Eating disorders or disordered eating behaviors frequently occur in the childbearing years. The few studies to date to examine the effects of eating disorders on neonatal outcome have reported conflicting results. For example, several studies
(1–
5) have reported complications or death, while others
(6,
7) have reported normal infant birth weights and a lack of medical complications. Stewart et al.
(4) found both lower Apgar scores and lower infant birth weights for babies of women with active episodes of anorexia nervosa and/or bulimia nervosa than for babies of women in remission. The two fetal deaths identified in this study occurred in women who had an eating disorder at conception. In 20 women entering pregnancy with an active eating disorder, Lacey and Smith
(8) reported nine women with hypertension, eight with breech deliveries, and three with deliveries by cesarean section.
Abraham
(9) found that, in relation to 43 nonpsychiatric comparison women, women with current or past histories of bulimia nervosa reported a significantly greater number of miscarriages and otherwise unsuccessful pregnancies and were more likely to be treated for postpartum depression. Using an innovative design, Conti and colleagues
(10) investigated the association of pregnancy outcome and eating behaviors in 88 women who delivered babies of low birth weight and 86 comparison women of normal weight. Three months before pregnancy, 32% of the women who later delivered smaller babies had an eating disorder, compared to 9% of the mothers of premature infants and 5% of the comparison mothers. The women who delivered smaller babies at term reported more disturbed eating behaviors both during pregnancy and in the postpartum period. In this study, the unique predictors of having an infant who was small for its gestational age included low prepregnancy weight, smoking, low maternal weekly weight gain, and a high score on a self-report questionnaire that screened for bulimia nervosa.
A retrospective study that compared pregnancy and obstetric outcomes in 66 anorexic patients and 98 comparison women
(11) found a higher number of miscarriages, lower birth weights, more premature births, and more cesarean sections in the group with anorexia nervosa. When the anorexic group was divided into those who restricted food intake and those who purged, those who restricted intake were found to have babies of lower gestational weight. No significant differences were found between the patients with active and remitted symptoms of anorexia nervosa on any variable.
Waugh and Bulik
(12) interviewed and videotaped 10 mothers with current or past eating disorders and 10 comparison mothers and their children. Both the birth weights and lengths of the infants born to the mothers with eating disorders were significantly less than those of the babies of comparison women; however, no premature births were recorded. Anorexic and bulimic mothers reported more problems breast-feeding, and the videotapes revealed that they made fewer positive comments during feeding than did the comparison subjects.
Morgan et al.
(13) recently reported on a cohort of 113 subjects who were interviewed retrospectively to determine the impact of their pregnancies on their bulimia nervosa. Their bulimic symptoms improved as the pregnancies progressed for a majority of the subjects; however, 57% of the group had worse symptoms after pregnancy than they had had before. Severe bulimic symptoms at conception, a history of anorexia nervosa, gestational diabetes, and unplanned pregnancy all predicted relapse. Postpartum depression occurred in one-third of the group and was predicted by the presence of a lower body mass index at conception, a higher frequency of bingeing after delivery, and a higher weekly alcohol intake at conception. Two-thirds of those with a history of anorexia nervosa reported postpartum depression.
The findings in the studies published to date, while somewhat inconsistent, suggest that both anorexia nervosa and bulimia nervosa may negatively affect fetal outcome. Together, these data suggest that having a past or current eating disorder may put a woman and her infant at risk for problems during pregnancy. Retrospective study designs and modest group sizes have limited the generalizability of the findings in the studies published to date.
The current study is unique in that it examines the course of pregnancy and neonatal status for babies born to women with eating disorders. The availability of prospectively collected data from a large cohort of women with anorexia and/or bulimia nervosa who were studied longitudinally adds new information to the existing body of literature. This study employed a rigorous tracking of symptom levels of eating disorders 9 months before, 9 months during, and 9 months after pregnancy. These data were supplemented with self-report questionnaires and medical records regarding the outcome of each subject’s pregnancy. Together, these data provide a detailed description of the course of pregnancy and the neonatal health of the offspring of these women.
Method
Participants
Data were obtained from the 102 pregnancies identified to date in the entire group of 246 women participating in a longitudinal study of anorexia and bulimia nervosa. Sixty-three of these pregnancies had resulted in live births. We obtained medical records and/or self-report information concerning the course of pregnancy and neonatal status for 49 (77.7%) of these live births. Medical records and self-report data were obtained for 45 of the subjects; for four subjects, only self-report data were available.
The original study group was drawn from 554 women who initially sought treatment for their eating disorder at Massachusetts General Hospital between October 1987 and June 1990. All of the participants met DSM-III-R criteria for anorexia nervosa or bulimia nervosa. A total of 268 (48.4%) met the criteria for anorexia nervosa and/or bulimia nervosa, and 229 (85.4%) of those agreed to participate, four of whom dropped out before the first follow-up interview. An additional 21 participants were recruited into the study in 1991, resulting in an anorexia nervosa group of 62 and a total group of 246. Since that time, the subjects have been reclassified with the use of DSM-IV criteria, resulting in 51 with anorexia nervosa who restrict food intake, 85 with anorexia nervosa who binge or purge, and 110 with bulimia nervosa.
For inclusion into the study, the participants had to be female and speak English, be at least 12 years of age, and reside within 200 miles of the study site. Exclusion criteria were evidence of organic brain syndrome or terminal illness.
All the participants are interviewed at approximately 6-month intervals over the course of their participation in the longitudinal study. All women who self-reported pregnancies were identified during interviews, and data were collected by using the same procedure as in the longitudinal study. The subjects who reported live births were later sent a letter inviting them to participate in a substudy on pregnancy and eating disorders for which consent for a review of medical records and completion of a brief self-report questionnaire on pregnancy complications were requested. Forty-nine consent forms for review of medical records and questionnaires were sent to the subjects who reported pregnancies; 44 consent forms and questionnaires were completed and returned to the investigators. Requests for both mothers’ and children’s medical records were sent along with consent forms to the hospitals in which the births had occurred. Thirty-six of the mothers’ medical records and 30 of the children’s medical records were obtained. The Pregnancy Study Questionnaire is an eight-item measure that we developed to assess both live birth statistics and birth-related complications. After a complete description of the study was given to the subjects, written informed consent to participate was obtained.
The participants were categorized in two ways: by diagnosis at the time of entry into the longitudinal study and by diagnosis in the 9 months preceding conception. By intake diagnoses, two live births occurred for women with anorexia nervosa with restricted intake, 16 for women with anorexia nervosa with bingeing or purging, and 31 for women with bulimia nervosa. Because some women recovered from their eating disorder before pregnancy, the participants were also diagnosed on the basis of symptom levels at the time of conception. Twenty-two of the live births occurred for women with an active eating disorder (either full or partial) when entering their pregnancy (averaged over the 9 months before conception). Of these, five women met the criteria (either full or partial) for anorexia nervosa with restrictive intake, three met the criteria for anorexia nervosa with bingeing or purging, and 14 met the criteria for bulimia nervosa. Just over half (N=25, 51.0%) of the live births occurred for subjects without an active eating disorder in the 9 months before conception. Two of the subjects had missing symptom data for some portion of the 9 months before conception.
Thirty-seven (75.5%) of the live births occurred for married women. The majority (N=27, 55.1%) of the women were college graduates. The mean age at pregnancy was 28 years (SD=4, range=19–41).
Instruments
Trained clinical interviewers (S.S.D., K.T.E., A.T.F., and D.N.G) evaluated the subjects with the Eating Disorders Longitudinal Interval Follow-Up Evaluation, a modified version of the Longitudinal Interval Follow-Up Evaluation
(14). The Eating Disorders Longitudinal Interval Follow-Up Evaluation is a semistructured interview that assesses eating disorder symptoms, comorbid psychopathology, treatment, and psychosocial functioning. The instrument follows the course of eating disorders using the Psychiatric Status Rating Scale, an ordinal, symptom-oriented scale based on Research Diagnostic Criteria ratings. Psychiatric Status Rating Scale scores range from 0 to 6 for eating disorders on the following scale: 0=no history of the disorder, 1=a past disorder, 2=residual symptoms, 3=partial symptoms, 4=marked symptoms, 5 and 6=definitely meet criteria, depending on severity. Depression, including major depressive disorder and probable major depressive disorder, are rated by use of this scale as well. Other depressive disorders, including minor depressive disorder, intermittent depressive disorder, intermittent depressive features, and depressive disorder not otherwise specified, are rated on a scale of 0–3: 0=no history of the disorder, 1=a past disorder, 2=partial symptoms (subthreshold), and 3=definitely meet criteria. The Metropolitan Insurance Company’s height and weight norms
(15) were used to calculate the participants’ percentage of ideal body weight. SPSS 6.1 (SPSS Inc., Chicago) was used for data analysis.
Procedure
The trained research assistants identified pregnancies during the course of regular follow-up interviews. Following the standard interview conducted for all subjects, described in detail elsewhere
(16), the assistants elicited information relevant to the pregnancy. This included data concerning menstrual status (for exact date of pregnancy), restrictive eating, compulsive exercise, overconcern with body weight and shape, frequencies of bingeing and vomiting, and Psychiatric Status Ratings for anorexia and bulimia nervosa.
This study spanned three 39-week periods, which were divided into three trimesters each. The three periods were as follows: 39 weeks before pregnancy, 39 weeks during pregnancy (more or less, depending on length of pregnancy), and 39 weeks postpartum. For pregnancies longer than 39 weeks’ gestation, the third trimester included weeks beyond 13. For example, for a live birth of gestation 42 weeks, data were averaged during each of two trimesters of 13 weeks each, while data from the third trimester of pregnancy were averaged over 16 weeks.
Ratings for restrictive eating, compulsive exercise, and overconcern with body weight and shape, frequencies of bingeing and vomiting, and Psychiatric Status Ratings for anorexia and bulimia nervosa were averaged over 13-week periods or collapsed over 39 weeks.
Measures describing pregnancy course, obstetric complications, and neonatal outcome status were obtained from either the medical records or through subject self-report. Pregnancy course variables included hypertension during pregnancy and hyperemesis. Obstetric complications included breech presentation, use of forceps, delivery by cesarean section, induction of labor, vaginal bleeding, eclampsia, preeclampsia (toxemia), postpartum hemorrhage, dilation and curettage, placenta previa, and premature rupture of the membrane. Neonatal outcome variables included Apgar scores at 1 and 5 minutes after birth, birth defects, fetal heart rate abnormality, prolapsed cord, and nuchal cord. We also assessed postpartum depression and its relationship to the variables regarding pregnancy and eating disorders.
Results
Neonatal Status
For all 49 live births, the following descriptive statistics were obtained. Twenty-six subjects were primiparous, 15 had second pregnancies, seven had third pregnancies, and one had a fourth pregnancy. The mean length of pregnancy was 38.7 weeks (SD=2.7), the mean birth weight was 7.6 lb (SD=1.0), the mean Apgar score 1 minute after birth was 8.2 (SD=1.1), the mean Apgar at 5 minutes was 9.0 (SD=0.6), and the mean number of pregnancy problems or birth complications was 1.3 (SD=1.1). Twelve of the 49 pregnancies resulted in delivery by cesarean section; three of the 12 were repeat Caesarian sections, and there were no Caesarian sections after a prior vaginal delivery. Three babies (6.1% of the group) had birth defects. Seventeen (34.7%) of the women experienced postpartum depression.
Eating Disorders
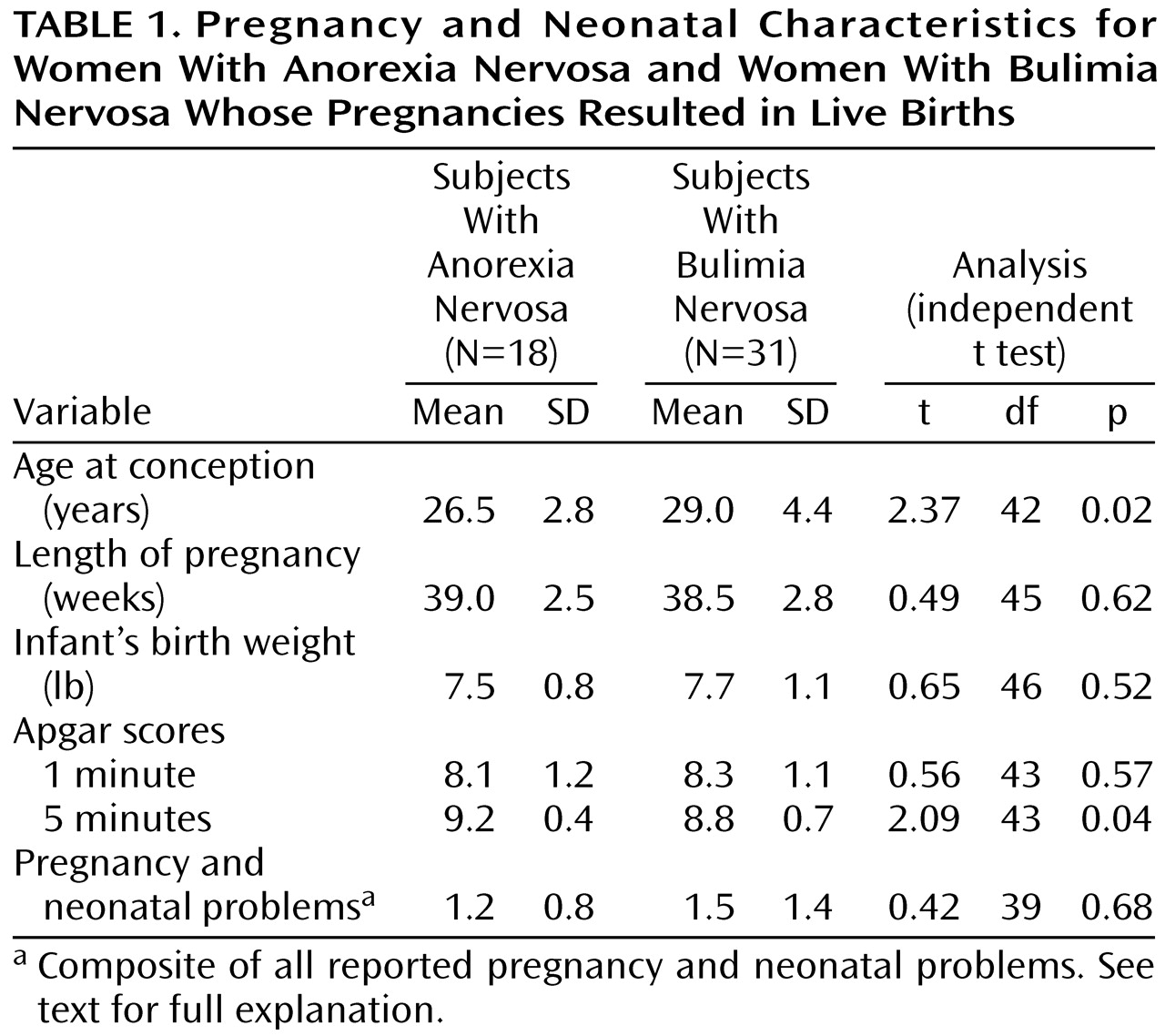
Because of the small number of subjects with anorexia nervosa who restricted intake (N=2), those and the subjects with anorexia nervosa who binged or purged were combined to form a single anorexia group (N=18). When the subjects were grouped by their diagnoses at intake, we found that the anorexic and bulimic subjects differed on only two dependent variables: Apgar score at 5 minutes and age at conception (
Table 1). Apgar scores at 5 minutes were significantly higher in the anorexic subjects (t=2.09, df=43, p=0.04). The bulimic subjects were significantly older at the time of conception than the anorexic subjects (t=2.37, df=42, p=0.02).
Symptom Severity
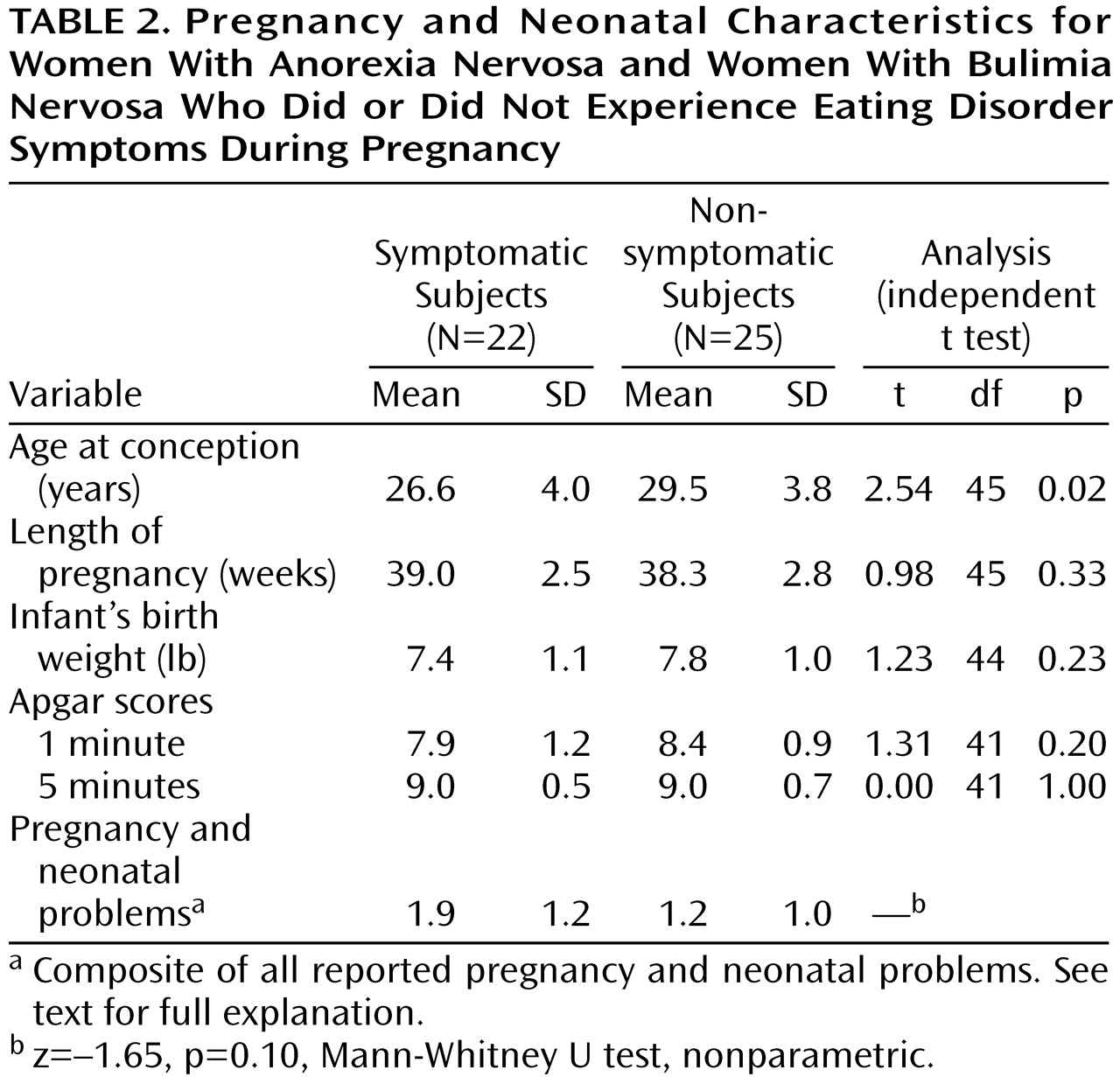
We divided the group of 49 pregnancies into those of women who had and did not have symptoms of eating disorders (
Table 2). A series of independent t tests revealed that the symptomatic group (N=22) was significantly younger at the time of pregnancy (t=2.54, df=45, p=0.02) than the nonsymptomatic group (N=25). We compared the frequency of a composite of all reported pregnancy and neonatal complications for symptomatic and nonsymptomatic women using a nonparametric test, but this difference was not significant (z=–1.65, p=0.10, Mann-Whitney U test, nonparametric).
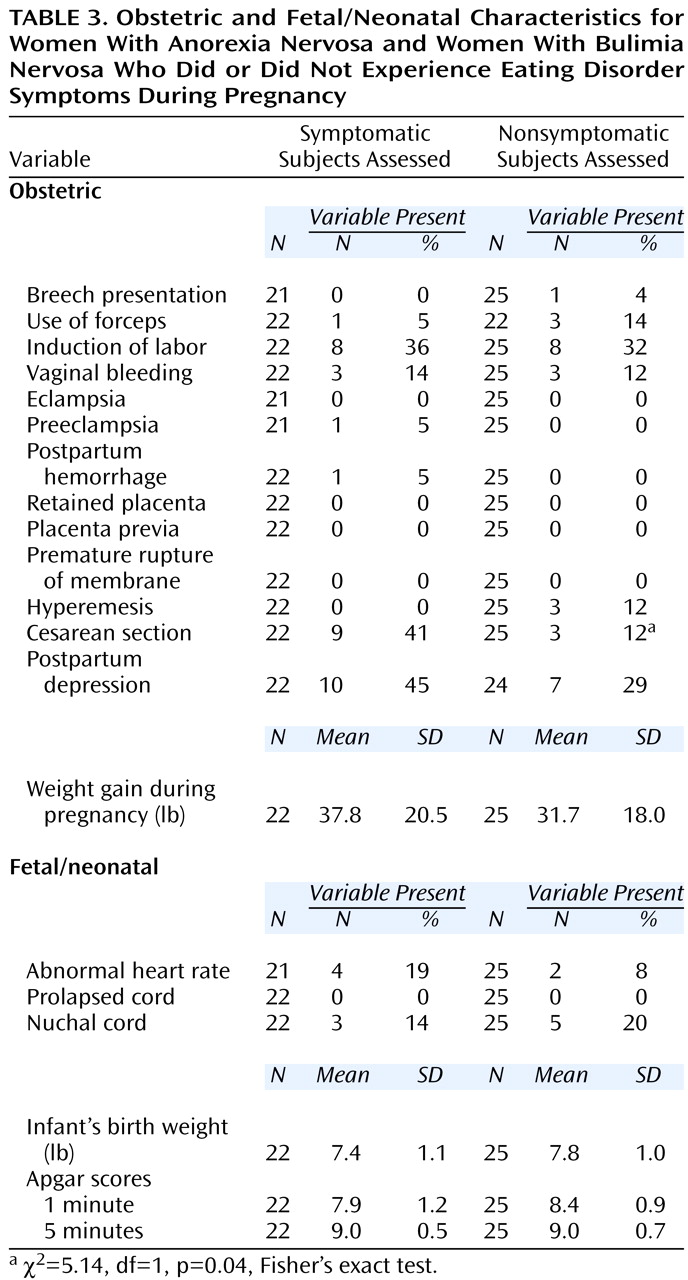
We also examined the specific type and frequency of complications for the groups with and without symptoms and found a statistically significant difference between the groups on only one variable (
Table 3). The number of cesarean sections was significantly higher in the symptomatic subjects (χ
2=5.14, df=1, p=0.04, Fisher’s exact test). Although not a significant difference, postpartum depression occurred in 10 (45.5%) of 22 symptomatic subjects and seven (29.2%) of 24 asymptomatic subjects. Within the symptomatic group, the amount of weight gained during pregnancy correlated with baby’s birth weight (r=0.42, df=21, p=0.06).
We were interested in the use of substances (alcohol and drugs), medications, and laxatives in the women with and without symptoms. A history of alcohol or drug abuse before pregnancy was found in 11 of 22 pregnancies in the women who were symptomatic of an eating disorder during pregnancy. In contrast, in the asymptomatic group, a history of substance abuse was reported in only five of 25 pregnancies (χ2=4.75, df=1, p=0.03). With regard to use of substances during pregnancy, three women in the symptomatic group reported use of alcohol (no drugs), and none of the women in the asymptomatic group reported using alcohol or drugs during pregnancy (χ2=3.64, df=1, p=0.10). Similarly, psychotropic medications were used during three of the 22 pregnancies in the symptomatic group, compared to none of the 25 in the asymptomatic group (χ2=1.40, df=1, p=0.24). Although none of the asymptomatic group used laxatives during their pregnancies, laxative use was reported in four of the pregnancies in the symptomatic group (χ2=4.97, df=1, p=0.04). Apgar scores or birth weights did not differ between the women with and the women without a history of substance abuse (all t<2.0, df=43–46, all p>0.05). Unfortunately, we did not collect information on tobacco use.
Three infants in the group were born with birth defects. These included an undescended right testicle (subject 1), persistent hyperplastic primary vitreous (subject 2), and a ventricular septal defect (subject 3). The records of these three subjects were reviewed for symptoms of eating disorders, medication, and substance use before and during pregnancy. Subject 1 reported no symptoms, medication use, or alcohol or drug use during these time periods. Subject 2 was extremely symptomatic both before and during pregnancy, engaging in bingeing and purging behaviors, including laxative abuse, up to two times per day. She was taking medication (50 mg/day of trazodone and 1200 mg/day of lithium carbonate) until week 6 in the 9 months before conception, after which time she was medication free until after delivery. She did not use alcohol or drugs during these periods. Subject 3 reported no symptoms of eating disorders or medication use but drank heavily both during the prepregnancy period and throughout the first trimester.
We examined history of depression in the women who reported postpartum depression. All (N=17) had a lifetime history of either major depressive disorder (N=9), minor depressive disorder (N=5), or hypomania/mania (N=3). It is of interest that the presence of depressive features in the 6 months before pregnancy was not associated with postpartum depression (χ2=1.88, df=1, p=0.17). During pregnancy, nine (52.9%) of these 17 subjects were diagnosed with either major depressive disorder or reported depressive features, compared to 13 (48.1%) of 27 subjects who did not have postpartum depression (χ2=0.47, df=1, p=0.55). Four subjects in the postpartum group were taking antidepressant medication for some period (range=3–13 weeks) during the first trimester, and one subject was taking antidepressant medication throughout her pregnancy. We also compared the marital status of the women with postpartum depression to those without postpartum depression and found no differences on this variable (χ2=2.49, df=1, p=0.15).
Discussion
The majority of the women with eating disorders in this group had pregnancies of average length, which resulted in infants of normal birth weight with good Apgar scores. Although 6.1% of the babies in our group had a reported birth defect, there were otherwise minimal obstetric or fetal/neonatal complications. One caveat, however, is that the rate of birth by cesarean section and the frequency of postpartum depression were higher than population norms. Clinical differences were found between the women who were symptomatic during pregnancy and those who were not.
Although the symptomatic women reported more obstetric and fetal/neonatal complications and a higher rate of complicated deliveries than the nonsymptomatic subjects, this difference did not reach statistical significance. There were, however, more births by cesarean delivery in the symptomatic group than in the nonsymptomatic group. Nearly one-half of the symptomatic group reported postpartum depression. Only within the symptomatic group was pregnancy weight gain significantly positively correlated with birth weight. Overall, these data suggest that women who continue to manifest symptoms of eating disorders may fare worse than those who do not, but only in relation to some outcome measures. There were no differences, for example, in Apgar scores, infant birth weight, or length of pregnancy between the two groups of subjects.
The number of birth defects found in the group was greater than the population-based rate of 2.5%
(17). Two of the three subjects who had babies with birth defects engaged in potentially teratogenic behaviors during pregnancy, including severe purging (by means of vomiting and laxative use) and alcohol abuse. However, the small number of subjects and the correlational nature of the data do not permit causal inferences to be made with respect to the etiology of the birth defects.
A serious finding in this study was the high rate of postpartum depression. The prevalence of clinical depression in the postpartum period in the general population is estimated to be between 3% and 12%
(18). Our group reported more than three times that rate (34.7%). This high rate of postpartum depression is consistent with the findings of earlier studies
(9,
13). Of particular note was the higher frequency of postpartum depression found in the subgroup with the symptoms of an eating disorder. Nearly one-half of the women with the symptoms of an eating disorder reported postpartum depression that was confirmed by medical records. It is likely that the high rate of postpartum depression in this group was a function of the subjects’ lifetime history of affective disorders
(19). It is also possible that the physiological and psychological stresses of having an active eating disorder exacerbated this risk in the symptomatic group. The vulnerability to postpartum depression may have been increased by the medical complications of eating disorders (e.g., dehydration and electrolyte instability), as well as by environmental factors such as poor social support
(20). Our data limit our ability to comment on the relative contribution of biological and environmental factors, but they suggest that the relationship between postpartum depression and eating disorders deserves further study.
There are a number of limitations to this study. A larger group size, particularly with more anorexic subjects, would increase the power and allow for comparisons between the groups with eating disorders. The ability to determine predictor variables was also limited by the group size. Because we had only two subjects with anorexia who restricted food intake, our results might not be generalizable to this subtype. Most notably, the absence of a comparison group did not allow us to make any comparisons to women without eating disorders. The use of a comparison group is strongly recommended for any future studies. The use of medical records for data collection and the lack of data from some subjects were further limitations. Furthermore, although the data were prospectively collected, the information in this study was extracted rather than planned for at the outset of the longitudinal study. It would have been useful to obtain information on use of over-the-counter medications and tobacco use during pregnancy. Prospective monitoring of the infants throughout the neonatal period, measuring gestational compliance, and having ongoing liaisons with the obstetricians would have added important information to this study.
Additional research is needed to follow up on and extend these findings. Future studies might include an exploration of the quality of marital and parental relationships that might affect the outcome of pregnancy and the ways in which pregnancy and obstetrical complications might be related to having an eating disorder. Questions examining the extent to which postpartum depression can be predicted by specific eating disorder behaviors and postpartum social support would be of interest in future research. These areas of inquiry might best be examined by using methods that assess both women with and without eating disorders at more frequent intervals throughout their pregnancies by means of a variety of quantitative and qualitative measures.
Several conclusions can be drawn on the basis of the data obtained in this study. It is somewhat surprising that the majority of the women with eating disorders had normal pregnancies, resulting in healthy babies. Although there was a high percentage of birth defects, most of the babies were of average birth weight and had good Apgar scores. However, the women who were symptomatic during their pregnancies fared worse than the nonsymptomatic women and appeared to be at greater risk for delivery by cesarean section and postpartum depression. Consistent with the findings of other researchers
(9,
11,
13), we argue for close observation throughout pregnancy by obstetrical and mental health providers, particularly for patients with active eating disorders. We recommend this for both anorexic and bulimic patients. Furthermore, we suggest that obstetricians screen routinely for eating disorders and inquire carefully when a pregnant woman has a history of an eating disorder to determine if she is currently experiencing the symptoms of anorexia or bulimia nervosa
(21). It appears that women currently experiencing symptoms may be at the greatest risk and require the closest monitoring, both during and after pregnancy, in order to optimize maternal and fetal outcomes.