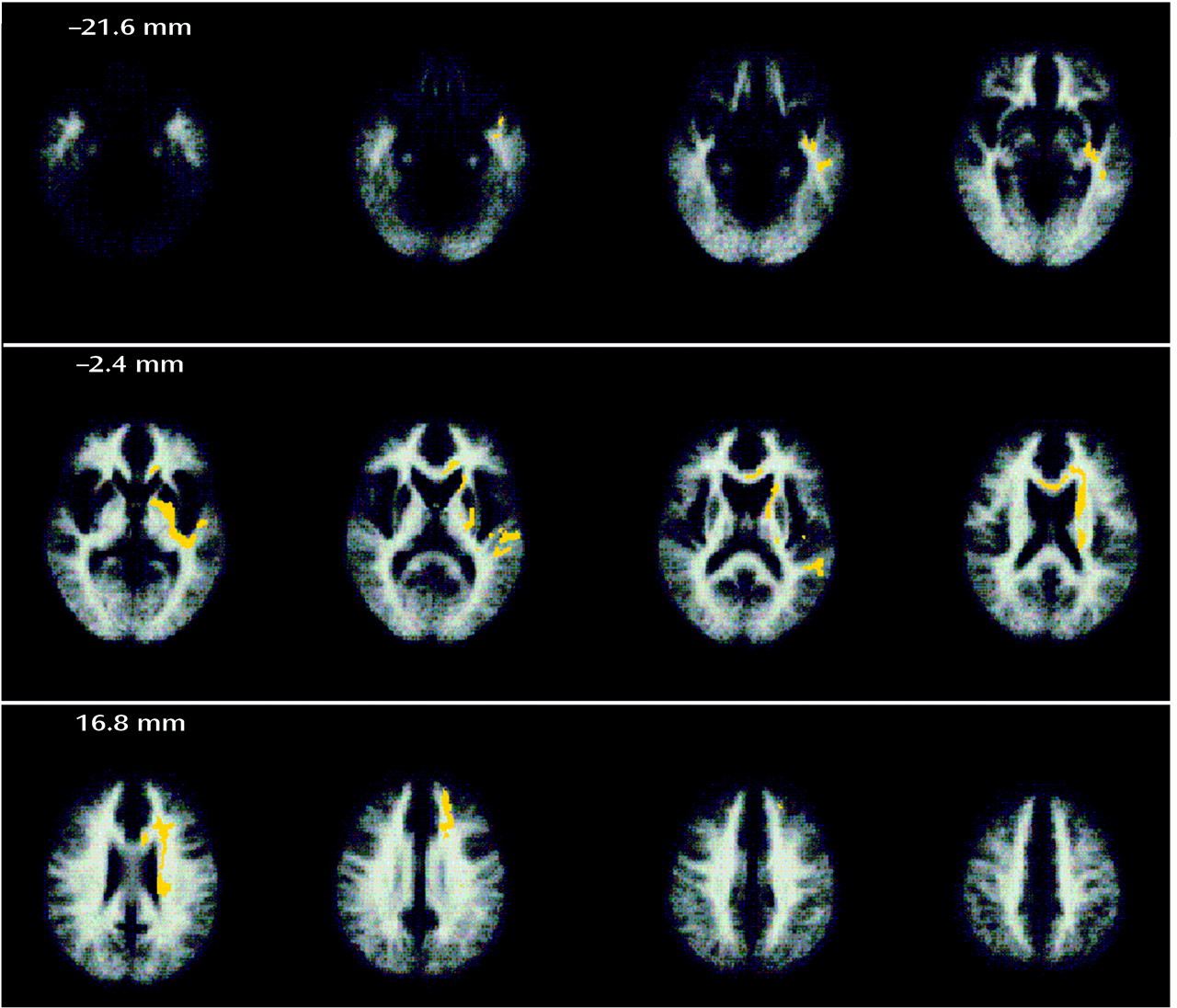
Another important finding was an area of excess gray matter volume in the left basal ganglia. Similar findings have previously been reported following region-of-interest analysis of the caudate and putamen
(53,
54) and generally are attributed to the hypertrophic effects of prolonged dopamine D
2 receptor antagonism by antipsychotic drugs. In our data, both bilateral enlargement of basal ganglia gray matter and a unilateral (right-sided) deficit of adjacent white matter were positively correlated with positive symptom scores. This relationship might reflect the tendency for patients with more severe positive symptoms to receive larger doses of antipsychotic medication, although this could not be confirmed in our study. Alternatively, these subcortical abnormalities may be a cause rather than an effect of positive symptoms.
Other areas of relative gray matter increase in relation to increased positive symptom scores identified in the patient group cannot be readily discounted as side effects of medication. Higher gray matter volume in the right superior temporal gyrus and insula, for example, has not previously been linked to antipsychotic drug exposure, but there is some functional imaging evidence to suggest relatively enhanced activation of these areas by auditory-verbal stimulation in patients with schizophrenia and a history of auditory-verbal hallucinations
(55). Speculatively, these data are consistent with the hypothesis of Crow et al.
(56) that a pathological process involving the left (normally dominant) perisylvian area, perhaps early in the course of development, may be partly compensated by a reorganization of contralaterally homologous areas, leading to abnormally increased right (normally nondominant) temporal cortical size and functional responsivity.
There are several methodological questions arising in consideration of these results, probably the most important of which concern the methods of computational morphometry we have used. To summarize the image processing “pipeline” used here: 1) we constructed a template image in Talairach space by spatially normalizing and averaging images from a subset (N=6) of the comparison subjects; 2) after locally adaptive and automated tissue classification in native space, we registered all images (N=54) with this template by an affine transformation; 3) finally, we tested for differences in tissue classification by permutation tests on spatial statistics. These procedures have been described and validated in detail elsewhere
(1,
4,
16,
17). As we have noted, many of our results are corroborated by prior imaging studies of schizophrenia that used region-of-interest morphometry (although results of regional abnormality in the medial frontal cortex, insula, and white matter are difficult to cross-validate in this way, since these regions have not often been regarded a priori as interesting
[5]). However, this sequence of image processing operations does not represent a unique solution to the complex problem of extracting anatomical information from MRI datasets. In particular, we have used a relatively simple (affine) transformation to match images to a template. This linear transformation globally resizes and reorients the images relative to the template; it does not rescale voxel gray scale values. It is intended to minimize global size and orientation differences between images but not to eliminate more local differences. It is these residual (postregistration) differences that were comprehensively measured and tested for evidence of a main effect of diagnostic group. A fundamentally different analytic strategy is to use a higher-order or nonlinear transformation that aims to match images to a template so precisely that there are zero residual differences between images. In this case, the test statistic for anatomical disparity between groups must be the deformation applied at each voxel to bring it into perfect registration with the template rather than the residual difference in voxel values after a deliberately imperfect registration (see Thompson et al.
[57] for an example of this approach and references to related work). Methods for computational morphometry are an actively developing focus of brain mapping research, and there is insufficient comparative data to allow a fully informed choice between these two rather different strategies—analysis of residual differences after linear transformation and analysis of the nonlinear transformations required to eliminate residual differences
(22). We conclude that our methods are wholly reliable and valid insofar as we have evaluated them, but they do not represent the only possible solution to the problem they address.
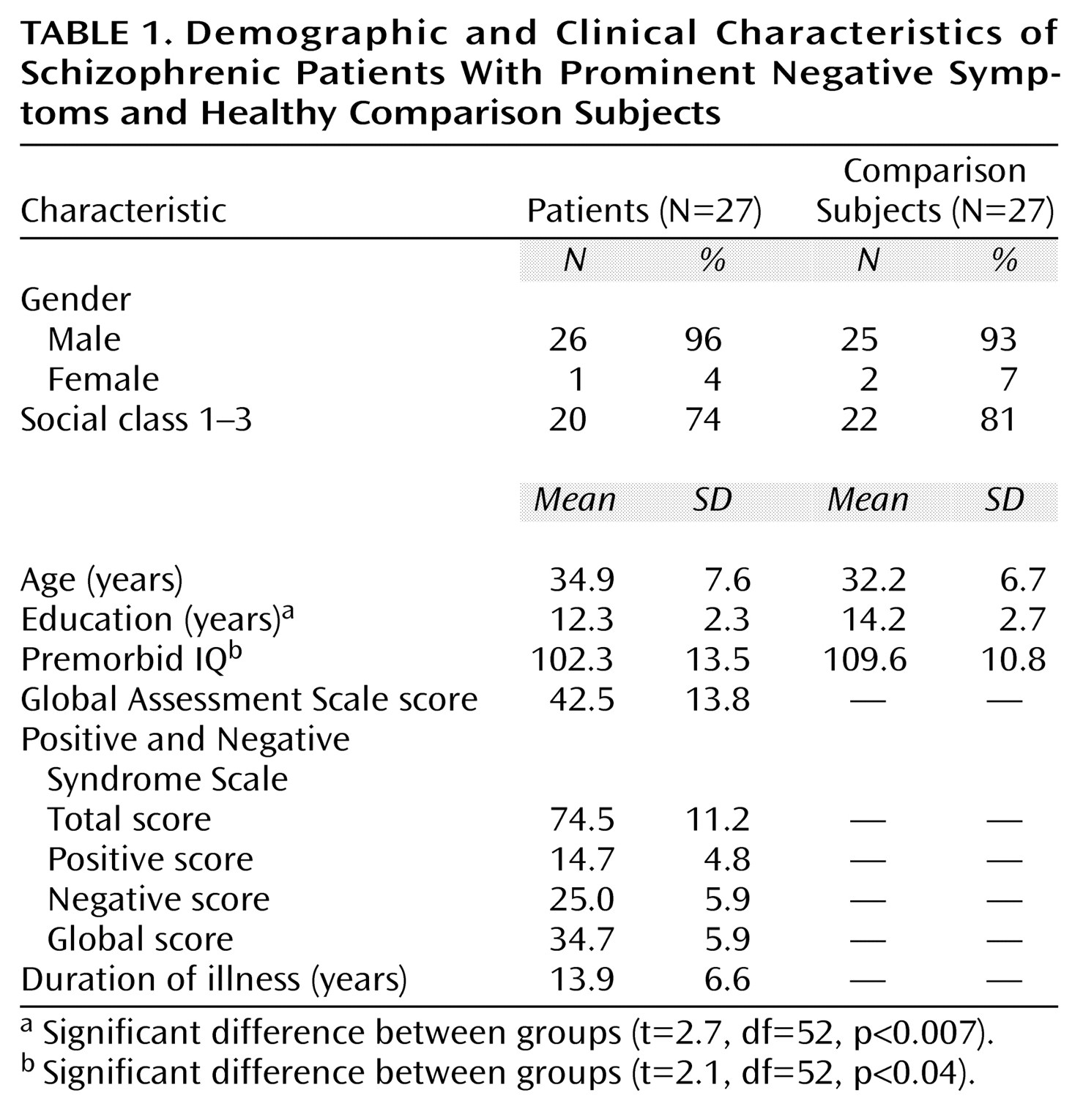
There are a number of subsidiary issues to consider methodologically. First, the study group size was small. One implication of this is that some of the nonsignificant differences we have reported may be type II (false negative) errors. This seems particularly likely to be the reason for our failure to show significant between-group differences in global tissue and CSF volumes, which were of the same order as those previously reported as significant in a larger study
(2). Second, the patient group was not representative of the population of patients with schizophrenia; rather, we selectively recruited a subset of patients with marked negative symptoms that justified a supplementary diagnosis of deficit syndrome. This aspect of the study design was dictated by our desire to maximize power to detect any true differences between the schizophrenic patients and the comparison subjects by minimizing heterogeneity within the patient group. However, it means that our results may not generalize to the population of patients with schizophrenia. Furthermore, the lack of variability in the patient group with respect to negative symptoms may account for our failure to demonstrate any significant anatomical associations with negative symptom scores. Third, all the patients were taking antipsychotic drugs both at the time of the study and for many years previously and, as noted earlier, antipsychotic medication is likely to cause changes in brain structure that can confound interpretation of case-control differences. This issue can only be resolved with certainty by future studies of antipsychotic drug-naive patients. Fourth, we have presented most of our results in the form of hypothesis tests rather than confidence intervals. The use of hypothesis testing to summarize large quantities of imaging data is an almost universal practice but must be considered critically. Whether a region shows up as “significant” or not on an inferential brain map will obviously depend on the size of test applied to the data. We have here used very stringent probability thresholds, for which we expected less than one false positive test per map, to focus attention on the most salient (least questionable) effects of schizophrenia as expressed in this patient group. However, this means that the probability of type II errors will be considerable, or, more plainly, there may well be other abnormalities in brain structure than those we have reported.
In summary, we have used contemporary image analysis tools to demonstrate significant deficits of both gray and white matter in a refined group of patients with marked negative symptoms of schizophrenia. The anatomical abnormalities are spatially distributed in a network of neocortical and limbic regions and interconnecting white matter tracts. These data are compatible with supraregional models for the pathology of schizophrenia.