A substantial number of patients with major depressive disorder fail to respond to adequately performed treatment with antidepressants. One of the preferred treatment strategies for such refractory depression is addition of lithium to the antidepressant
(1). The addition of lithium to an established course of an antidepressant has substantial advantages, compared with strategies involving switching of antidepressants. Time is saved because there is no need for tapering the previous treatment or for a washout period, and possible partial response to the antidepressant is preserved. Many open studies and at least 11 double-blind studies have suggested that 50%–60% of patients with refractory depression may respond to lithium addition; those studies were included in two meta-analyses that confirmed the efficacy of this strategy
(1,
2). Lithium has been found to augment the therapeutic effect of several antidepressants, including tricyclic antidepressants
(1), selective serotonin reuptake inhibitors (SSRIs)
(3,
4), and, possibly, venlafaxine
(5). There is some controversy concerning the speed of onset of the effect of lithium addition. An early open study reported a high frequency of a response within 48 hours
(6), whereas later placebo-controlled studies have suggested that only a small minority of subjects responds within 1 week
(3,
7). A recent meta-analysis reported that maximum benefit from lithium addition is reached after 7 days
(1).
In depressed inpatients, we previously compared the effects of imipramine with those of mirtazapine, followed by lithium addition for patients without response to the antidepressants
(8). We found that the imipramine-lithium strategy proved significantly superior to the mirtazapine-lithium strategy. For patients with mood-congruent psychotic depression, the imipramine-lithium strategy was especially effective. The present study compares imipramine with fluvoxamine, both followed by lithium addition. To our knowledge no other study has compared the efficacy of lithium addition to two different antidepressants with monitoring of the response to the first phase of treatment with the antidepressants alone. This design is important because the efficacy and clinical value of lithium addition can be properly evaluated only in the light of the efficacy of the first treatment step. Fluvoxamine was chosen for this study because it has been reported to be effective in the treatment of depressed inpatients
(9). The present report focuses on achieving remission during this two-phase treatment, in which phase I compares imipramine with fluvoxamine and phase II investigates the addition of lithium to both antidepressants for patients without remission in phase I. The results of the phase I study comparing the effects of imipramine and fluvoxamine are presented elsewhere
(10).
Method
The study protocol was approved by the medical ethics boards of the two centers where the study took place, and the study was performed in accordance with the ethical standards laid down in the World Medical Association Declaration of Helsinki.
Eligible for inclusion were patients age 18–65 years who fulfilled the DSM-IV criteria for major depressive disorder, which was diagnosed by administration of the depression part of the Schedule for Affective Disorders and Schizophrenia
(11), and who had a Hamilton Depression Rating Scale score ≥17. All assessments were done by the three research psychiatrists (W.W.vdB., J.A.B., T.K.B.). During the study, interrater sessions took place six times per year. Subject exclusion criteria were schizophrenia, schizoaffective disorder, bipolar disorder, organic brain syndrome, chronic alcohol or drug abuse, relevant somatic illness, lack of response to previous adequate treatment with a tricyclic antidepressant or fluvoxamine, and pregnancy or inadequate contraception for women in the fertile age group. Eligible patients had to be drug free for at least 3 days before the baseline assessment. None of the patients had been using fluoxetine in the 4 weeks before study entry. After study procedures were fully explained, all patients provided written informed consent.
The study was performed between April 1997 and July 2001 at the inpatient depression unit of two centers: the Department of Psychiatry at University Hospital Rotterdam (W.W.vdB., J.A.B.) and Parnassia Psychomedical Center, The Hague (T.K.B.). During the study period in the first center, 201 patients met the criteria for major depressive disorder. Of those, 95 (47%) patients fulfilled one or more exclusion criteria and 28 (14%) patients refused participation. Thus, from the first center, 78 (39%) patients participated in the study. In the second center, 145 patients met the criteria for major depressive disorder. Of those, 78 (54%) fulfilled at least one exclusion criterion and seven (5%) refused participation, leaving 60 participants (41%) from the second center. Eligible patients were randomly allocated to double-blind treatment with either imipramine or fluvoxamine. After a 4-day single-blind placebo run-in period, the 17-item Hamilton depression scale was administered again. Patients who still met the inclusion criteria of a Hamilton depression scale score reduction ≤50% and a score ≥17 started treatment with either imipramine or fluvoxamine.
Phase I: Imipramine Versus Fluvoxamine (5–7 Weeks)
The medication comprised tablets, in two sizes, of imipramine and fluvoxamine of identical appearance, taste, and weight, containing 75 mg (the larger tablets) or 25 mg (the smaller ones) of the antidepressant.
Preparation of the study medication tablets and randomization from a random-number table was done by the Department of Pharmacy at the first center. Patients received 75 mg/day of imipramine or fluvoxamine during days 1–2 and then 150 mg/day for days 3–8, unless any severe side effects emerged. Plasma levels of both antidepressants were monitored weekly, and doses were adjusted to obtain plasma levels of 200–300 ng/ml for imipramine plus desmethylimipramine and 150–200 ng/ml for fluvoxamine. Fluvoxamine plasma levels were assayed by high-performance liquid chromatography, as described by Pullen and Fatmi
(12). The range used in the present study was derived from a study with a limited number of subjects
(13), in which a relationship between plasma level and clinical response was found. Other studies failed to confirm this relationship
(14,
15). To ensure adequate blinding, the Department of Pharmacy at the first center presented plasma levels as a percentage of a target plasma level. The 100% plasma level was 250 ng/ml for imipramine plus desmethylimipramine and 175 ng/ml for fluvoxamine. Adequate plasma level was between 80% and 120%, with the lower margin considered more important. Scoring of the 17-item Hamilton depression scale and the Clinical Global Impression (CGI) of severity and response was performed weekly. Excellent interrater reliability (kappa=0.95) was achieved between the participating psychiatrists regarding the total score on the Hamilton depression scale. The use of concomitant psychotropic medication was strongly discouraged, although its use was not a reason for exclusion. Some patients with severe insomnia received 1–6 tablets containing an extract of valerian per day; this extract was assumed to be without antidepressant properties. Less than 10% of the patients received either 1–3 mg/day of lorazepam for excessive anxiety or 1–5 mg/day of haloperidol for intolerable psychotic symptoms.
Phase II: Lithium Addition (4–5 Weeks)
Four weeks after achievement of an adequate plasma level of imipramine or fluvoxamine, lithium was added to the antidepressant for patients who did not have remission during phase I. Nonremission is often defined as a Hamilton depression scale score >7. For pragmatic reasons, a Hamilton depression scale score >13 was chosen as the threshold for lithium addition: in patients with a Hamilton depression scale score around 10, the adverse effects of lithium addition may well outweigh its benefits. Furthermore, in patients with entry scores below 14, the treatment effect is difficult to measure. Patients entering phase II continued their double-blind medication and had lithium added in an initial dose of 600 mg at 8:00 p.m. Blood lithium level was measured on day 7 and weekly thereafter, 12 hours postdose. The dose was adjusted to achieve a lithium level of 0.6–1.0 mmol/liter as soon as possible. Weekly assessment of the 17-item Hamilton depression scale and the CGI was performed until 3 weeks after the patient reached the target lithium level, at which point in time response was evaluated. Weekly measurement of the antidepressant plasma level continued throughout phase II. As in phase I, the use of concomitant psychotropic medication, although permitted, was strongly discouraged.
Statistical Analysis
The efficacy of both treatment strategies was compared with survival analysis by using the Cox proportional hazards regression model. The duration of treatment until the response criterion was met was the survival time variable. The two outcome criteria used were response and remission. Response was defined as a reduction in the Hamilton depression scale score of at least 50%, compared to baseline. Remission required a posttreatment Hamilton depression scale score ≤7.
During phase I, patients were assessed weekly with the Hamilton depression scale until 4 weeks after achieving the target antidepressant plasma level. During phase II they were assessed weekly until 3 weeks after attainment of an adequate blood lithium level. The first time a patient met the response criterion was scored as a terminal event. Dropouts were censored at the time of dropout. Patients without treatment response were censored at the end of phase II. The following prespecified covariables were included with the treatment strategy in the statistical analyses: center, the duration of the index episode, adequate previous treatment during the index episode, and the presence of psychotic features. Adequate previous treatment during the index episode was defined as a score ≥3 on the Antidepressant Treatment History Form
(16,
17). A p value <0.05 (two-sided) was considered statistically significant.
Results
Subjects
A total of 138 depressed inpatients were randomly assigned to receive either imipramine (N=70) or fluvoxamine (N=68).
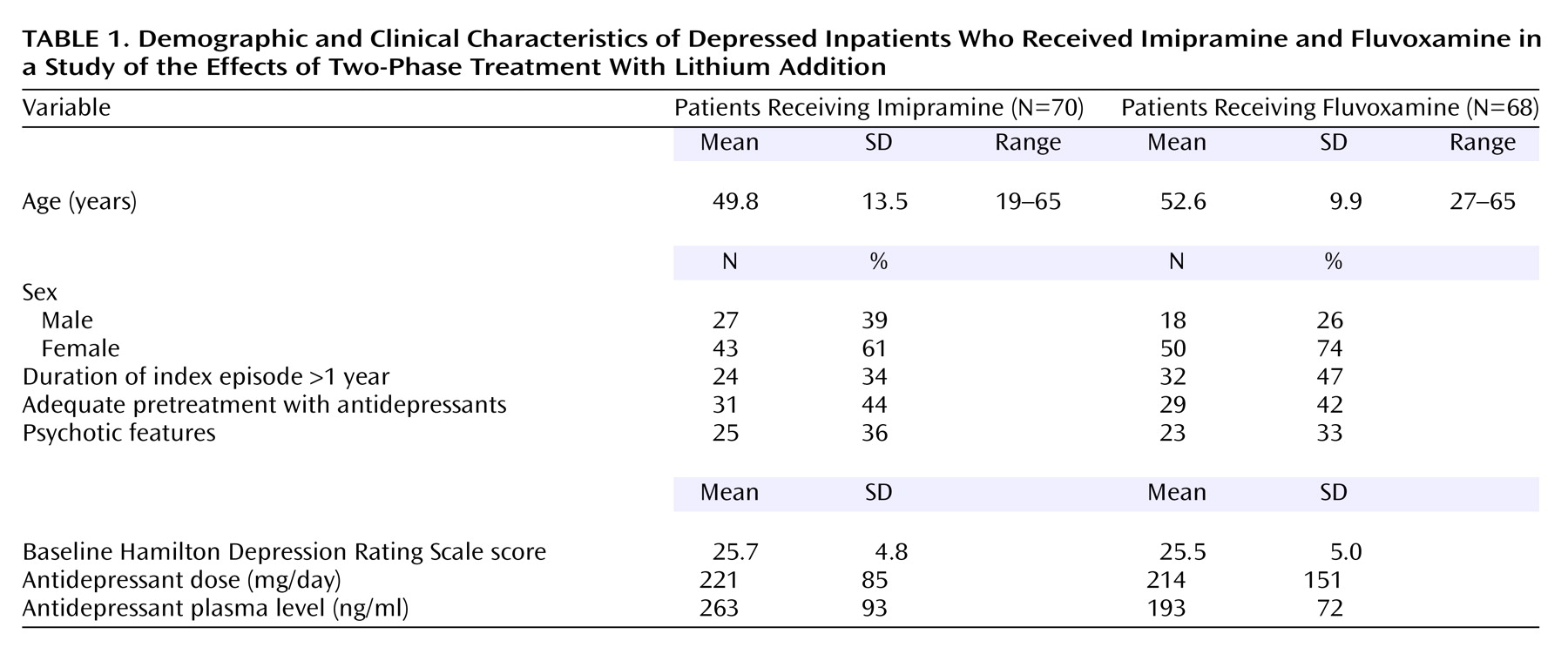
Table 1 summarizes the demographic and clinical characteristics of the 138 study participants. The two treatment groups were balanced for age, sex, entry Hamilton depression scale score, and proportion of patients with psychotic features. Of the 138 study participants, 131 completed the study. The mean doses after achievement of the target plasma level were 253 mg/day (SD=77, range=75–450) for imipramine and 287 mg/day (SD=265, range=150–1800) for fluvoxamine.
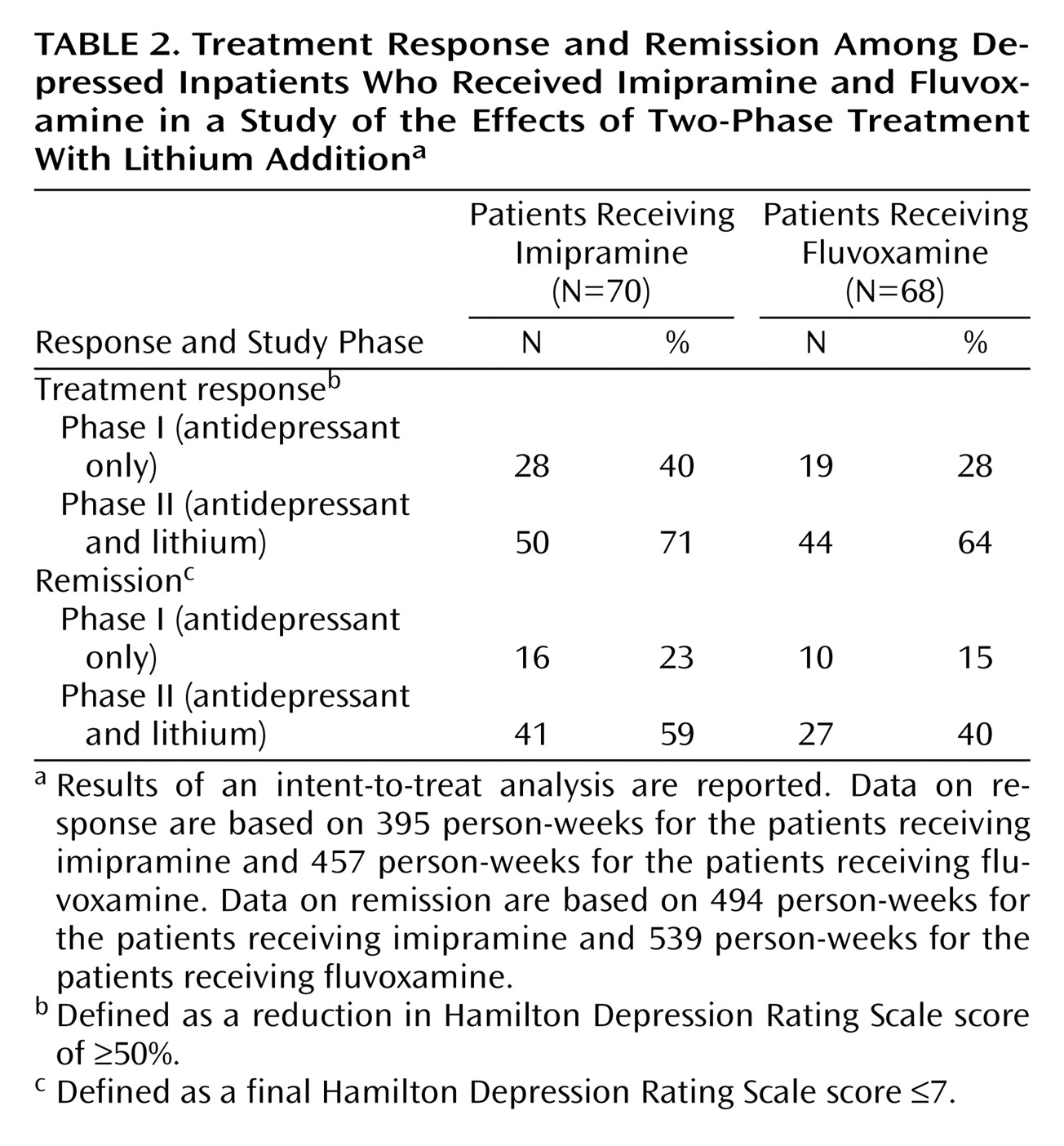
Table 2 summarizes rates of treatment response and remission at the end of phase I. Twenty-eight (40%) of 70 patients who received imipramine and 19 (28%) of 68 patients who received fluvoxamine had a response to treatment. Remission was achieved by 16 (23%) of 70 patients who received imipramine versus 10 (15%) of 68 patients who received fluvoxamine.
Of the 131 phase I completers, 78 (59%) met the inclusion criteria for phase II and 71 of them received lithium addition. Seven patients who met the entry criteria for phase II did not participate: three received electroconvulsive therapy because of a deteriorating condition, two discharged themselves without our consent, and two refused lithium addition. The mean Hamilton depression scale scores at the beginning of phase II were 20.6 (SD=5.4) (N=35) for the imipramine group and 23.8 (SD=5.3) (N=36) for the fluvoxamine group. During phase II four patients in the imipramine-lithium group dropped out: one because of adverse effects and another because of hypomania; two others refused further cooperation. Three patients in the fluvoxamine-lithium group dropped out: one dropped out because of a deteriorating condition, one was discharged without our consent, and one had an emerging somatic illness. The total dropout rate for phase II was seven (10%) of 71. Finally, 64 patients—31 patients who received imipramine and 33 patients who received fluvoxamine—completed phase II.
Plasma Antidepressant and Lithium Levels
Antidepressant plasma levels were available for all 138 patients who took study medication. The mean plasma level after achievement of the target level was 262.7 ng/ml (SD=93) for imipramine (N=70) and 193.2 ng/ml (SD=71.8) for fluvoxamine (N=68). The mean time to achieve the target plasma level was 13.3 days (SD=4.4) for imipramine and 12.6 days (SD=6.2) for fluvoxamine.
Plasma lithium levels are available for 68 (96%) of the 71 patients for whom lithium was started; three patients dropped out before the first plasma level determination. The mean lithium level after achievement of the target level was 0.81 mmol/liter (SD=0.13) for the imipramine group and 0.78 mmol/liter (SD=0.14) for the fluvoxamine group. Six patients (two who received imipramine and four who received fluvoxamine) who completed phase II had a lithium level <0.6 mmol/liter on at least one occasion, while only one patient (who received fluvoxamine) had a final lithium level <0.6 mmol/liter (0.53 mmol/liter). The mean time to attain the target lithium level was 15.5 days (SD=6.3).
Concurrent Medication
During phase I, 12 (9%) of 138 patients received concurrent medication. Lorazepam was prescribed for four patients in the imipramine group and five in the fluvoxamine group. Three of the 48 patients with psychotic depression (one patient in the imipramine group and two in the fluvoxamine group) were treated with 2.5–10 mg/day of haloperidol; all three had no treatment response by the end of phase I. During phase II, seven (10%) of 71 patients were treated with concurrent medication. Two patients in each group received lorazepam. Three patients in the fluvoxamine-lithium group received haloperidol; none of the patients who received haloperidol had treatment response by the end of phase II.
Efficacy
Table 2 summarizes rates of treatment response and remission at the end of phase II. Response (≥50% decrease in Hamilton depression scale score) occurred in 50 (71%) of the 70 patients who received imipramine and lithium versus 44 (64%) of 68 patients who received fluvoxamine and lithium. Remission was achieved in 41 (59%) of the 70 patients in the imipramine-lithium group versus 27 (40%) of 68 patients in the fluvoxamine-lithium group. The mean reduction in Hamilton depression scale score during phases I and II together was 13.8 (SD=9.3) for the patients who received imipramine and 11.9 (SD=9.2) for the patients who received fluvoxamine, a nonsignificant difference. The Cox regression analysis of response in the entire study group (N=138) for phases I and II together, with the type of treatment as an independent variable and with adjustment for the prespecified covariables, showed no significant difference between the imipramine group and the fluvoxamine group (p=0.22, 95% confidence interval [CI]=0.51–1.17). The Cox regression analysis of remission in the entire study group revealed a significant difference between the imipramine and fluvoxamine groups in favor of the imipramine strategy (p<0.05, 95% CI=0.37–0.99). A similar regression analysis, which excluded the 12 patients who received concomitant psychotropic medication, also showed a significant difference in favor of the imipramine strategy (p=0.01, 95% CI=0.29–0.87).
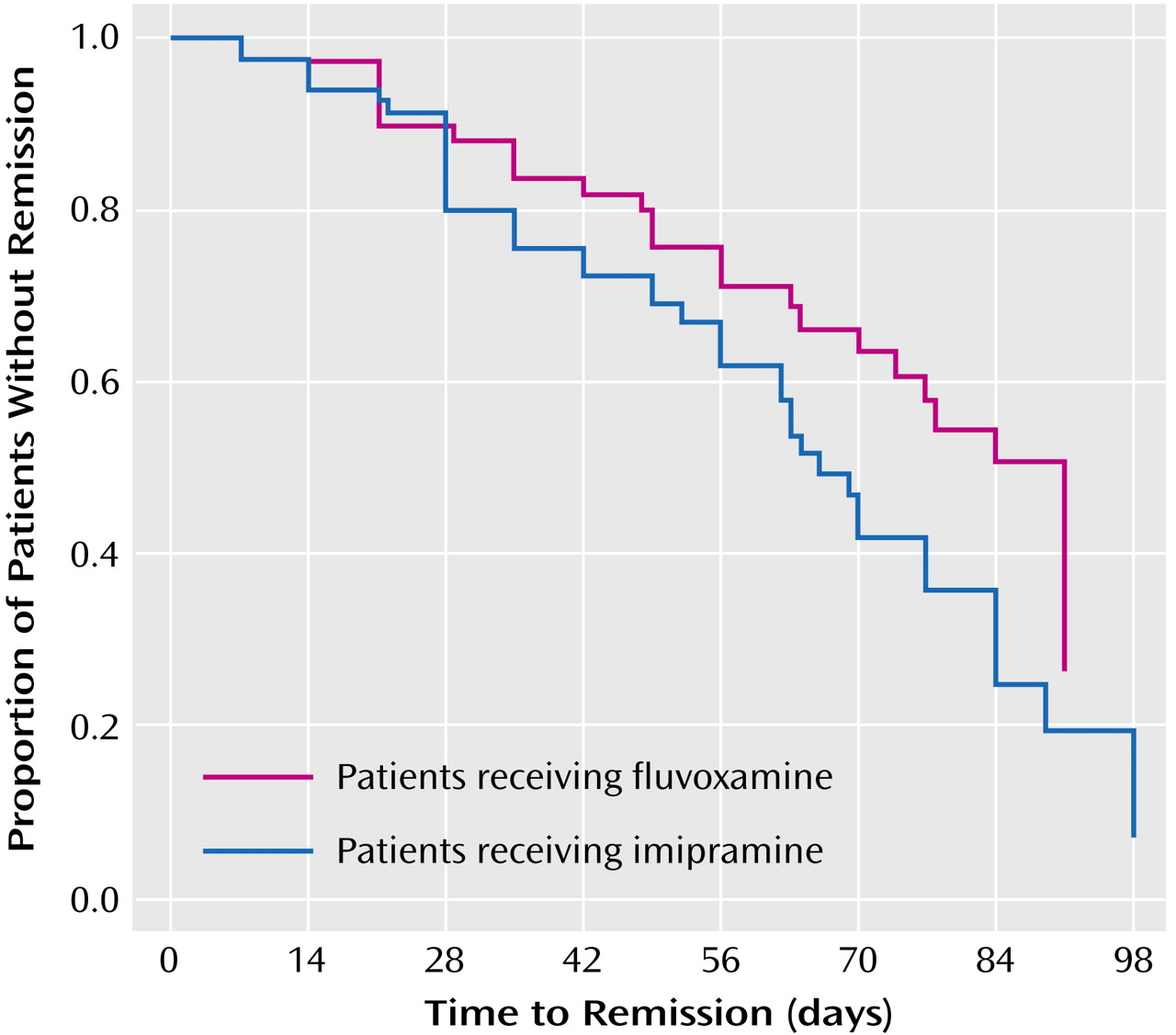
The probability of nonremission in the two treatment groups over time is shown in
Figure 1. None of the prespecified covariables (center, presence of psychotic features, duration of the index episode over 1 year, and adequate pretreatment of the index episode) had a significant effect on the difference found between imipramine and fluvoxamine. Considering phase II separately, the efficacy of lithium addition was high in both treatment groups. The remission rate was 25 (71%) of 35 for patients without response to imipramine in phase I and 17 (47%) of 36 for patients without response to fluvoxamine in phase I.
A considerable proportion of patients met the criteria for response after only 1 week of lithium addition. Ten (33%) of 31 imipramine-treated patients and seven (26%) of 33 fluvoxamine-treated patients had treatment response after 1 week of lithium addition. No significant difference in response at this point in time was found between the two treatment groups. Another 30 patients responded during the remaining weeks of lithium addition, compared to 17 patients in the first week. Thus, the patients with early treatment response constituted about one-third of the overall group of patients with treatment response in phase II.
Discussion
Lithium addition is recommended as a first choice for depressed patients who do not respond to therapy with conventional antidepressants
(1). This combination treatment strategy is in line with the practice guidelines of the American Psychiatric Association
(18). To our knowledge, the present study is the first to provide meaningful quantification of the effect of lithium addition, because the research design controlled for prior treatment with antidepressants in the first phase of the study. In addition, the present study provides a comparison between imipramine and fluvoxamine, followed by lithium addition for patients who did not achieve remission with either agent. The efficacy of lithium addition can be evaluated properly only if treatment with the antidepressant, including dosing, in the first phase is optimal. Optimal antidepressant treatment was achieved in the first phase of this study
(10,
16).
The remission rates for patients with lithium addition were 71% in the imipramine group (25 of 35 patients without remission in phase I) and 47% in the fluvoxamine group (17 of 36 patients without remission in phase I). Our figure for the imipramine group is more favorable than the 50% rate usually cited for the efficacy of lithium addition
(18). In clinical practice, lithium will most often be added to the antidepressant that did not provide sufficient response. Therefore, it is important to note that in our study two-phase treatment with imipramine followed by lithium was more effective than the two-phase treatment with fluvoxamine followed by lithium, with remission rates of 59% (41 of 70 patients included in phase I) and 40% (27 of 68 patients included in phase I), respectively.
In the present study, the difference was not as large as the difference noted in an earlier study of two-phase treatment with imipramine and lithium, compared with mirtazapine and lithium
(8). Both studies included severely depressed inpatients, of whom 35% and 32%, respectively, had psychotic features. The combination of a tricyclic antidepressant and an antipsychotic often is recognized as first-choice pharmacotherapy for psychotic depression
(19). However, in a previous study by our group
(20), the response rate to imipramine with fixed plasma level in patients with psychotic features was superior to that in nonpsychotic patients—nine (64%) of 14 and 14 (44%) of 32, respectively—and lithium addition to imipramine resulted in a response rate of 86% in the group with psychotic features
(8). We, therefore, chose a similar strategy in the present study for depressed patients both with and without mood-congruent psychotic features. One factor that may affect treatment results is the variation in diagnostic criteria for psychotic depression between studies. Possibly the inclusion of depressed patients with mood-incongruent psychotic features could account for some of the differences in the response rate to tricyclic antidepressant monotherapy between studies
(20,
21).
In the present study, about one of three patients responded to lithium addition during the first week, which is in accordance with a recent meta-analysis
(1). Although lithium addition as second-step treatment is remarkably effective, the choice between lithium addition and ECT is dependent on the patient’s condition. Almost 60% of the patients in our study achieved remission after 9–12 weeks of two-phase treatment with imipramine and lithium, and more than 70% had a treatment response to this combination. This high percentage of patients in remission is important in view of the vulnerability of patients with residual symptoms for relapse
(22). As also reported by others
(8,
23), the patients in our study who had a longer duration of the present episode had significantly lower rates of treatment response and remission, not only after treatment with the antidepressant, but also after addition of lithium. However, this covariable, as well as the covariables adequate pretreatment and psychotic features, did not contribute significantly to the difference between the two-phase treatments with imipramine or fluvoxamine. The two-phase treatment with imipramine resulted in a slightly higher discontinuation rate (13%), compared to fluvoxamine (7%), although in absolute numbers the difference was small. The SSRI-lithium combination was tolerated well
(4,
6), with no indications for a substantial risk of a serotonin syndrome described previously during fluvoxamine-lithium treatment
(24).
The overall response rate and remission rate during phase I were relatively low, 34% and 19%, respectively. These low rates may be due to the inclusion of many patients who had been pretreated with antidepressants and/or who were referred to inpatient units specialized in the treatment of severe and treatment-resistant depression. The inclusion of these patients makes the remission rate of 50% for lithium addition even more remarkable and clinically relevant. The dose of both antidepressants was adjusted to attain a target plasma level. This adjustment could be considered a disadvantage for the fluvoxamine-treated group, because no plasma level-response relationship has been proven for that drug, in contrast with imipramine
(25). Such a disadvantage does not seem likely, however, since the mean daily dose for fluvoxamine was relatively high (287 mg) and the target plasma level technique itself resulted in exclusion of low and possibly subtherapeutic plasma levels in fast metabolizers of fluvoxamine
(26).
In conclusion, treatment with imipramine with dosing targeted by plasma levels and addition of lithium for patients without remission while taking imipramine alone proved to be a highly effective treatment for severely depressed inpatients, including those with psychotic features. The two-phase combination of imipramine and lithium is slightly, but significantly, more effective than the same two-phase strategy with the SSRI fluvoxamine and lithium; the difference between two-phase treatment with imipramine and two-phase treatment with mirtazapine is more pronounced
(8). The greater effectiveness is at the expense of a higher discontinuation rate with imipramine, but this rate is only about 10%. We, therefore, consider imipramine with plasma-level-targeted dosing and lithium addition for patients without remission to be a first choice in the treatment of these patients. Since a similar two-phase treatment with fluvoxamine is not very much less effective and leads to less discontinuation, fluvoxamine as a first choice is justifiable, especially in less severely depressed patients.