Clinical research is premised upon free, informed, and competent participation of patient volunteers. Therefore, research involving persons with potential impairments in decision-making abilities, such as those with Alzheimer’s disease, has come under considerable ethical scrutiny
(1). An essential element in the ethical assessment of research protocols is a consideration of the risk/benefit consequences of participation
(2). However, relatively little is known about the perspective of persons with Alzheimer’s disease regarding their willingness to participate in studies involving varying levels of risk or potential benefit
(3).
The preferences and enrollment decisions of persons with dementia are ethically important. One concern is that loss of decisional abilities may decrease an Alzheimer’s disease patient’s sense of caution and appreciation of risks and burdens
(4). While attempts to find a relationship between cognitive impairment and treatment preferences have had mixed results
(5,
6), one recent study that used a validated method of assessing capacity found that cognitive impairment is associated with an increase in preference for treatment interventions
(4). If decision-making impairment leads to ill-judged risk-taking, it would be ethically important to anticipate this phenomenon and take it into account during the informed consent process. On the other hand, if Alzheimer’s disease patients with significant impairment can still meaningfully discriminate between risk/benefit scenarios, as the results from another study seem to indicate
(7), it would be important to have informed consent procedures sensitive to preserving as much autonomy as possible for such persons.
Our study sought to answer two questions. First, how do subjects with Alzheimer’s disease and healthy elderly subjects compare in their willingness to participate in research studies that have varying risk/benefit profiles? Second, within the Alzheimer’s disease group, do impairments in decision-making or cognitive abilities have any influence on subjects’ willingness to participate in research?
Method
Subjects
This study was one part of an overall project that studied various aspects of decision making in 37 persons with Alzheimer’s disease and 15 elderly comparison subjects without dementia. In the present study, we focus on the research participation preferences of Alzheimer’s disease subjects compared with healthy subjects and examine whether impairments in cognitive and decision-making abilities are associated with those preferences.
The patient subjects were recruited from the outpatient Geriatric Neurology and Psychiatry Clinic at the Monroe Community Hospital, an affiliate of the University of Rochester. Each patient subject carried a diagnosis of probable Alzheimer’s disease according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association
(8). The 15 comparison subjects for the study were recruited from the same clinic and were caretakers of persons with Alzheimer’s disease. It was reasoned that such a group would not only be comparable in age and education as the Alzheimer’s disease subjects but also have personal experience regarding Alzheimer’s disease to draw from in answering questions related to Alzheimer’s disease research. Additional details of subject recruitment and characteristics are described in a previous report
(9).
This study was approved by the University of Rochester’s Research Subjects Review Board. After full description of the study to the subjects and their caregivers, written informed consent was obtained from each participant. Whenever there was any doubt about the subject’s capacity, the primary caregiver’s simultaneous written consent was also obtained.
Measures
The abilities essential to decision making were assessed by using the MacArthur Competence Assessment Tool—Clinical Research Version, an instrument previously shown to be valid and reliable
(9–
11). The MacArthur Competence Assessment Tool contains 21 pertinent elements of informed consent, each given a rating of 0 (inadequate), 1 (partial), or 2 (adequate). The items are structured under the four-abilities model of competence discussed by Grisso and Appelbaum
(12). These abilities are 1) understanding of disclosed information about the nature of the research project and its procedures (13 items); 2) appreciation of the effects of research participation (or failure to participate) on subjects’ own situations (three items); 3) reasoning about participation (four items); and 4) communicating a choice (one item). The MacArthur Competence Assessment Tool must be adapted for specific research protocols. In this study, it contained information regarding a hypothetical clinical trial of a new drug for the treatment of Alzheimer’s disease. The version used in this study can be obtained from the first author.
The degree of cognitive impairment was measured by the Mini-Mental State Examination (MMSE)
(13). While it has notable limitations
(14), the MMSE is a widely used screening tool for establishing the level of cognitive functioning in patients with Alzheimer’s disease.
We elicited subjects’ willingness to participate in research by using four hypothetical clinical research vignettes of varying risk/benefit profiles: a blood draw, a drug clinical trial, a study involving a drug challenge and a positron emission tomography (PET) scan, and brain surgery. Information was presented in both written and oral form so that the subjects could read along with the interviewer, although the subjects were not allowed to have the written description in front of them while answering questions. The drug clinical trial vignette was part of the MacArthur Competence Assessment Tool. The blood draw and brain surgery vignettes were taken from Sachs et al.
(7).
For the blood draw vignette, subjects were asked whether they would allow a tube of blood to be drawn so that it could be used to develop a diagnostic test for Alzheimer’s disease. The minor risks, the lack of direct benefit to the subject, and the potential for future benefit to others were explained.
For the drug challenge with PET scan, subjects were asked whether they would participate in a drug challenge study that could temporarily worsen their cognitive symptoms as well as cause side effects such as dry mouth and constipation; the subjects were then told that after taking the drug, they would also be exposed to a very low dose of radiation in order to image the brain. The subjects were informed that the purpose of the study was to gain knowledge and not for direct therapeutic benefit of participants.
For the brain surgery vignette, subjects were asked whether they would participate in a clinical trial that involved drilling a small hole in their skull under anesthesia in order to deliver a drug through a slow-infusion pump installed under the scalp. The risks of surgery and anesthesia, including the risk of death, were explained; the study was described as being of potential benefit to the subjects if the treatment worked.
The answers to the vignettes were coded as “willing to participate,” “unwilling to participate,” or “unsure/don’t know.”
Analyses
Statistical analyses were performed on data from subjects who completed all of the measures; SPSS Version 10.0 software (Chicago) was used. Three of the 37 Alzheimer’s disease subjects had incomplete data. For these three subjects, decisional ability scores (from the MacArthur Competence Assessment Tool) ranged from 0 to 3 (out of a possible 26) on understanding, 0 to 1 (out of a possible 6) on appreciation, and 0 to 2 (out of a possible 8) on reasoning. One comparison subject declined to answer the research vignette questions. As a result, 34 Alzheimer’s disease subjects and 14 comparison subjects were included in the analyses.
Comparisons between the Alzheimer’s and the healthy subjects, as well as comparisons within the Alzheimer’s disease group, were performed by using either a t test or Fisher’s exact test, depending on the nature of the variable. For the Alzheimer’s within-group comparisons that assessed the influence of cognitive and decisional impairment on willingness to participate in research, two groups were created for each vignette: those willing to participate and those who were unwilling/unsure. Those responding “unsure/don’t know” were lumped into the unwilling group, since in practice only participants providing explicit consent would be enrolled in an actual study. (Since the normal group showed no cognitive or decisional impairment, this analysis was done only for the Alzheimer’s disease group.)
Multivariate logistic regression models were constructed to assess the effect of decisional impairment on responses to research vignettes. Because significant correlations between the ability measures of the MacArthur Competence Assessment Tool are known to occur
(11), we tested for and found significant correlations between the understanding, appreciation, and reasoning scores (Kendall’s tau range=0.50 to 0.55, N=34, p=0.01 for all three pairwise correlations). Thus, separate models were constructed with each ability measure as the predictor variable and MMSE score, gender, and education as covariates. A total of 12 regression models were therefore constructed (four vignettes and three ability measures). Age was not included as a covariate in any of the models because this was already an elderly study group.
Results
There were no significant differences between the Alzheimer’s and the healthy comparison subjects in terms of age (mean=78.5 years [SD=6.0] and 75.3 years [SD=4.9], respectively), gender (proportion of women: 56% and 71%), or education (mean=14.2 years [SD=3.0] and 14.0 years [SD=2.1]). All subjects were white, reflecting the nature of the clinic from which they were recruited. The Alzheimer’s disease group had a significantly lower MMSE score (mean=23.3, SD=3.7, range=16–28) than did the healthy comparison group (mean=29.0, SD=1.7) (t test assuming unequal variance: t=7.34, df=45.6, p<0.001).
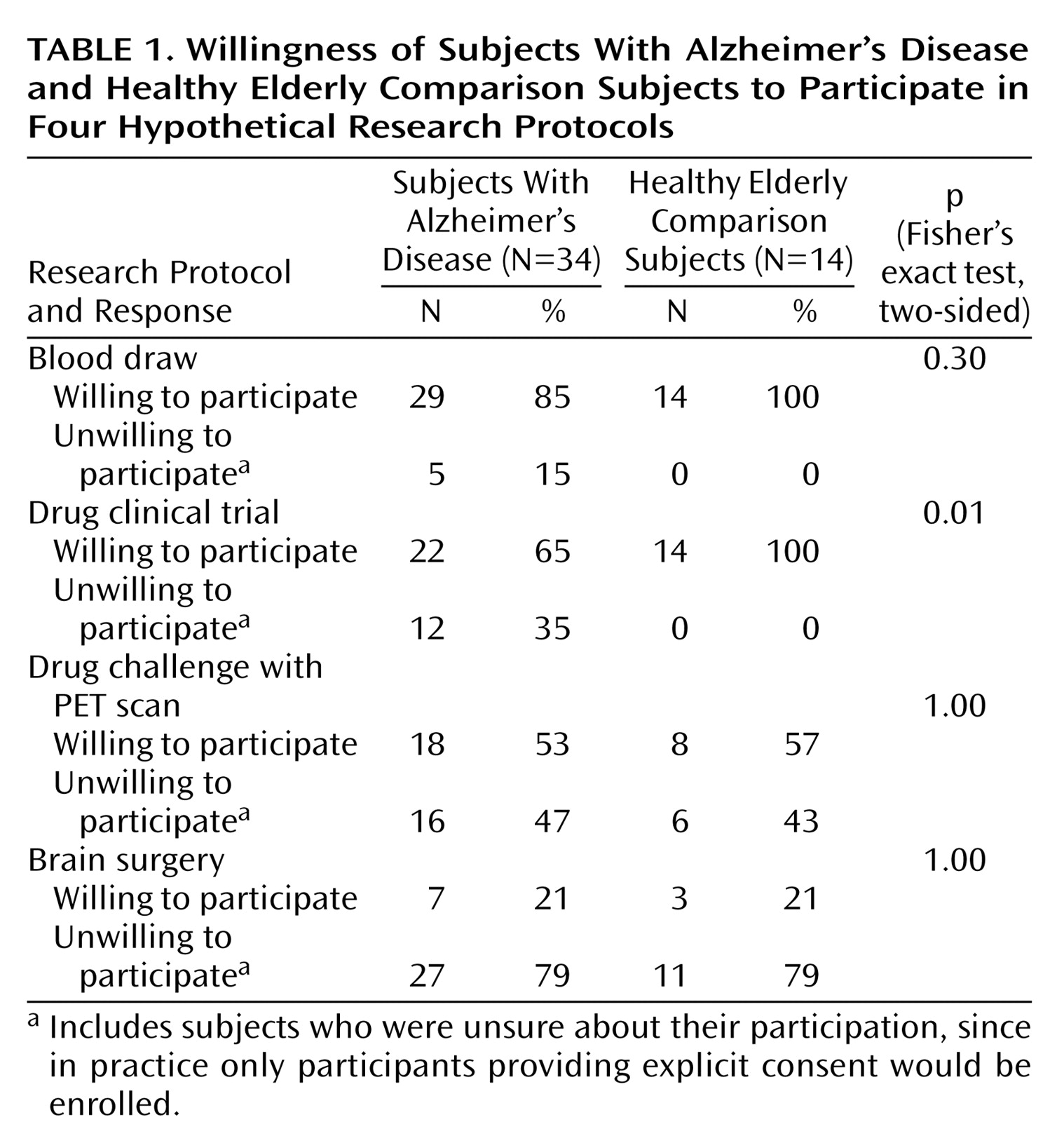
There were no significant differences between the Alzheimer’s and the comparison subjects in the proportion willing to participate in three of the four vignettes (
Table 1). Only for the drug clinical trial vignette was there a significant difference, with the healthy subjects more willing to participate. It is notable that for both the healthy and the Alzheimer’s disease groups, the willingness to participate declined as the risks increased.
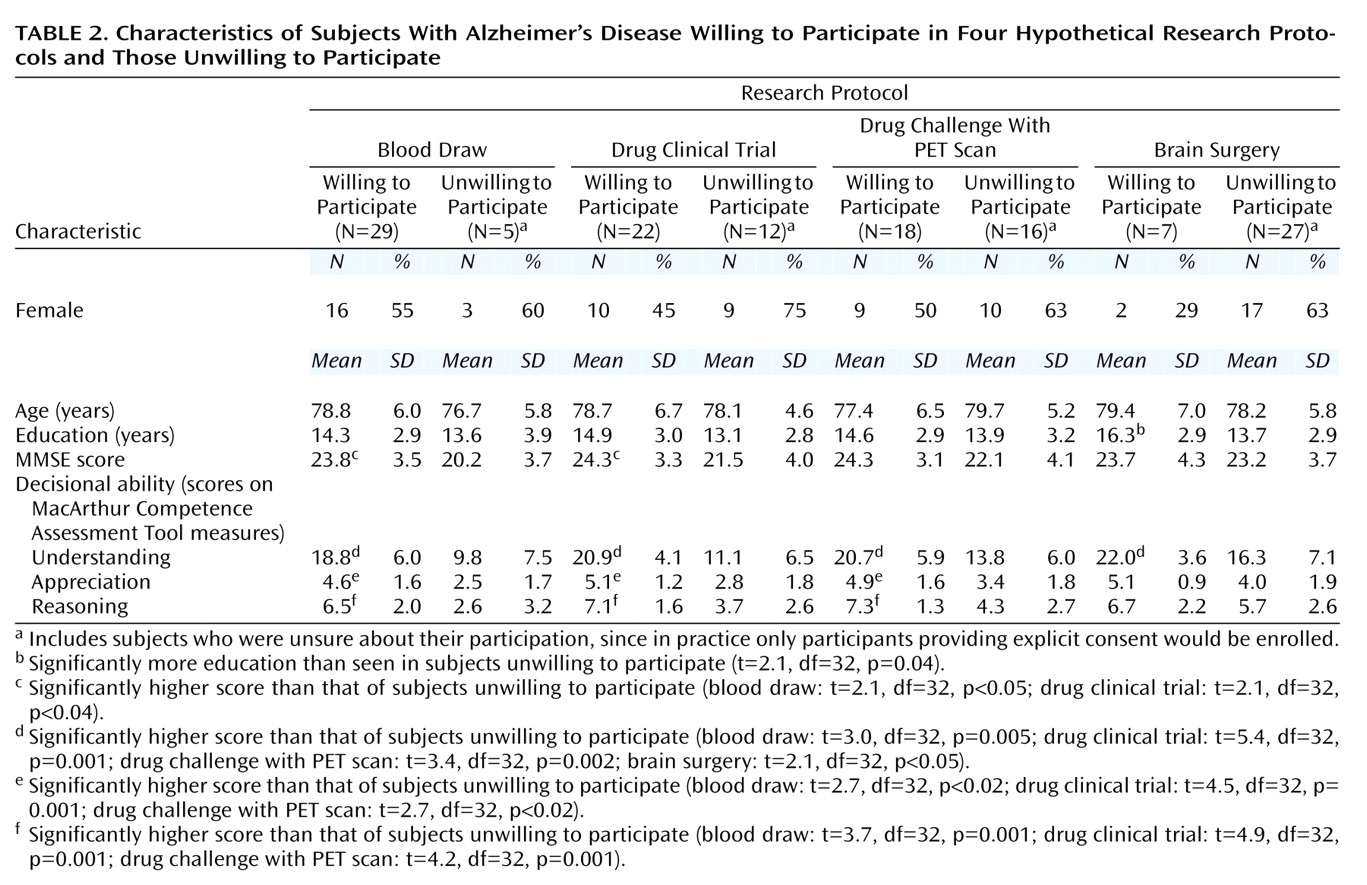
Comparisons of characteristics of Alzheimer’s disease subjects willing to participate in a study versus those unwilling/unsure are presented in
Table 2. Age, education, and gender were not significantly different between the willing and unwilling groups for each research protocol except for the brain surgery vignette, in which those willing to participate had significantly more education. The MMSE scores were significantly higher in the willing than in the unwilling group for the blood draw and drug clinical trial vignettes. Decisional ability (understanding, appreciation, and reasoning scores of the MacArthur Competence Assessment Tool) was significantly higher in the Alzheimer’s subjects willing to participate in the blood draw, drug clinical trial, and drug challenge with PET scan studies; for the brain surgery vignette, only understanding scores were significantly higher in the willing group.
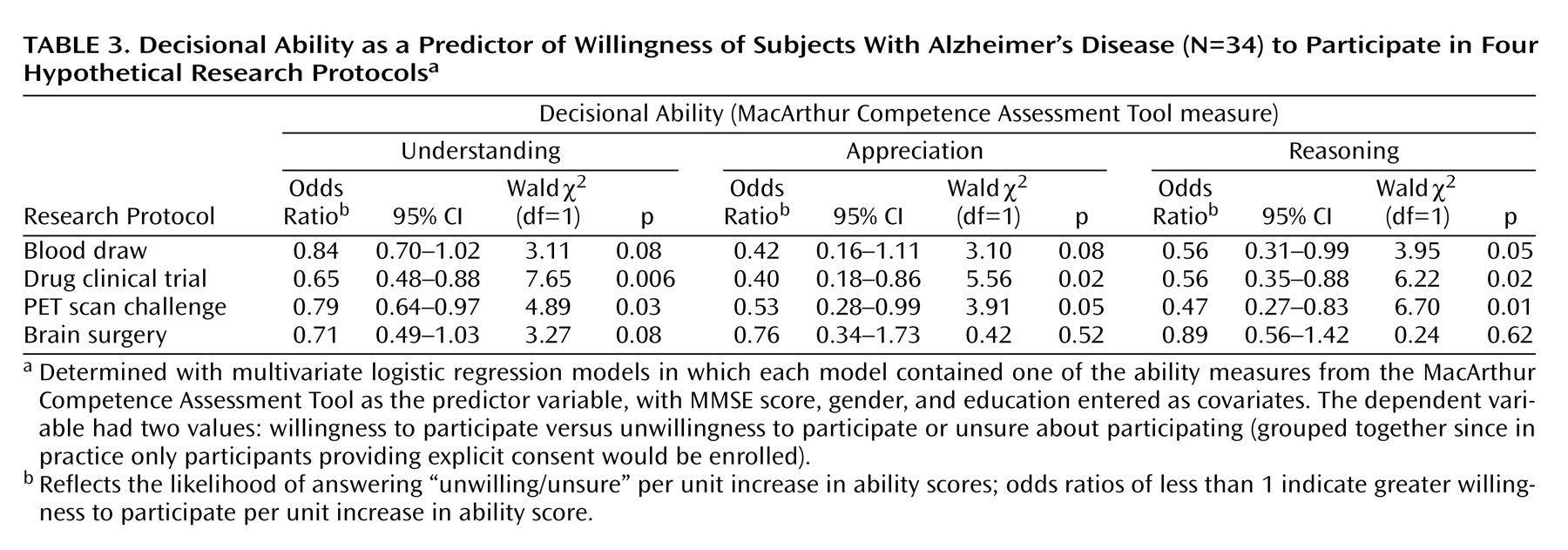
In a multivariate analysis with gender, education, and MMSE score as covariates, each measure of decisional ability still retained significant effects (
Table 3). All significant and near-significant effects were in the direction of higher ability predicting higher rates of willingness to participate. For the protocols involving a drug clinical trial and a drug challenge with a PET scan, all three ability measures were significant predictors of willingness to participate. For the blood draw vignette, only reasoning had a significant effect, although the effects of understanding and appreciation approached statistical significance. For the brain surgery vignette, no ability measure had a significant effect, although the effect of understanding again approached significance. It is of interest that viewed as a predictor variable, the MMSE score did not have a significant effect on willingness to participate in any of the 12 regression models.
Discussion
Relatively little research has been done on the effects of cognitive and decisional impairment on the research preferences of persons with Alzheimer’s disease or other dementing disorders. Sachs et al.
(7) showed that even among Alzheimer’s disease patients with moderate dementia (mean MMSE score of 16.7), their willingness to participate in research declined as risks increased. Sachs et al. found that Alzheimer’s disease patients had a lower willingness to participate than a healthy comparison group for three of the four vignettes that they tested. (Only two of those four vignettes were used in our study.) They found no association in the Alzheimer’s disease group between willingness to participate and age, race, education, or MMSE score. However, they did not use a validated capacity assessment, and the relationship between decisional capacity and research preferences was not examined.
Fazel et al.
(4) recently showed that, among a mixed group of dementia patients and community-residing elderly individuals, those who preferred higher rates of end-of-life treatment interventions tended to be significantly more cognitively and decisionally impaired.
Our study replicated the finding that persons with Alzheimer’s disease are able to distinguish among research studies of varying risk/benefit profiles
(7). As a group, the Alzheimer’s disease subjects responded similarly to the healthy comparison subjects for three of the four vignettes, in contrast to findings by Sachs et al
(7). For the protocol in which they differed (drug clinical trial), 12 of 34 Alzheimer’s disease patients would have refused to participate, whereas all of the healthy comparison subjects would have entered the study.
In terms of the effects of decisional impairment on willingness to participate in persons with Alzheimer’s disease, we found that loss of decisional abilities seemed to increase, rather than decrease, sensitivity to unfavorable risk/benefit ratios. Such effects were independent of factors such as education, gender, and even cognitive impairment (as measured by the MMSE). The lack of effect of cognitive impairment should be interpreted with caution, since it is quite possible that other cognitive measures, especially those testing so-called “executive functions,” may have produced different results, since they are more closely correlated with decision-making abilities
(15–
17).
Before further discussing the potential significance of the findings, we note several limitations of this study. First, the study group size was small. Thus, findings of no difference between groups must be interpreted with caution, and there is a need to replicate the findings in different and larger groups. Second, our study group was quite homogeneous; our subjects were white and well educated. This seems to be an issue for Alzheimer’s research in general
(18). However, it is notable that one of our primary findings—that even significantly impaired Alzheimer’s disease patients can, as a group, discriminate between studies of varying risk/benefit profiles—was also reported by Sachs et al.
(7), whose subjects were predominantly African American. Third, while we were able to show the general effects of decisional impairment on willingness to participate in research, we did not examine the unique contributions of each of the abilities relevant to decisional capacity on research preferences of our Alzheimer’s disease subjects. Finally, although the use of hypothetical scenarios in eliciting preferences is widely accepted
(19–
21), it is not the same as measuring real preference-based decisions. Hypothetical vignettes cannot control for the nature and quality of the relationship between the subject and the investigator
(22), or between patient-subjects and their caregivers
(3), which may influence the research preferences of Alzheimer’s disease patients. However, our study is consistent with a previous study that used an actual drug treatment situation to assess decision making by persons with dementia. Stanley et al.
(23) found that persons with Alzheimer’s disease, relative to healthy elderly subjects, showed significantly worse comprehension of purpose and procedures for the proposed treatment but similar comprehension of risks and benefits.
There are two “take away” findings in this study. First, as judged by their decisions to participate or not, our Alzheimer’s disease subjects judged risk/benefit profiles in a fashion generally similar to our healthy comparison subjects, even in the face of diminished decisional abilities. This speaks to a relative preservation of one important aspect of decision making in persons with Alzheimer’s disease who show diminished decision-making abilities overall
(20). If anything, the Alzheimer’s disease participants were more conservative; relatively fewer were willing to participate in a hypothetical drug trial. Second, those Alzheimer’s disease patients with more intact decisional abilities were more often willing to participate in studies, whereas those with more diminished abilities tended to have a greater aversion to risks. Thus, if the current widely accepted practice
(1) of requiring assent from incapable Alzheimer’s disease subjects is followed, the concern that such vulnerable subjects as a group will be exposed to unusually high risks because of their poor judgment may not be warranted.
Given the apparent connection between impaired decision-making abilities and decreasing willingness to participate in research, the comparable preferences (in three of four vignettes) between our Alzheimer’s and healthy comparison subjects may deserve a more complex interpretation, one that appreciates the dynamic nature of Alzheimer’s disease and the changing impact of gradually diminishing cognitive abilities across the course of this progressive illness. For the relatively intact Alzheimer’s disease subjects, i.e., those with substantial insight and little or no decisional impairments, there may be a greater degree of willingness to participate than in subjects without Alzheimer’s disease. This conjecture has theoretical appeal: having an illness likely increases one’s willingness to participate in research into that illness. During the early stages of Alzheimer’s disease, when insight is relatively preserved, the desire to take active steps to fight the disease may be strong. As the disease progresses, however, it appears that Alzheimer’s disease patients have less willingness to participate. The sense of individual desperation may decrease as insight declines, even while the more basic inclination of self-preservation may play a dominant role. Thus, the comparable performance between different Alzheimer’s disease and healthy comparison groups may vary according to the composition of the Alzheimer’s disease group. If the Alzheimer’s disease group had been more impaired, the overall willingness rates may have been lower than for the healthy comparison group. Indeed, this may explain the divergence of some of our findings from those of Sachs et al.
(7), whose Alzheimer’s disease subjects had an average MMSE score of 16.7, whereas subjects in this study had a mean MMSE score of 23.3.
Our findings do not contradict those studies that suggest that greater decisional impairment among dementia patients leads to greater preferences for clinical therapeutic interventions
(4,
24). It is essential to differentiate between treatment and research contexts. The same person may seek more clinical interventions, believing those to be self-preserving, while refusing to participate in new or experimental procedures, which are perceived to be full of risk.
While it is reassuring that, at least for the stages of Alzheimer’s disease examined in this study, increasing impairment seems to create more caution, those obtaining informed consent from Alzheimer’s disease patients and their surrogates should anticipate this possibility. This may be particularly relevant when enrolling persons with potential decisional impairment into protocols of high risk or burden with no, or uncertain, potential benefit, such as symptom exacerbation challenge studies
(25) or phase I experiments involving neurosurgery
(26). The informed consent process will need to be particularly sensitive to this issue, perhaps with specially designed educational procedures to address the increased fears of the subjects
(27).
Despite the aforementioned limitations, this study showed a significant connection between decisional impairment and willingness to participate in research in persons with mild-to-moderate Alzheimer’s disease. The results have intriguing theoretical dimensions, are consistent with results from related studies, and may have implications for procedures for enrolling decisionally impaired Alzheimer’s disease subjects into research protocols involving significant degrees of risk or burden.