In the past decade, velocardiofacial syndrome has received increasing attention in psychiatric and neurocognitive research. This syndrome, which occurs in 90% of cases from a large 3Mb deletion on chromosome 22 (del 22q11.2), affects cognitive, behavioral, and neurological functioning and predisposes affected individuals to psychiatric disorders
(1–
3). Individuals with velocardiofacial syndrome have impaired cognition and particular deficits in arithmetic reasoning and visuospatial abilities
(4,
5). Neurological symptoms such as poor psychomotor, tactile, and perceptual skills also are reported in velocardiofacial syndrome
(4).
Previous studies have shown that individuals with velocardiofacial syndrome are at greater risk for developing serious psychiatric disorders
(6–
8). Between 15% and 25% of individuals with velocardiofacial syndrome will develop schizophrenia during adolescence or early adulthood
(1–
3). Higher rates of psychotic bipolar disorder or schizoaffective disorder also have been reported in the velocardiofacial syndrome population
(2,
9). Velocardiofacial syndrome is the first genetic disorder proposed as a model for a genetically mediated subtype of schizophrenia
(7).
Although the biological determinants of the neurocognitive and behavioral features of velocardiofacial syndrome are not yet fully understood, this disorder has measurable effects on brain structure and function
(5,
10–14). In particular, at a gross morphological level, the 22q11.2 deletion appears to affect white matter to a greater extent than gray matter. Several volumetric magnetic resonance imaging (MRI) studies of individuals with the 22q11.2 deletion reported a reduction in white matter volume, particularly in parietal regions of the brain
(10,
12,
14). However, there are no currently published findings about the structure and organization of white matter tracts in brains of individuals with velocardiofacial syndrome.
In the current study, we used diffusion tensor imaging to further delineate the white matter tracts potentially altered in individuals with velocardiofacial syndrome. Diffusion tensor imaging is a powerful tool for the investigation of white matter tract structure and coherence in vivo. This method uses MRI to visualize the diffusion of water molecules within axons, thereby allowing investigation of white matter tract structure beyond simple volumetric measurements. On the basis of previous MRI studies, we expected to find white matter aberrations in parietal regions. In addition, since schizophrenia is a relatively high-frequency psychiatric outcome that affects up to 25% of subjects with velocardiofacial syndrome, we expected to find similar white matter aberrations to what has been previously reported in preliminary diffusion tensor imaging investigations of schizophrenia. Specifically, we expected to observe aberrations in frontal and temporal tracts as well as in fibers connecting the frontal and temporal lobes
(15–
18).
Method
Subjects
Participants included 19 subjects with velocardiofacial syndrome ranging in age from children to young adults and 19 typically developing comparison subjects individually matched for age and gender. Thirteen male and six female subjects were included in each group, ranging in age from 7.2 to 21.8 years (velocardiofacial group: mean age=12.2 years [SD=3.9], mean IQ=70 [SD=13.5]; comparison group: mean age=14.4 years [SD=4.2], mean IQ=117.5 [SD=8.9]). The mean age difference was not significant between groups (F=2.2, df=1, 36, p<0.15). The participants with velocardiofacial syndrome were recruited through the VCF Support Group Network of Northern California and through advertisements on our web site (www.cap.stanford.edu/research/). Only individuals with velocardiofacial syndrome with the 22q11.2 microdeletion, as shown by fluorescent in situ hybridization, were included in the study. All individuals with velocardiofacial syndrome had the “classic” 3Mb deletion. Comparison subjects were recruited through advertisements in local newspapers and parent networks. A minimum IQ of 85 (one standard deviation below the population mean) and the absence of previous neurological or psychiatric disorders were used as inclusion criteria for comparison subjects.
After completely describing the study protocol to all participating subjects and their parents, we obtained written informed consent as approved by the institutional review board of Stanford University.
Assessment
To measure cognition, participants were given the WISC-III (ages 6–17)
(19) or the WAIS-III (ages 17 and over)
(20). To assess psychopathology, the parents of all velocardiofacial syndrome subjects were interviewed using the computerized Diagnostic Inventory for Children and Adolescents
(21) or, for individuals older than 18 years, the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID).
Following administration of the Diagnostic Inventory for Children and Adolescents or SCID, a psychiatrist further interviewed the parents for evidence of psychosis and mood cycling, using the screening question portion of the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL)
(22). If any symptom category from the screening interview was positive according to the parents’ responses, the section of the K-SADS-PL relevant to the symptom was administered to investigate a possible diagnosis. Detailed description of the clinical procedure is reported elsewhere
(23).
Among the velocardiofacial syndrome population included in the present study, the following diagnoses were observed: attention deficit hyperactivity disorder (42%, N=8), oppositional defiant disorder (26%, N=5), past/present major depressive disorder (21%, N=4), generalized anxiety disorder (26%, N=5), separation anxiety disorder (21%, N=4), simple phobia (26%, N=5), some evidence for hallucination (N=4) and/or delusions (N=3) (26%, N=5), enuresis (16%, N=3 [associated with encopresis in one case]), and obsessive-compulsive disorder (11%, N=2). Five subjects (26%) had no diagnosis, and 10 (53%) had more than one diagnosis.
MRI Protocol
Magnetic resonance images of each subject’s brain were acquired by using a GE Signa 1.5-T scanner (General Electric, Milwaukee). A diffusion tensor imaging sequence was based on a single-shot, spin-echo, echo-planar imaging sequence with diffusion sensitizing gradients applied on either side of the 180º refocusing pulse. Imaging parameters for the diffusion-weighted sequence were as follows: field of view=24 cm, matrix size=128×128 (19 slices) or 256×256 (18 slices), TE/TR=106/6000 msec, 18 or 19 axial-oblique slices, slice thickness=5 mm, interslice distance 1 mm. The scan was prescribed from the top of the brain and included only the most superior part of the cerebellum. Diffusion gradient duration was δ=32 msec, diffusion weighting was b=900 s/mm. T2-weighted images were acquired by removing the diffusion sensitizing gradients. Diffusion was measured along six noncollinear directions: XY, XZ, YZ, –XY, –XZ, and –YZ. This pattern was repeated four times for each slice with the signs of all diffusion gradients inverted for odd repetitions.
To aid in the localization of white matter differences, high resolution T1-weighted spoiled gradient recall three-dimensional MRI sequence with the following parameters was used: repetition time=35 msec, echo time=6 msec, flip angle=45°, number of excitations=1, matrix size=256×192, field of view=24 cm2, slice thickness=1.5 mm, 124 slices.
Image Processing
Raters who were blind to diagnosis first inspected the raw data. Scans were examined for motion artifacts and corrupted images. Nine scans (out of 28 acquired) in the velocardiofacial syndrome group were unusable due to image corruption occurring at the stage of image encoding at the scanner console. Eddy current effects in the diffusion-weighted images (i.e., geometric distortions that vary from one diffusion direction to the next) were unwarped before averaging
(24,
25). Averaging of the four magnitude images effectively removed the effect of gradient cross-terms between the diffusion sensitizing and imaging gradients. For each slice, two T
2 images with no diffusion weighting (b=0 seconds/mm) were acquired and averaged.
In this study, fractional anisotropy was the variable of interest. Fractional anisotropy is an intravoxel measure that yields values between 0 (perfectly isotropic diffusion) and 1 (perfectly anisotropic diffusion). The degree of anisotropy in a voxel is determined by microstructural features of the tissue in that particular voxel, including fiber diameter and density, degree of myelination, and macrostructural features such as intravoxel fiber-tract coherence. Greater anisotropy within a measured voxel corresponds to a higher fractional anisotropy value.
Fractional anisotropy was calculated for each voxel according to Basser and Pierpaoli
(26) to produce a fractional anisotropy image. The fractional anisotropy images were further processed by using statistical parametric mapping software (SPM 99, Wellcome, U.K.). Voxel-by-voxel type analysis was chosen as the primary method of analysis in the present study. The T
2-weighted image map was used to determine normalizing parameters subsequently applied to the fractional anisotropy images using SPM 99. Resampling in the normalization process eliminates potential differences due to different matrix sizes. The normalized fractional anisotropy images were smoothed with a 4-mm kernel to increase the signal-to-noise ratio. These smoothed images for healthy subjects and subjects with velocardiofacial syndrome were compared by using voxel-wise two-tailed t tests. Results were normalized to z scores to provide a statistical measure of differences between voxels that is independent of sample size. Finally, the joint expected probability distribution of the height and extent of z scores, with height (z>2.33, p<0.01) and extent (p<0.05) thresholds, was used to determine the presence of significant clusters of difference and correct for spatial correlation in the data. We created a white matter mask with the averaged normalized spoiled gradient recall image and used it to highlight changes in white matter tracts by eliminating noise and edge effects. The mask excluded the cerebellum and the brainstem, which were not completely scanned in the sequence prescribed.
A confirmatory analysis was subsequently conducted by using regions of interest. Spherical regions of interest with a diameter of 2 mm were placed on the individual fractional anisotropy maps in pathways that were delineated as having lower fractional anisotropy values in our velocardiofacial syndrome group in the whole-brain, voxel-based statistical parametric mapping analyses. This included the left and right external capsules, left supramarginal gyrus white matter, left and right superior-parietal white matter, left and right temporal stem, and right frontal lobe white matter corresponding to the superior longitudinal fasciculus. Finally, “control” regions of interest were placed bilaterally in the occipital optic radiations. A nonparametric test (Mann-Whitney U) was used to examine group differences. A p value of 0.01 (two-tailed) was chosen as the significance threshold.
Results
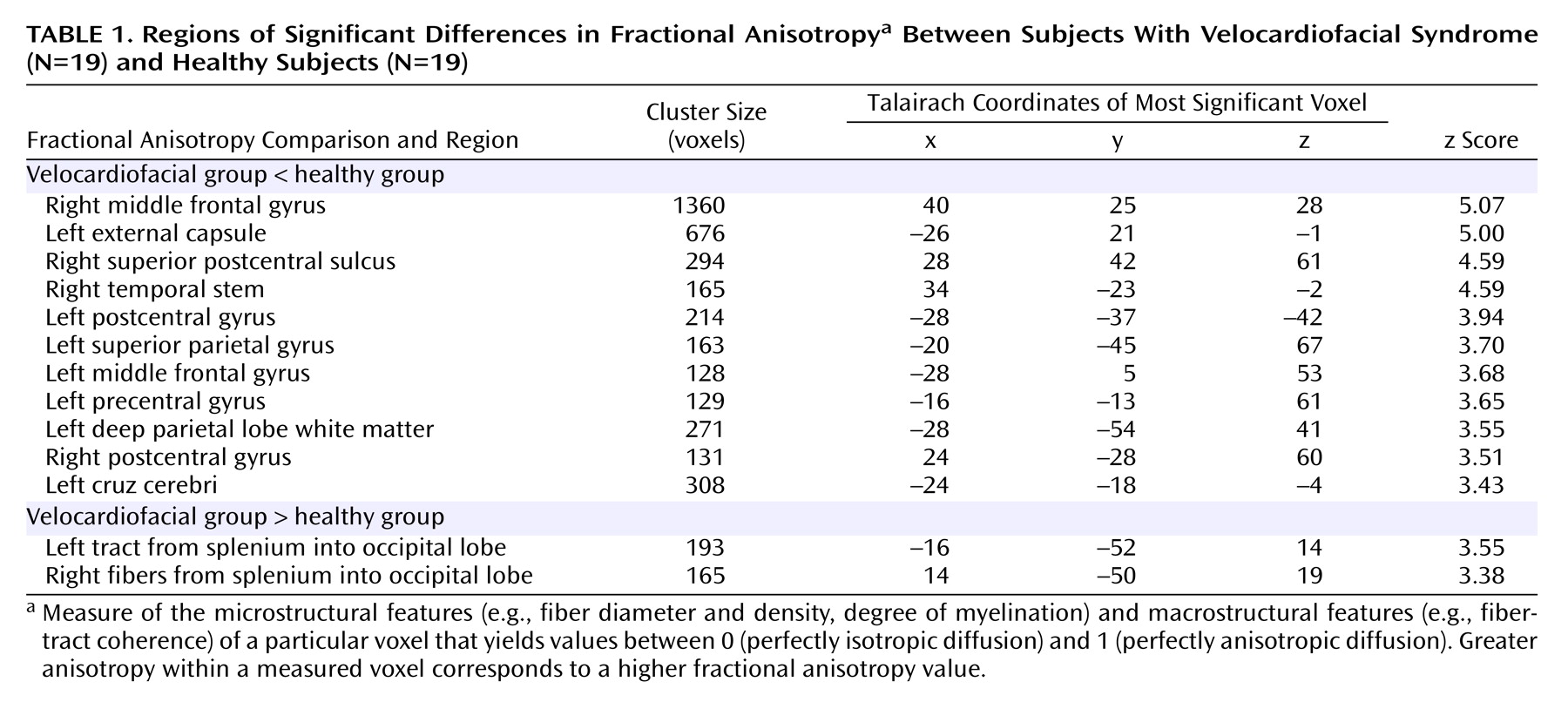
We found decreased fractional anisotropy values distributed throughout the frontal, temporal, and parietal lobes in both the left and right hemispheres of subjects with velocardiofacial syndrome (
Table 1 and
Figure 1).
Relative to the comparison subjects, the velocardiofacial syndrome group had lower fractional anisotropy values in two bilateral clusters in the middle frontal gyrus. The right middle frontal cluster extended into the right external/extreme capsule corresponding to white matter tracts from the superior longitudinal fasciculus connecting frontal and temporal lobes. A separate cluster was seen in the left external/extreme capsule that included the superior and inferior longitudinal fasciculus and possibly the tapetum
(27). Reduced fractional anisotropy values in the velocardiofacial syndrome group were also seen in the left precentral gyrus extending into the superior frontal gyrus. Significant group differences were observed in the temporal lobes, with decreased fractional anisotropy values for the velocardiofacial syndrome group in the temporal stems extending into the cruz cerebri. These tracts, probably too posterior to include the fasciculus uncinatus, correspond to the inferior longitudinal fasciculus or the infero-external peduncle (thalamocortical tracks)
(27,
28). In the parietal lobes, bilateral reduced fractional anisotropy values were seen in the postcentral gyrus, with the left cluster extending partially into the precentral gyrus. Reduced fractional anisotropy values were also seen in the left superior parietal gyrus and in the posterior part of the left centrum semiovale. Several of these bilateral reductions in fractional anisotropy values for the velocardiofacial syndrome group may indicate alterations of the corticospinal tracts, extending from the pre- and postcentral gyri into the crus cerebri.
Examination of group differences by region of interest confirmed the significant fractional anisotropy differences observed in the voxel-based statistical parametric mapping analyses. Significant between-group differences in fractional anisotropy values were seen in the left external capsule (U=25, p<0.0001), right external capsule (U=42, p<0.0001), left supramarginal gyrus (U=60, p<0.0005), left superior parietal gyrus (U=57, p<0.0005), right superior parietal gyrus (U=39.5, p<0.0001), left temporal stem (U=70, p<0.001), right temporal stem (U=34, p<0.0001), and right frontal lobe (U=34, p<0.0001). Average values generated from the “control” regions of interest, placed in left and right optic radiations, did not differ between the two groups.
The healthy comparison group had reduced fractional anisotropy values compared with the velocardiofacial syndrome group in two regions that bilaterally spanned the splenium of the corpus callosum (
Table 1).
Discussion
In the present study we found reduced fractional anisotropy values in bilateral prefrontal tracts, in parietal tracts (particularly on the left), and in bilateral external capsules and temporal stems in velocardiofacial syndrome subjects. The regional location of the observed differences is compatible with an alteration of the superior longitudinal fasciculus as well as the inferior longitudinal fasciculus in the temporal region
(27). Both tracts participate in frontotemporal connections
(28,
29). Reduced fractional anisotropy values were also seen in pre- and postcentral gyri. Increased fractional anisotropy values were seen in the splenium of the corpus callosum in the velocardiofacial syndrome group relative to the comparison subjects. These regional differences in fractional anisotropy values reflect localized alterations in density, coherence, or myelinization in the velocardiofacial syndrome group.
This study supports previously reported findings of white matter alterations in velocardiofacial syndrome imaging studies
(14,
30). However, these studies reported reduced white matter volumes particularly in posterior regions of the brain, whereas our findings were more widespread and included tracts in the posterior frontal areas. This apparent discrepancy can be explained by both the difference in the construct measured between cited volumetric studies and a diffusion tensor imaging study as well as by the differences in analytical methods used in the volumetric studies and in our diffusion tensor imaging study. The analytical unit in the previous morphometric studies was an indicator of volume; the analytical unit in the current study is an indicator of water movement, reflecting the integrity of white matter tracts. The volume of white matter in a region may, in part, be a function of the integrity of white matter tracts, but there can be other contributing factors of white matter integrity that are not related to volume (e.g., white matter tract organization or coherence). Furthermore, methodological differences also could contribute to differences between studies. Eliez et al.
(30) and Kates et al.
(14) used a region of interest analysis corresponding to entire lobes. Region of interest-based methods may not be sensitive to localized volumetric differences in frontal lobe white matter, since larger areas of nonaffected white matter within the frontal lobes may obscure these changes. The voxel-by-voxel type analysis used in our study is better suited for investigating abnormalities of quality in distinct white matter tracts. One previous voxel-by-voxel analysis compared 10 adults with velocardiofacial syndrome and 13 subjects with learning and psychiatric problems
(10) and observed white matter volume reductions in areas similar to those observed here (frontal, parietal, and temporal regions). Moreover, Amelsvoort et al.
(10) also reported increased white matter density in the splenium of the corpus callosum, consistent with our findings. However, the increased density in the splenium of the corpus callosum should be interpreted with caution, since it may reflect a technical problem inherent in the common methodology used in both studies. With statistical parametric mapping, all brain scans are imperfectly normalized to a common template. Potential posterior displacement of the splenium of the corpus callosum in velocardiofacial syndrome, secondary to unusual shape of the brain, may result in voxels that represent the corpus callosum in the velocardiofacial syndrome group but not in the comparison group. Since the corpus callosum has high fractional anisotropy values, these areas would spuriously appear to have higher fractional anisotropy values in the velocardiofacial syndrome group. Shape analysis of the brain and the corpus callosum in velocardiofacial syndrome would help elucidate this issue.
The reduced white matter anisotropy in frontal and parietal regions in our study together with reduced volumes of gray and white matter in parietal regions of the brain in velocardiofacial syndrome
(12,
14) is intriguing to consider in light of the neurocognitive and neurologic phenotype of velocardiofacial syndrome. Arithmetic reasoning and visuospatial abilities, which are known to be disrupted in velocardiofacial syndrome
(4,
31,
32), have been shown to involve a distributed network consisting of the parietal and prefrontal cortex
(33–
37). Disrupted connectivity within this network could contribute to the cognitive impairment in skills served by the parietal and prefrontal cortex network. A recent functional MRI (fMRI) study of brain activation during mathematical problem solving demonstrated abnormal left posterior parietal brain activation in young people with velocardiofacial syndrome relative to comparison subjects
(5). Impaired function in this area is consistent with decreased left parietal volumes
(12,
14) and with our finding of suboptimal circuitry in parietal areas, particularly on the left. Taken together, these findings indicate that left parietal impairment along with disrupted frontal and parietal networks may underlie arithmetic difficulties seen in subjects with velocardiofacial syndrome.
While neurological deficits are documented in velocardiofacial syndrome
(38–
40), the basis for these impairments is not well understood. Our findings show that white matter connectivity is aberrant in velocardiofacial syndrome in pre- and postcentral gyri, regions that are important for tactile perceptual abilities and psychomotor skills. This suggests that impairment in tactile perceptual abilities and psychomotor skills in velocardiofacial syndrome may be associated with aberrant white matter connectivity.
In our group of individuals with velocardiofacial syndrome, there was bilateral reduction in fractional anisotropy values in the external and extreme capsule, a region known to contain fibers connecting the frontal and temporal lobes
(41). This finding suggests disrupted frontotemporal connectivity in velocardiofacial syndrome, which is of interest considering recent findings reported in samples of patients with schizophrenia. Preliminary studies using structural and functional imaging modalities reported that frontotemporal connectivity could be disrupted in schizophrenia
(23,
42,
43). Investigating white matter tract integrity, a recent diffusion tensor imaging study demonstrated aberrant connectivity in the uncinate fasciculus, one of the frontotemporal pathways, in individuals with schizophrenia
(15). It has been hypothesized that disruption in frontotemporal connectivity may underlie failure to recognize inner speech as self-generated
(43,
44). Multimodal neuroimaging studies (diffusion tensor imaging and fMRI) will have to investigate the impact of frontotemporal white matter tract disruption on auditory hallucination symptoms in schizophrenia and velocardiofacial syndrome.
One limitation of the current study is the difference in cognitive ability between subject groups. The effect of cognition on white matter anisotropy has not been studied. However, to better define white matter differences attributed to the 22q11.2 deletion, future studies should include a comparison group that is cognitively matched to the velocardiofacial syndrome group. Further research also is needed to discern the pathology of white matter tracts. The presence of fractional anisotropy alterations in velocardiofacial syndrome signifies various potential white matter structural alterations. Reduced density, coherence of fiber organization, or reduced myelinization could all influence anisotropy. Histopathologic investigation of velocardiofacial syndrome brain tissue of recently developed mice models for velocardiofacial syndrome
(45) will help determine which of these causes is responsible for fractional anisotropy reduction in velocardiofacial syndrome. Also, new fiber tracking techniques would help us understand the relationship between gray matter alterations and white matter tract organization.
Our results suggest white matter alterations in velocardiofacial syndrome. Understanding the influence of the 22q11.2 deletion on white matter circuitry will improve our understanding of the velocardiofacial syndrome phenotype. Ultimately, linking genetic alterations to changes in brain structure and function, as well as to neurobehavioral and neuropsychiatric profiles, will improve our diagnostic specificity and outcome prediction for children with velocardiofacial syndrome.