Recent theories about the neurobiology of schizophrenia have emphasized
involvement of the thalamus in the pathophysiology of schizophrenia
(1,
2). A growing body of evidence indicates that the thalamus is smaller, has fewer neurons, and exhibits metabolic disturbances in schizophrenia patients relative to healthy comparison subjects
(3–
15). From a functional standpoint, the thalamus is multifaceted, as it mediates sensory perception and gating via ventrobasal sensory relay nuclei
(16), coordinates motor activity through basal ganglia and cerebellar relays in the ventral anterior and ventral lateral nuclei
(17–
19), and contributes to other cognitive functions via extensive reciprocal connections with associational cortices, particularly the prefrontal cortex
(20–
23). Some postmortem studies have reported reductions in volume and neuronal number within nuclei that make major contributions to cortical input, such as the mediodorsal nucleus
(9,
11,
24), anterior nucleus
(9), pulvinar
(11), and the ventral lateral nucleus
(12). These findings suggest that thalamic abnormalities may contribute to the cognitive disturbances that are characteristic of schizophrenia
(25).
Controversy continues to exist as to the severity and pattern of thalamic abnormalities in subjects with schizophrenia, since not all postmortem and in vivo neuroimaging investigations have found evidence of volume reduction
(12,
26–29). A meta-analysis of thalamic volume in schizophrenia and healthy comparison subjects indicated that the effect size was modest relative to effect sizes for volume reductions in other brain structures
(30). Moreover, volume reductions of the thalamic complex have been reported to be asymmetric (i.e., left-sided)
(28,
29) or restricted to subregions such as the mediodorsal nucleus and pulvinar
(7,
29). Also, Gur and colleagues reported thalamic volume reductions in neuroleptic-naive but not neuroleptic-treated patients, suggesting that drug treatment could increase thalamic volumes and mask otherwise significant differences between schizophrenia and healthy comparison subjects
(31).
The tools of computational anatomy are being increasingly used to characterize neuroanatomical abnormalities associated with neuropsychiatric disease
(32,
33). With these methods, assessment of neuroanatomical shapes as well as volumes is possible; thus, subtle abnormalities in the organization of a structure that may have little impact on volume can be assessed
(34,
35). We have previously used one of these methods, high-dimensional (large-deformation) brain mapping
(36–
38), to characterize shape abnormalities in the hippocampus in patients with schizophrenia
(34,
35), depression
(39), and dementia of the Alzheimer type
(40).
In the present study, we used high-dimensional brain mapping to compare the shape, symmetry, and volume of the thalamus in a group of schizophrenia subjects (in whom we previously characterized hippocampal abnormalities
[34]) with a group of healthy subjects. Relationships between these neuroanatomical measures and the clinical and cognitive features of schizophrenia were also examined.
Method
Subjects
The study group consisted of 52 schizophrenia subjects and 65 comparison subjects, matched for age, gender, race, and parental socioeconomic status. All subjects gave written informed consent for participation in this study after the study’s risks and benefits were explained to them. The mean age of the 30 male and 22 female schizophrenia subjects was 38 years (SD=1.7), and their mean age at onset of illness was 23 years (SD=1.2). A more detailed summary of the clinical characteristics of the schizophrenia and comparison subjects can be found in a prior publication
(34). The DSM-IV diagnosis of each subject was determined by the consensus of a research psychiatrist and a trained research assistant who used the Structured Clinical Interview for DSM-IV Axis I Disorders
(41). Exclusion criteria were presence of an unstable medical condition, a neurologic disorder, head injury with loss of consciousness, or substance abuse or dependence in the 3 months preceding the study. Comparison subjects were also excluded if they had first-degree relatives with a psychotic disorder.
All schizophrenia subjects were clinically stable (i.e., their symptoms had remained unchanged for at least 2 weeks)
(42), and all but three had been treated with antipsychotic drugs. The severity of three dimensions of residual psychopathology (i.e., negative symptoms, psychosis, and thought disorganization) was assessed in the schizophrenia subjects by using the Scale for the Assessment of Positive Symptoms
(43) and the Scale for the Assessment of Negative Symptoms
(44) as previously described
(34). Forty-three of the schizophrenia subjects and 59 of the comparison subjects were assessed with neuropsychological tests of memory and general intelligence: the Wechsler Memory Scale (immediate and delayed), Benton Visual Retention (immediate and delayed), and WAIS-III verbal and performance IQ.
Image Collection and Generation of the Neuroanatomical Template
Magnetic resonance (MR) scans were collected by using a turbo-fast low-angle shots sequence (TR=20, TE=5.4, flip angle=30°, number of acquisitions=1, matrix=256×256, voxel size=1 mm
3, scanning time=13.5 minutes)
(45). MR data were reformatted by using Analyze software (Rochester, Minn.). Signed 16-bit data sets were compressed to unsigned 8-bit data sets to maximize tissue contrast. In all MR scans, landmarks were placed at the anterior, posterior, superior, inferior, and lateral brain boundaries and at points where the anterior and posterior commissures intersected the midsagittal plane. Points at the anterior and posterior boundaries of the thalamus were also demarcated, and a line connecting them was used to create an anterior/posterior axis. The thalamus was then divided into seven equally distanced slices along this axis, and five landmarks surrounding the thalamus were placed in each slice.
The template for the human thalamus was generated by using a T
1-weighted MR sequence collected from another healthy comparison subject that was not otherwise included in the analysis. The thalamus was manually outlined in this MR scan by three authors (M.K.S., L.W., and M.G.) according to atlas guidelines
(46).
High-Dimensional Brain Mapping
Transformation of the template onto the target MR scans occurred in two steps
(33,
36,
37) First, it was coarsely aligned to each target scan by using the previously placed landmarks, and then a high-dimensional transformation was applied to achieve an optimal voxel-by-voxel match
(36). During the transformation, the movement and deformation of template voxels were constrained by assigning them the physical properties of a fluid
(47). The reliability and validity of high-dimensional brain mapping for segmenting closed volume brain structures with respect to expert manual outlining has been previously demonstrated
(37). To check the validity of using high-dimensional brain mapping for mapping the thalamic template to the target scans in this study, we compared the segmentations generated by this process to those produced by an expert (M.G.) in the MR scans of three of the comparison subjects. The average overlap between the segmentations generated by high-dimensional brain mapping and the segmentations generated by an expert was 84.80% (minimum value was 72.81%).
To quantify thalamic shape and volume, a triangulated graph was superimposed onto the surface of the thalamus within the template; this graphic surface was then carried along as the template was transformed onto the target scans. Left and right thalamic volumes in the target scans were determined by calculating the volumes enclosed by the transformed surfaces. Fields of vectors were also derived from the displacements of the triangulated graph points during the transformations. To compare thalamic shapes between subject groups, a pooled within-group covariance matrix was derived from the transformation vectors, and the dimensionality of this matrix was reduced by using singular value decomposition to identify the major dimensions of shape variation (i.e., eigenvectors)
(35,
36).
To examine thalamic asymmetry, vector fields were also generated by reflecting the transformed graphic surfaces from the thalamus in one hemisphere across the midsagittal plane (i.e., the plane yielding the least sum of squared errors between the thalamic surface points in the two hemispheres) onto the thalamus in the opposite hemisphere. An asymmetry covariance matrix was then computed using these vector fields, and the principal dimensions of thalamic asymmetry (i.e., eigenvectors) were determined
(38).
Total cerebral volumes (excluding the brainstem and cerebellum) were derived by using a landmark-based elastic transformation
(36) of the template scan, with total cerebral volume as a covariate in volume analyses. During these transformations, the entire template scan was globally registered with each target scan using scalar points at the boundaries of the brain and in the midline.
Statistical Analyses
Thalamic volumes were compared using two-way, repeated-measures analysis of variance, with diagnostic group as a between-subject factor and hemisphere as a within-subject factor. To test for a group difference in thalamic shape, the first 10 eigenvectors derived from the transformation vector field covariance matrix (explaining 78% of the variance) were selected a priori, and the statistical significance of the group difference was tested using Wilks’s lambda. A logistic regression model was then generated to select eigenvectors among the first 10 that yielded maximal discrimination between the groups, and log-likelihood ratio values for each subject were calculated. The eigenvectors selected by the logistic regression model were used in a “leave-one-out” discriminant function analysis to determine the percentage of correctly classified subjects in each group. For the comparison of thalamic asymmetry between groups, eigenvectors were developed and selected using the asymmetry vector fields and used in similar statistical analyses.
To visualize the physical patterns of thalamic deformity represented by the eigenvectors, maps of the composite thalamic surfaces in the schizophrenia and comparison subjects were reconstructed using the selected eigenvectors. The patterns of these deformities were then compared to “point-by-point” surface displacement maps. These displacements were calculated at each surface point as the difference between the means of the group vectors in magnitude.
Results
Thalamic Volume
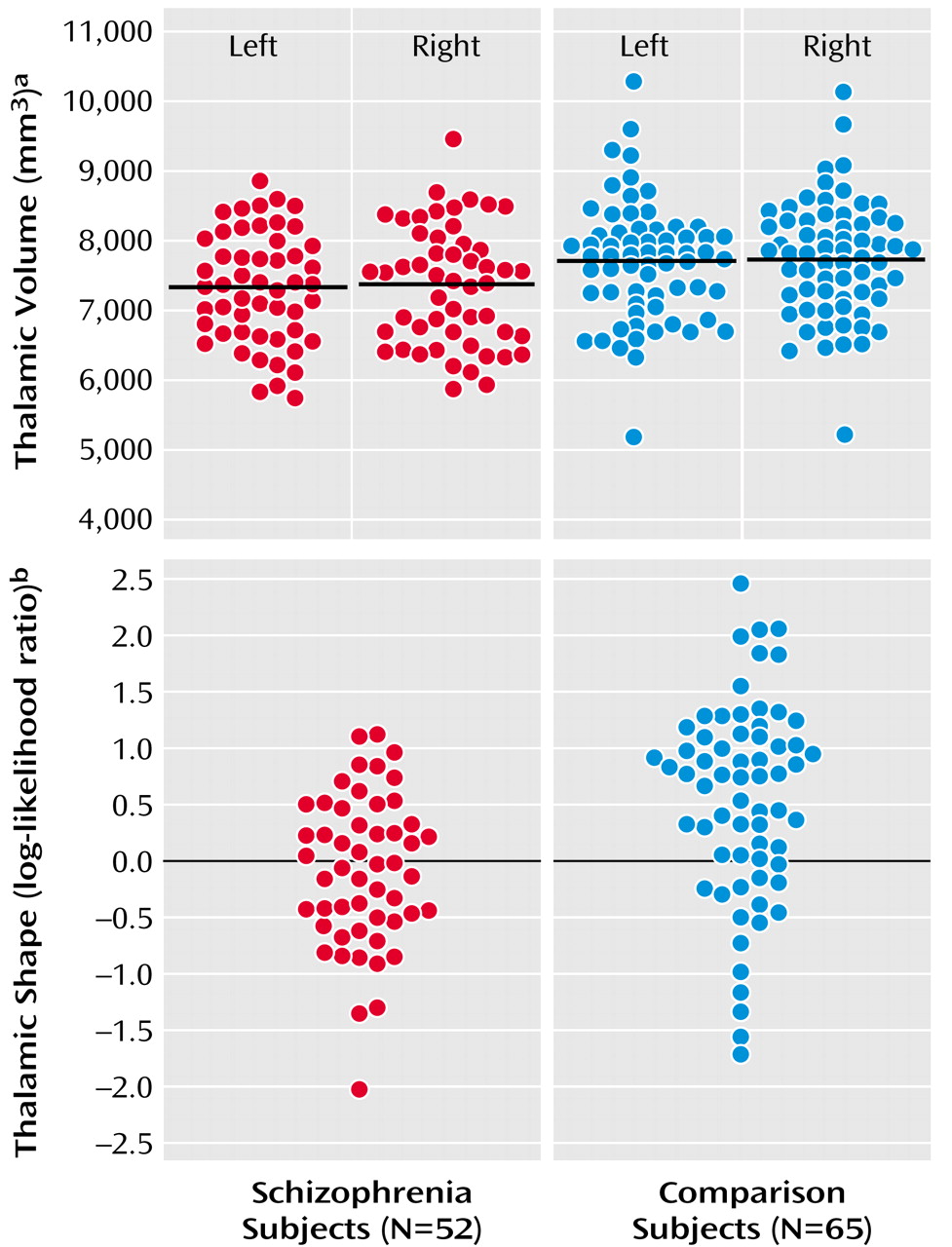
Mean thalamic volumes were 7307 mm
3 (SD=790) on the left and 7350 mm
3 (SD=846) on the right in schizophrenia subjects, and 7702 mm
3 (SD=851) on the left and 7734 mm
3 (SD=837) on the right in comparison subjects (
Figure 1). The group difference in thalamic volume was approximately 7% (effect size for left and right thalamus combined=0.48). The effect of diagnosis was significant (F=6.59, df=1, 115, p<0.02), and this effect remained significant after covarying thalamic volumes for age and gender. However, there was also a significant diagnosis effect for total cerebral volume (F=5.73, df=1, 115, p<0.02), and the effect of diagnosis on thalamic volume became nonsignificant after covarying thalamic volumes for total cerebral volume (F=1.30, df=1, 115, p=0.26). Post hoc correlations between the combined left and right thalamic volumes and total cerebral volumes were significant in the schizophrenia subjects (r=0.75, p<0.0001) and comparison subjects (r=0.75, p<0.0001). There was no significant effect of hemisphere (F=1.35, df=1, 115, p=0.25) nor was there a group-by-hemisphere interaction (F=0.03, df=1, 115, p=0.87). Including gender as a covariate in the analysis and the exclusion of left-handed subjects did not alter the results.
Thalamic Shape
Using the first 10 shape eigenvectors, a significant difference was found between the two subject groups (F=2.82, df=10, 106, p<0.004). Eigenvectors 1, 8, and 10 were then selected in a logistic regression model (χ
2=16.0, df=3) to maximally discriminate the two subject groups, and in a “leave one out” discriminant function analysis, 28 (53.4%) out of the 52 schizophrenia subjects and 49 (75.4%) out of the 65 comparison subjects were correctly categorized (
Figure 1). To test the stability of this “leave one out” analysis, 10 alternate comparisons were run after excluding in turn approximately 10% of the subjects in both groups. The overall mean rate of correct subject classification was 66.7% (SD=8.8%), which was significantly higher than the 50% correct categorization rate obtainable by chance (z=6.0, p<0.001). Also, in all of these alternate models, the selected eigenvectors were similar to those selected using the entire population (i.e., 1, 8, 10 in seven models; eigenvectors 1, 7, 10 in two models; eigenvectors 4, 8, 10 in one model). Including thalamic volume in this analysis, even as a forced variable, did not further improve the classification of schizophrenia and comparison subjects.
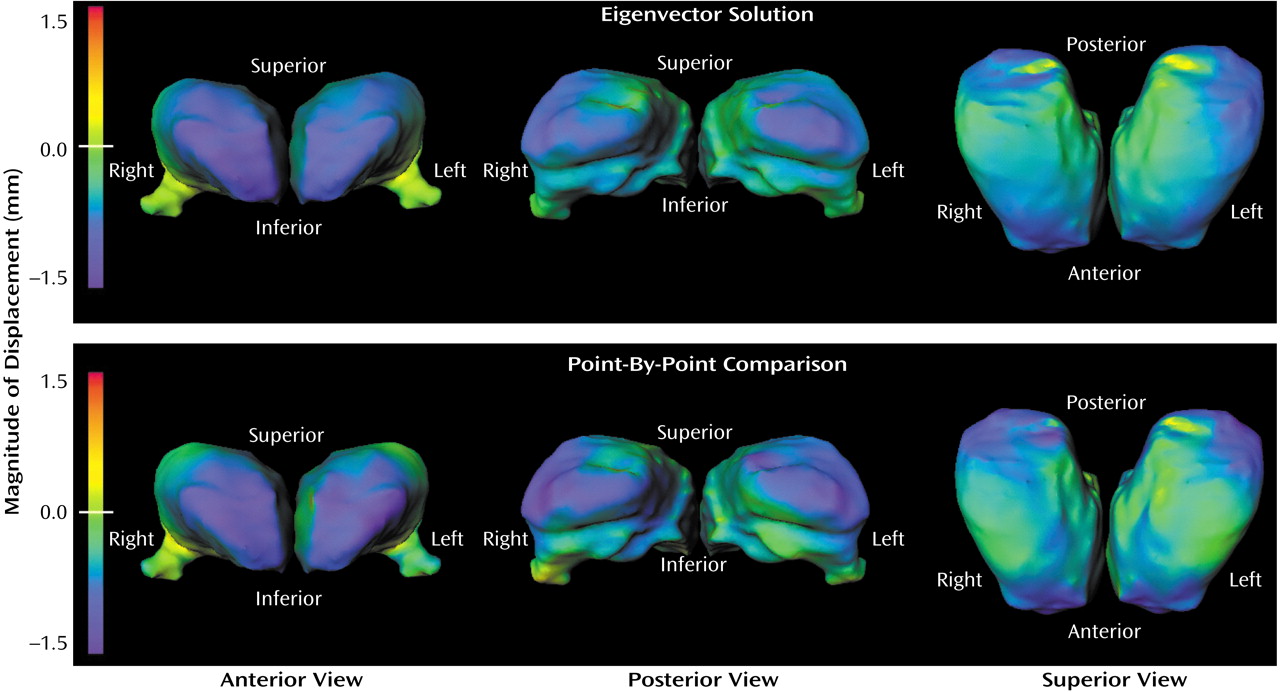
Visual representations of thalamic shape deformation in the subjects with schizophrenia suggested a loss of volume in the extreme anterior and posterior regions of the thalamus (
Figure 2). This pattern of deformity was similar regardless of whether the maps of thalamic surface deformation were reconstructed from the eigenvector solution (i.e., 1, 8, and 10) or generated using point-by-point maps of the thalamic surface.
Combining Thalamic and Hippocampal Shape Information
In a previous study of the same subjects
(34), 15 eigenvectors representing the shape of the surface of the hippocampus deformity were found to significantly distinguish the two groups of subjects (F=2.66, df=15, 101, p=0.002). From these hippocampal shape eigenvectors, eigenvectors 1, 5, and 14 (χ
2=21.6, df=3) were then selected in a logistic regression model, which allowed for the successful classification of 59.6% of the schizophrenia subjects and 80.0% of the comparison subjects.
Combining information about the shape of the thalamus with previously collected information about the shape of the hippocampus improved the proportion of subjects correctly classified. Using all 15 hippocampal eigenvectors and all 10 thalamic eigenvectors together, the two groups of subjects were again found to be significantly different (F=2.83, df=25, 91, p=0.0002). A logistic regression model was then developed using both the hippocampal eigenvectors 1, 5, 14 and thalamic eigenvectors 8 and 10 (χ2=32.5, df=5), and in a “leave one out” discriminant function analysis, 73.1% of the schizophrenia subjects (N=38 of 52) and 83.1% of the comparison subjects (N=54 of 65) were correctly classified. Also, correlations between the left and right hippocampal or thalamic volumes in both groups of subjects were very high (r=0.86–0.92), while correlations between the hippocampal and thalamic volumes on the left or right were lower in both groups of subjects (r=0.52–0.64).
Thalamic Asymmetries
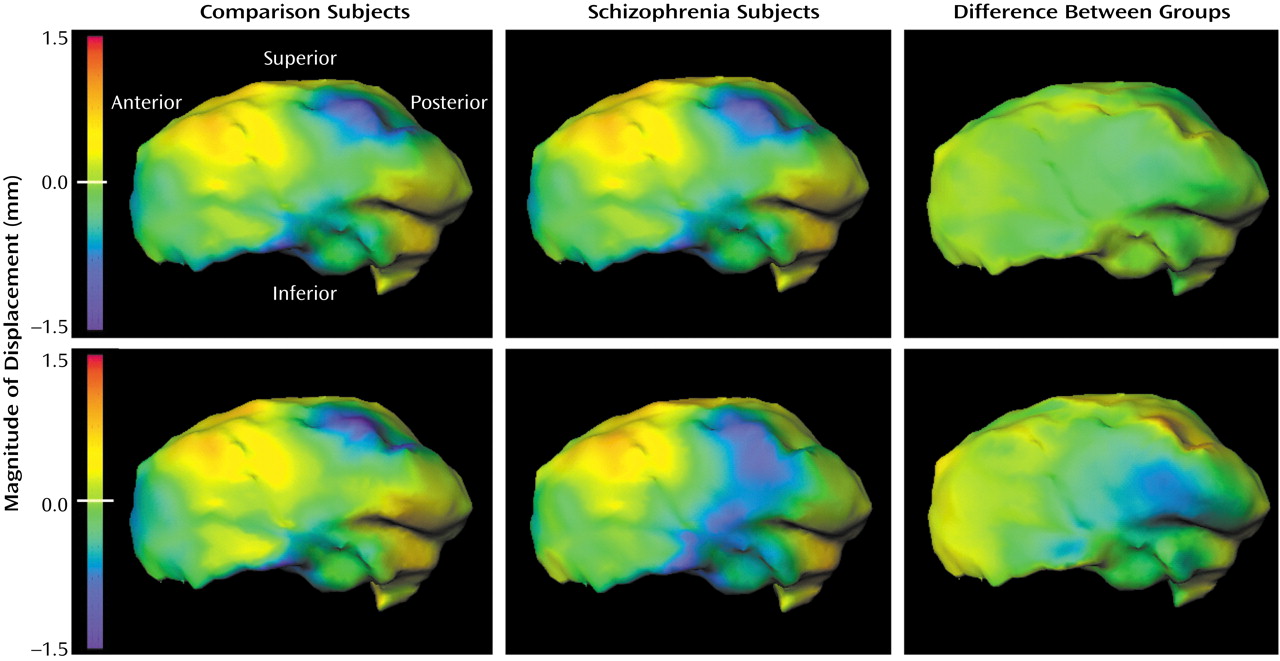
Development of a logistic regression model comparing patterns of thalamic asymmetry in the schizophrenia and comparison subjects resulted in the selection of eigenvectors 4, 6, 12, and 20 (χ
2=12.4, df=4), and the log-likelihood ratio values derived using these eigenvectors indicated a significant group difference in the pattern of thalamic asymmetry (F=3.31, df=4, 112, p=0.01). Visual representations of this group difference (
Figure 3) suggested that the subjects with schizophrenia showed an exaggeration of the normal pattern of asymmetry on the medial surface of the thalamus. This group difference in this pattern of thalamic asymmetry was more apparent in the point-by-point maps than in the maps generated from the eigenvector analysis, which suggests that the eigenvector-based analysis captured only a portion of the group difference in thalamic asymmetry.
Clinical Relationships
There were no significant correlations between thalamic volume or shape measures and the severity of any symptom dimension (i.e., negative symptoms, psychosis, or thought disorganization) in the schizophrenia subjects. Duration of illness in the schizophrenia subjects tended to be inversely correlated with the volume of the right thalamus (r=–0.26, df=47, p=0.07) but not the left thalamus (r=–0.13, df=47, p=0.38).
As previously reported
(34), the schizophrenia subjects demonstrated significant and substantial (approximately two standard deviations) deficits in performance on several cognitive tests (i.e., Wechsler Memory Scale immediate and delayed, Benton Visual Retention immediate and delayed, and WAIS-III verbal and performance IQ). Significant correlations were found between performance on the Benton Visual Retention test (delayed) and left (r=0.38, p=0.01) and right (r=0.30, p=0.05) thalamic volume. No other significant correlations were found between measures of thalamic volume or shape and performance on any neuropsychological test nor between thalamic volume and shape measures and performance on neuropsychological tests in the healthy comparison subjects.
Discussion
In this study, a small but significant reduction in thalamic volume (approximately 7%) was found in schizophrenia subjects relative to healthy comparison subjects, which is consistent with the results of prior neuroimaging studies
(5,
7,
13–15). While this difference became nonsignificant when thalamic volumes were covaried for total cerebral volumes, this result should not be taken to infer that the reduction in thalamic volumes was not of neurobiological significance. Indeed, given the robust connectivity between the thalamus and the cerebral cortex
(16–
22), alterations of thalamic structure would be expected to impact the cortex and vice versa. Moreover, the thalamic volume reduction was accompanied by significant deformities of thalamic shape and asymmetry, which suggests that schizophrenia may be characterized by abnormalities of specific thalamic nuclei.
The pattern of thalamic shape deformity observed in the schizophrenia subjects suggests involvement of nuclei in both the anterior and posterior extremes of the complex. In the case of the anterior extreme, this would implicate both the anterior and the dorsomedial nuclei, which have been previously shown to be smaller in volume and to contain fewer neurons in schizophrenia subjects
(4,
8,
9,
11,
12,
24,
29). The pulvinar nucleus at the posterior extreme of the complex has also been shown to have a reduced volume and neuronal population in schizophrenia subjects
(29). Involvement of these nuclei in schizophrenia is of particular interest because of their connections with the association cortices, particularly the prefrontal cortex. The anterior and mediodorsal nuclei of the thalamus have dense reciprocal connections with the prefrontal cortex
(20–
22), while the pulvinar has connections with parieto-occipital association cortices
(23,
48), the prefrontal cortex
(23,
48), and entorhinal cortex
(49). The involvement of these association relay nuclei in schizophrenia is consistent with recent reports of gray matter reductions in the prefrontal cortex
(50) and other heteromodal association cortices
(51). However, while inward deformations of the anterior and posterior extremes of the thalamic surface could have resulted from volume losses of nuclei at those extremes, such deformations could also have been the result of volume losses of other nuclei that shrank the overall anterior-posterior dimension of the structure. Discriminating between these two possibilities would require application of high-dimensional brain mapping to the individual thalamic nuclei and MR scans that offer more detailed resolution of such structures than the ones available for this study.
Our results also lend support to hypotheses that the pathogenesis of schizophrenia involves disturbances in the development of neuroanatomical asymmetries
(52). The disturbance of thalamic asymmetry (i.e., an exaggeration of the normal left > right pattern) observed in this study was similar to the disturbance of hippocampal asymmetry previously observed in the same subjects
(34), suggesting that a common mechanism may underlie both disturbances. Such disturbances of neurodevelopment could occur as the result of genetic factors
(53) or environmental insult
(54,
55). Discriminating between these two possibilities could be achieved by the study of populations of subjects at genetic risk for developing schizophrenia (i.e., relatives of patients with schizophrenia) and in whom there is a detailed gestational and developmental history.
There were few functional correlates of thalamic volume and shape measures in the schizophrenia subjects. The lack of any substantial correlation between duration of illness and thalamic neuroanatomic measures suggests that the deformities of thalamic structure observed were probably not the result of chronicity or long-term treatment factors. However, there were few untreated patients in this study, and the possibility that relatively short treatment periods can alter the structure of the thalamus cannot be excluded. The small but significant correlation between poor performance on a test of visual memory (i.e., Benton visual retention test, delayed type) is intriguing and may be related to the functional relationships that exist between the thalamus and association cortices involved in pattern recognition and retention.
The elucidation of abnormalities in neuroanatomical volume, shape, and asymmetry in schizophrenia using MR imaging and computational anatomy may help direct investigators who perform postmortem studies to particular subregions of neuroanatomical structures. Moreover, measures of neuroanatomical shape and asymmetry may one day be useful for improving clinical diagnosis or for selecting and monitoring the effects of treatment. However, before such measures can be used for clinical purposes, the degree to which abnormalities that appear during the illness are specific to schizophrenia must be determined, and how they are affected by treatment.