Mood disorders are among the most common mental disorders in the elderly population
(1). Both major depressive disorder, the most severe form of depression, and clinically significant forms of minor depression are responsible for considerable psychosocial morbidity in elderly patients
(2). These effects include frequent visits to physicians’ offices and emergency rooms, abuse of alcohol and tranquilizers, absenteeism, and suicide
(2). Mood disorders in elderly patients are also frequently associated with a broad spectrum of medical disorders, including cardiovascular, cerebrovascular, pulmonary, and musculoskeletal diseases
(3,
4). Despite these consistent epidemiological and clinical observations, the precise biological underpinnings of depression in late life remain largely unknown.
Both physiological and neuroanatomical approaches have been used to examine the neurobiological correlates of depression in late life
(5–
11). Studies with positron emission tomography and xenon-133 inhalation methods have shown lower levels of glucose metabolism and cerebral blood flow in neocortical and subcortical regions in elderly patients with major depression than in comparison subjects
(5,
6). Magnetic resonance imaging (MRI) studies have revealed two kinds of structural abnormalities in patients with late-life depression: lower brain volumes in circumscribed regions and a higher volume of high-intensity lesions in the parenchyma—areas that appear bright on T
2-weighted MRI findings
(7–
11). The lower brain volumes are more striking in the prefrontal lobes, hippocampus, and head of the caudate nucleus
(9). High-intensity lesions occur predominantly in the periventricular and deep white matter regions, although they have also been described in the subcortical nuclei
(10).
Discussion
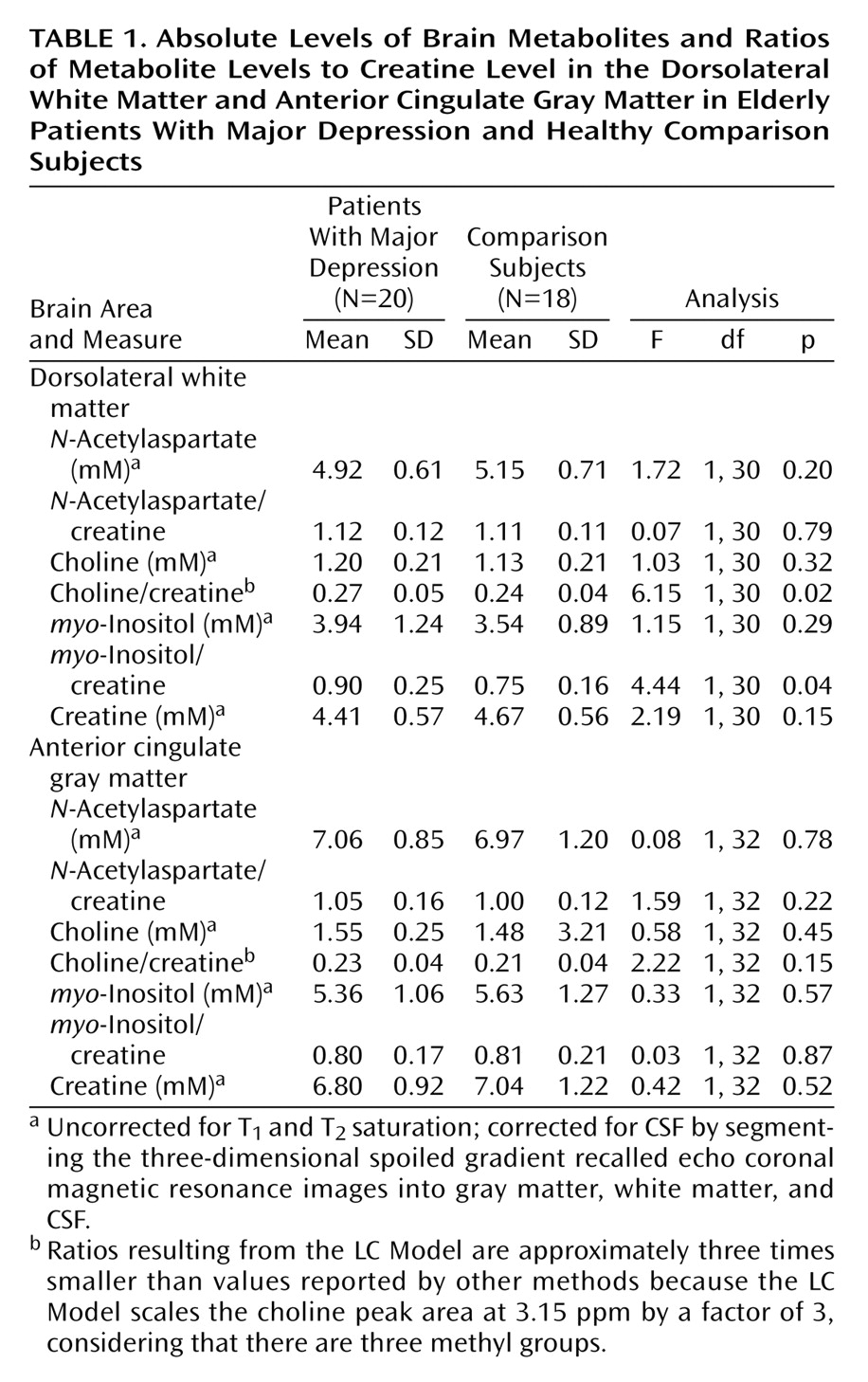
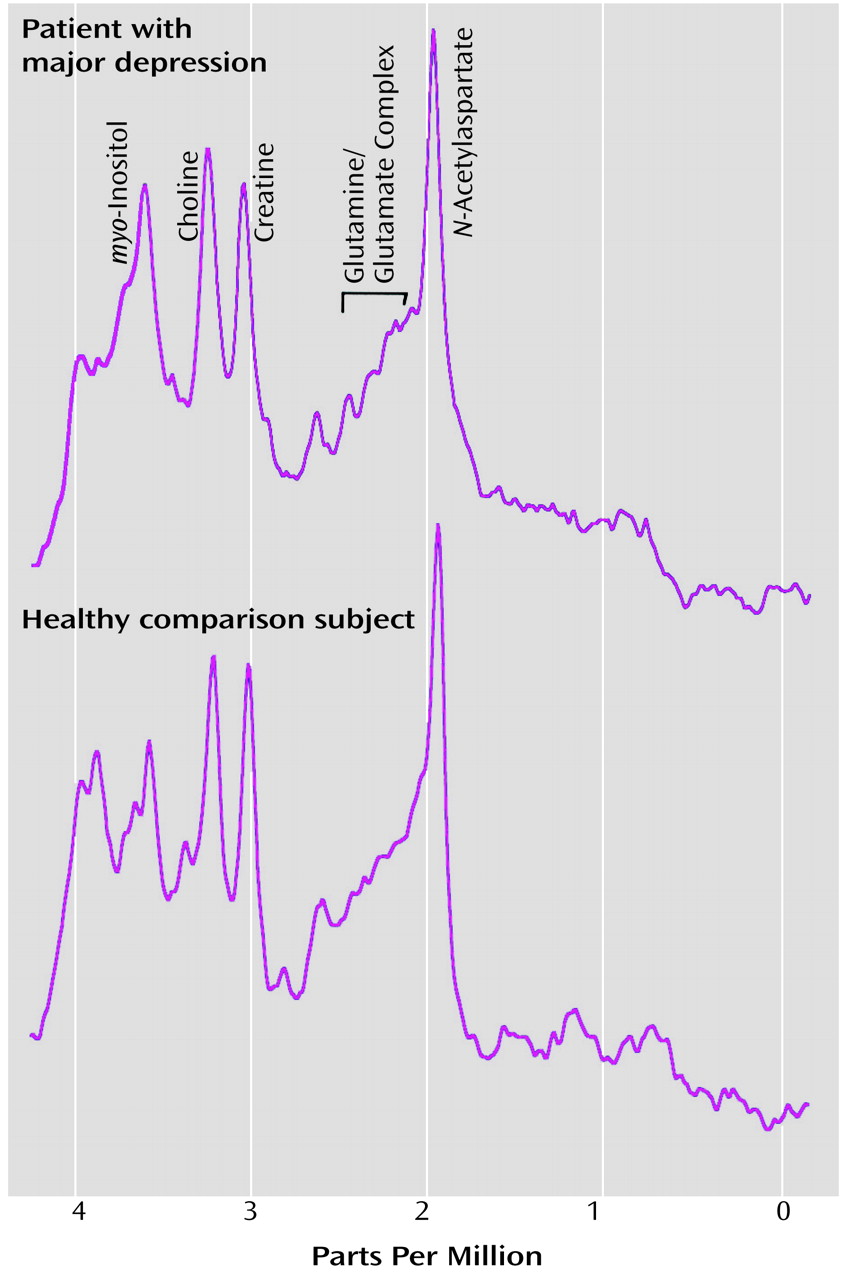
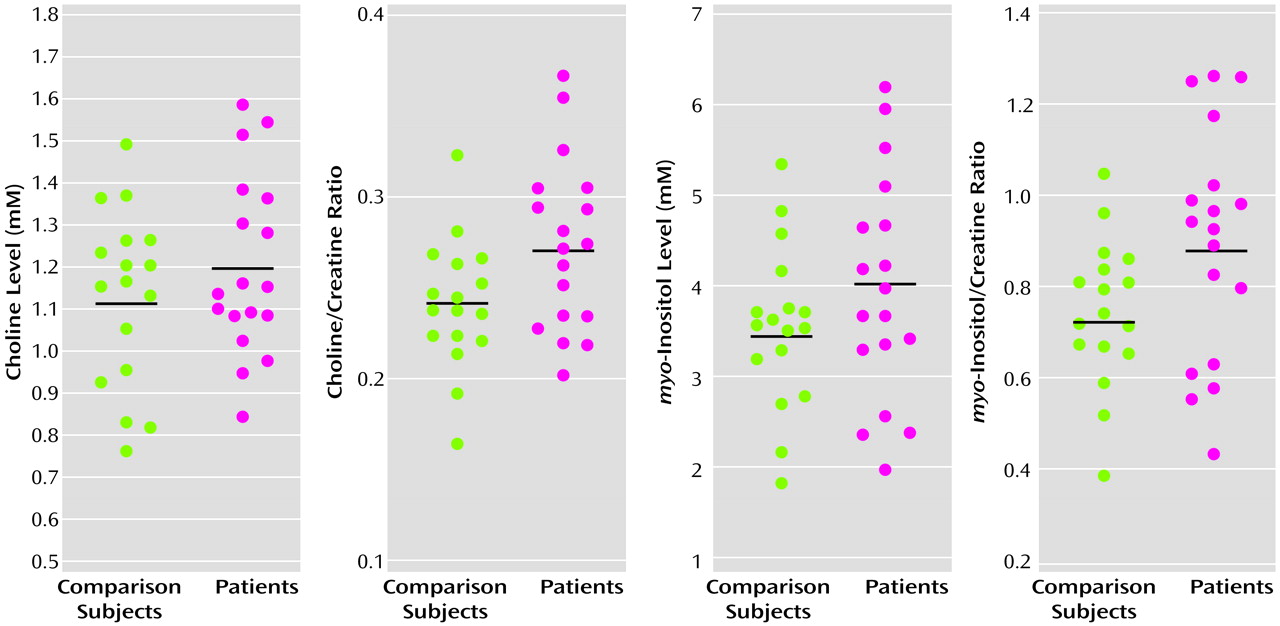
To our knowledge, our study is the first to demonstrate that frontal lobe biochemical abnormalities in patients with late-life major depression are more striking in the white than in the gray matter. The biochemical abnormalities observed in our study consisted of more prominent choline/creatine and myo-inositol/creatine signals in the left dorsolateral white matter in patients with major depression than in the comparison subjects. There was no significant difference in the white matter N-acetylaspartate levels between patients and comparison subjects. Also, there were no significant differences in any of the major metabolites in the gray matter between the two groups. These data suggest that physiological abnormalities in the white matter may provide an important neurobiological substrate to depression, especially in elderly patients.
Although higher choline resonances have been demonstrated in younger patients with both unipolar and bipolar depression, these results are controversial
(24–
26). The bulk of the evidence appears to support higher choline resonances in younger patients, relative to comparison subjects, especially in the basal ganglia/temporal lobe region
(24–
26). Another report, however, demonstrated lower choline/creatine ratios in the basal ganglia/temporal lobe regions in patients, especially in nonresponders to pharmacological intervention, than in comparison subjects
(27). The only published study of elderly patients that we could find
(28) demonstrated a higher choline/creatine ratio in a large voxel that encompassed several subcortical structures in seven patients with major depression, relative to comparison subjects. In this study, the high choline/creatine ratios normalized with pharmacological treatment.
The choline resonance obtained from in vivo MRS has been shown to reflect free choline, phosphocholine, and glycerophosphocholine
(29). It is therefore a composite signal from several choline-containing metabolic compounds
(30). Choline is an essential precursor of acetylcholine and has been implicated in the pathophysiology of mood disorders. Indirect evidence from several pharmacological studies suggests that choline may have a “depressogenic” effect on the central nervous system (CNS)
(31). It has been suggested that higher levels of choline resonance on MRS may reflect alterations in the structure of neuronal membranes of which choline components are an integral part. However, the precise relationship between the choline signal on MRS and acetylcholine transmission in the brain is unclear. The use of phosphorous (
31P) spectroscopy in conjunction with high-field proton MRS is likely to provide additional information in this regard.
The
myo-inositol signal in MRS is also likely to be a composite signal with the bulk of the contributions coming from
myo-inositol itself
(32,
33). The other two contributors to this signal are
myo-inositol monophosphate and glycine. Although inositol is presumed to play an important role in cerebral metabolism, its precise role remains unclear.
myo-Inositol may serve as the storage form of the inositol diphosphate that links receptors to intracellular activity, thereby serving as secondary or third messengers for hormones and neurotransmitters in the CNS
(32).
myo-Inositol is also the substrate for the mood stabilizing agent lithium, which reduces
myo-inositol levels by inhibiting the enzyme inositol monophosphate, a catalyst for converting inositol monophosphate hydroxyls to
myo-inositol.
myo-Inositol/creatine levels in the parietal cortex decrease in response to neuronal insult in conditions like hepatic encephalopathy
(33–
35). Explanations for the observed higher levels of
myo-inositol include higher levels of
myo-inositol uptake or retention or changes in the cellular/extracellular matrix. Alternative explanations include a possible perturbation in the coupling metabolism of the receptor-secondary messenger system complex, thereby providing an important biological substrate to mood disorders in late life.
Several subtle and overlapping biological factors need to be considered in interpreting the changes in metabolite levels in the brain in specific clinical brain disorders
(36–
41). The levels of some of these metabolites in certain brain regions have been reported to be higher in elderly subjects, compared with younger subjects
(37). There are also likely to be regional differences in the levels of metabolites in the CNS
(36,
39,
41). Further, the effect of aging might attenuate regional metabolite concentrations in the brain. The underlying tissue composition, gray versus white matter, has also been shown to influence metabolite levels
(38–
41). Consequently, the anatomical location and the underlying tissue composition of the region examined are critical to the validity and biological significance of these measurements. Age- and gender-matched comparison subjects need to be included in studies examining biochemical changes associated with specific brain disorders.
Although lower volumes of focal brain regions have been described in patients with late-life depression, reductions in
N-acetylaspartate are seldom seen in MRS studies of patients with significant mood disorders.
N-Acetylaspartate is traditionally considered a marker of the structural integrity of the neuron, both of the cell body and the axon, and the level of
N-acetylaspartate is typically low in degenerative disorders in which there is demonstrable cell loss
(42,
43). Higher levels of choline and
myo-inositol signals in the absence of changes in
N-acetylaspartate raise the question of the role of the nonneuronal compartment in the pathophysiology of mood disorders. The focus of in vivo and in vitro studies in the realm of behavior and higher cortical functions has typically been the neuron. The role of glia in the etiology of clinical brain disorders in general and psychiatric illness in particular remains unknown. The glial compartment and its physiological role have been receiving increasing attention recently
(44). The
myo-inositol system together with acetylcholine, glutamate, and other neurotransmitters may play an important role in glial cell function and in modulating synaptic activity
(44). Therefore, in vivo estimates of choline and
myo-inositol may also reflect biological changes in nonneural tissue, with implications for neuronal and synaptic function. Our findings corroborate physiological studies that demonstrate abnormalities in glucose metabolism and perfusion in the frontal lobes in patients with late-life major depression
(5,
6). More sophisticated in vivo and in vitro techniques need to be developed before definitive statements on the glial-axonal axis can be made.
Some limitations of our methodologic approach should be discussed. First, overlap of metabolites’ spectra is a major problem with one-dimensional MR spectroscopy. The spectra for glutamine,
N-acetylaspartate, γ-aminobutyric acid, and aspartate overlap in the 2–3 ppm region
(45,
46). Further,
myo-inositol, glutamate/glutamine, glucose, and aspartate spectral peaks overlap in the 3.5–4 ppm region. Time-domain fitting with the LC Model is more accurate for quantitation of overlapping peaks than conventional frequency domain fitting with the Marquardt algorithm. Despite this improvement, one-dimensional acquisition has intrinsic limitations that preclude a more precise characterization of brain metabolites. Localized two-dimensional correlated spectroscopy, especially if it is adapted to high-field systems, is expected to improve the analysis of several overlapping metabolites
(46). Second, although voxel placement was designed to maximize tissue homogeneity, the relatively large size of our voxel (2×2×2 cm
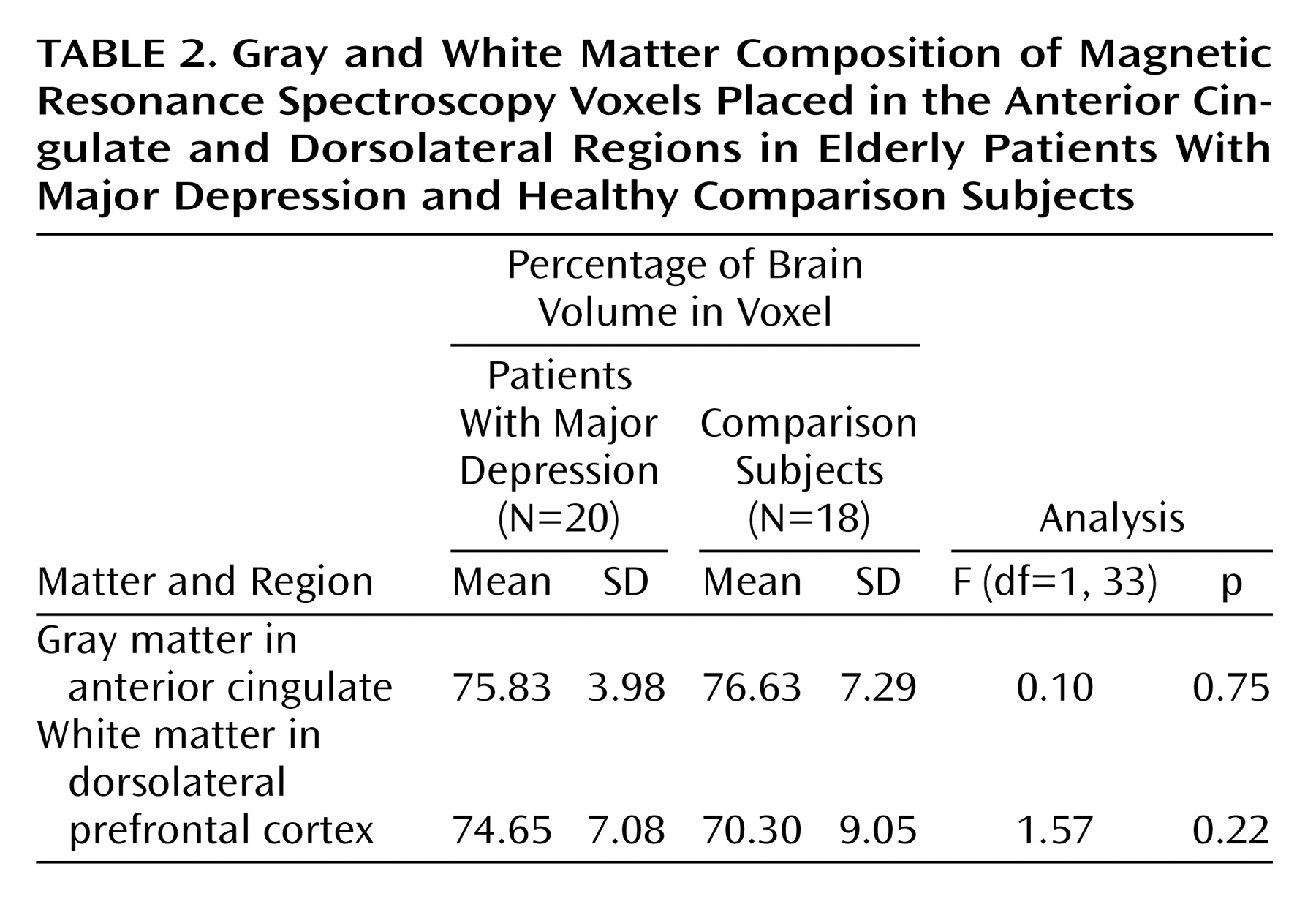
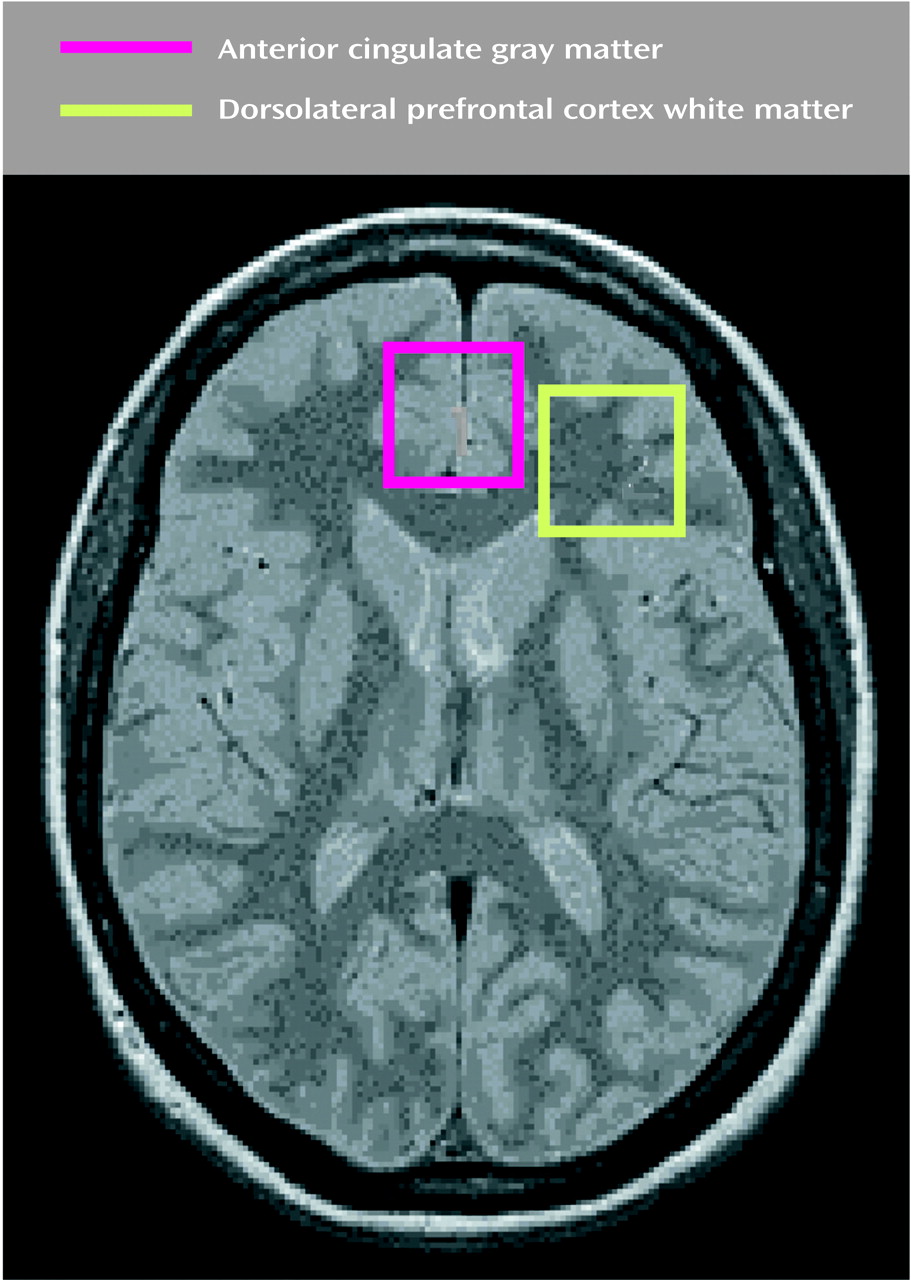
3) resulted in inclusion of both gray and white matter in each voxel location. However, since the voxel placed in the dorsolateral region comprised predominantly white matter, we feel confident that our findings primarily reflect biochemical changes in the frontal white matter. Smaller voxels obtained through chemical-shift imaging will allow more precise measurement of metabolite levels in circumscribed brain regions.
In conclusion, our observations suggest that some of the biochemical correlates of late-life major depression occur in the white matter of the frontal lobes. In general, the role of the white matter in most psychiatric diseases is underappreciated. The white matter has important roles in biological processes such as axoplasmic transport and neurotransmitter release. Disruption of these processes could lead to a cascade of changes that form the substrates of a clinical brain disorder. More focused approaches examining anatomical and biochemical changes in the white matter, both antemortem and postmortem, are likely to reveal the broad spectrum of changes that characterize mood and other psychiatric disorders.