Postpartum depression occurs in 13% of mothers
(1), and as many as 50% of affected women are treated with antidepressant medication
(2). Because 60% of mothers initiate breast-feeding
(3), substantial numbers of women with postpartum depression and women who require chronic antidepressant therapy may wish to continue breast-feeding while taking antidepressants. Unfortunately, there is little available information about the potential risk to the infant. The medical literature contains multiple case reports and case series involving measurements of antidepressant levels in maternal plasma, breast milk, and/or infant plasma. Although several published reviews summarize the available data
(4–
7), they focus on only one class of drug
(5), do not directly compare different drugs
(6,
7), or were published before data on newer drugs such as citalopram and paroxetine were available
(4). We present a pooled analysis of all available data on antidepressant levels in nursing mother-infant pairs.
Method
Published Data
Electronic searches of MEDLINE, PreMEDLINE, Current Contents, Biological Abstracts, and PsycINFO from 1966 through July 2002 were conducted by using the keywords “antidepressant drugs,” “antidepressive agents,” “human milk,” “lactation,” and “breast feeding” and the name of each antidepressant drug. Retrieved studies were examined for additional references. The inclusion criterion was the measurement of antidepressant levels in lactating mothers, breast milk, or nursing infants, alone or in any combination. Of 65 studies identified, two
(8,
9) were excluded because levels were measured by immunoassay only, which is an inaccurate method of quantification
(10,
11). Four others
(12–
15) were excluded because actual drug levels were not specified. A seventh
(16) was excluded because the patient had galactorrhea and was not breast-feeding. An eighth report
(17) contained data also included in a subsequent publication
(18). Data were analyzed from the remaining 57 articles (
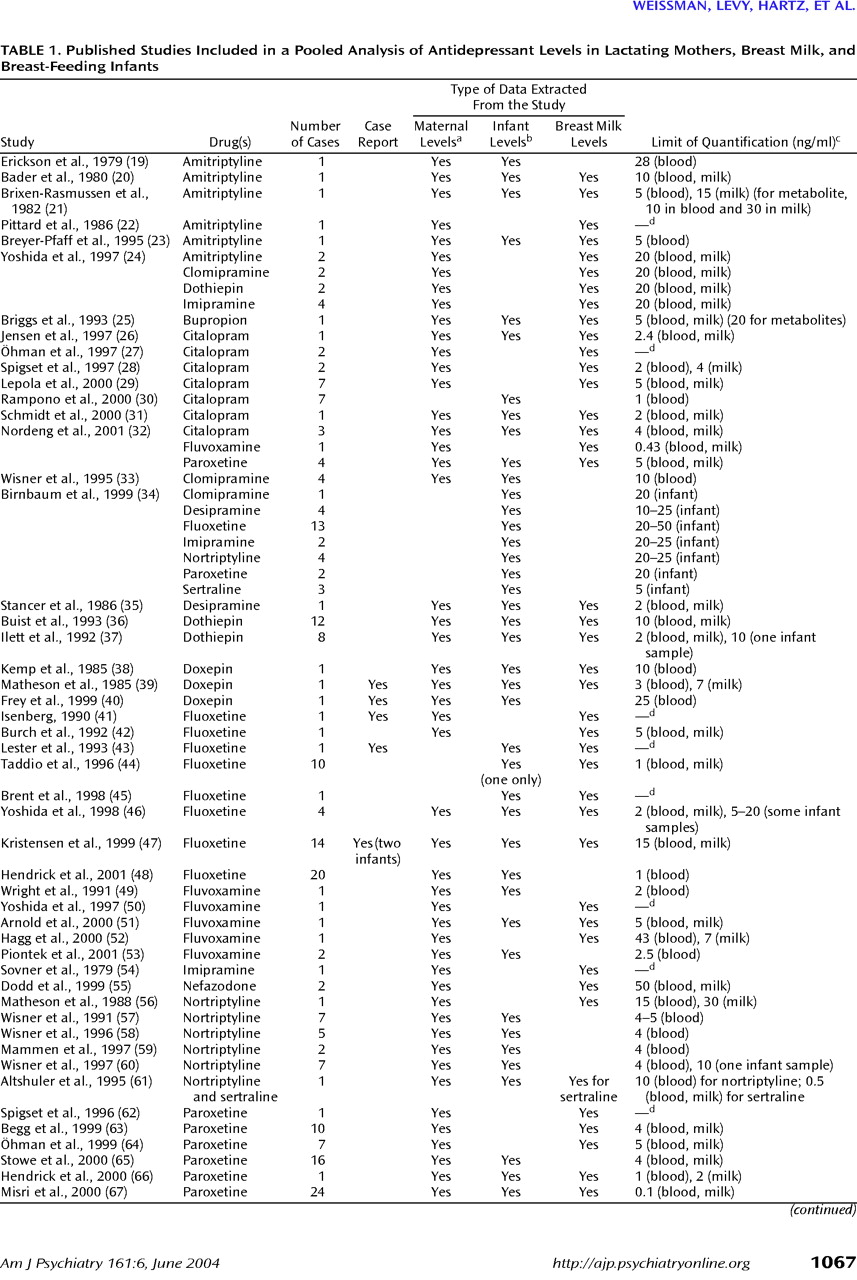
Table 1). Some authors measured antidepressant levels in plasma, and others measured levels in serum. Thus, in
Table 1 “maternal level” denotes a level measured in plasma or serum; similarly, “infant level” refers to a level measured in plasma or serum.
We contacted the seven authors who had published the largest series of data and the manufacturers of each drug produced in the United States. Two additional unpublished papers were discovered, but data on individual cases were not available from these studies.
Within the 57 articles we identified, some data could not be used because they included immunoassay data or drug levels averaged over several patients. One report
(63) presented some data only as the calculated area under the curve. Since these data could not be compared to one-time measurements, they were excluded. We were unable to obtain the original blood and breast milk levels for those subjects from the authors.
One subject, patient 9 from a study by one of us
(71), was excluded. In this case, the infant’s sertraline and norsertraline levels were 64 ng/ml and 68 ng/ml, respectively, which were more than 50% of the mother’s levels. In the 58 other published cases involving sertraline, the infant level did not exceed 4.9 ng/ml for sertraline or 24 ng/ml for norsertraline. The original investigators believed the unusually high sertraline levels in this infant were spurious.
Data were extracted by one author (M.D.) and checked on two occasions by another (A.M.W.). Of greatest value were data sets that included maternal blood levels, breast milk levels, and infant blood levels; however, many data sets included two and some only one of these measurements. Only 18 studies
(17,
23,
25,
31,
33,
39,
45,
51,
53,
56,
57,
59–
61,
66–
68,
71) specifically stated the proportion of breast milk versus bottle feedings; 11 infants
(67) were more than 80% breast-fed, seven infants
(17,
23,
31,
57,
68) were about 60%–80% breast-fed, one (exposed to fluvoxamine)
(53) was half breast-fed, and one (exposed to bupropion)
(25) was about 30% breast-fed; the other 49 infants were fully breast-fed. Only one publication
(51) reported a patient’s smoking status, and none commented on alcohol use.
Where possible, we categorized data sets as collected either from patients who were studied because the infant was symptomatic in some way (case reports) or from patients identified for study in other ways (research cases). When possible, we also noted which infants had been exposed to the same antidepressant before birth.
Unpublished Data
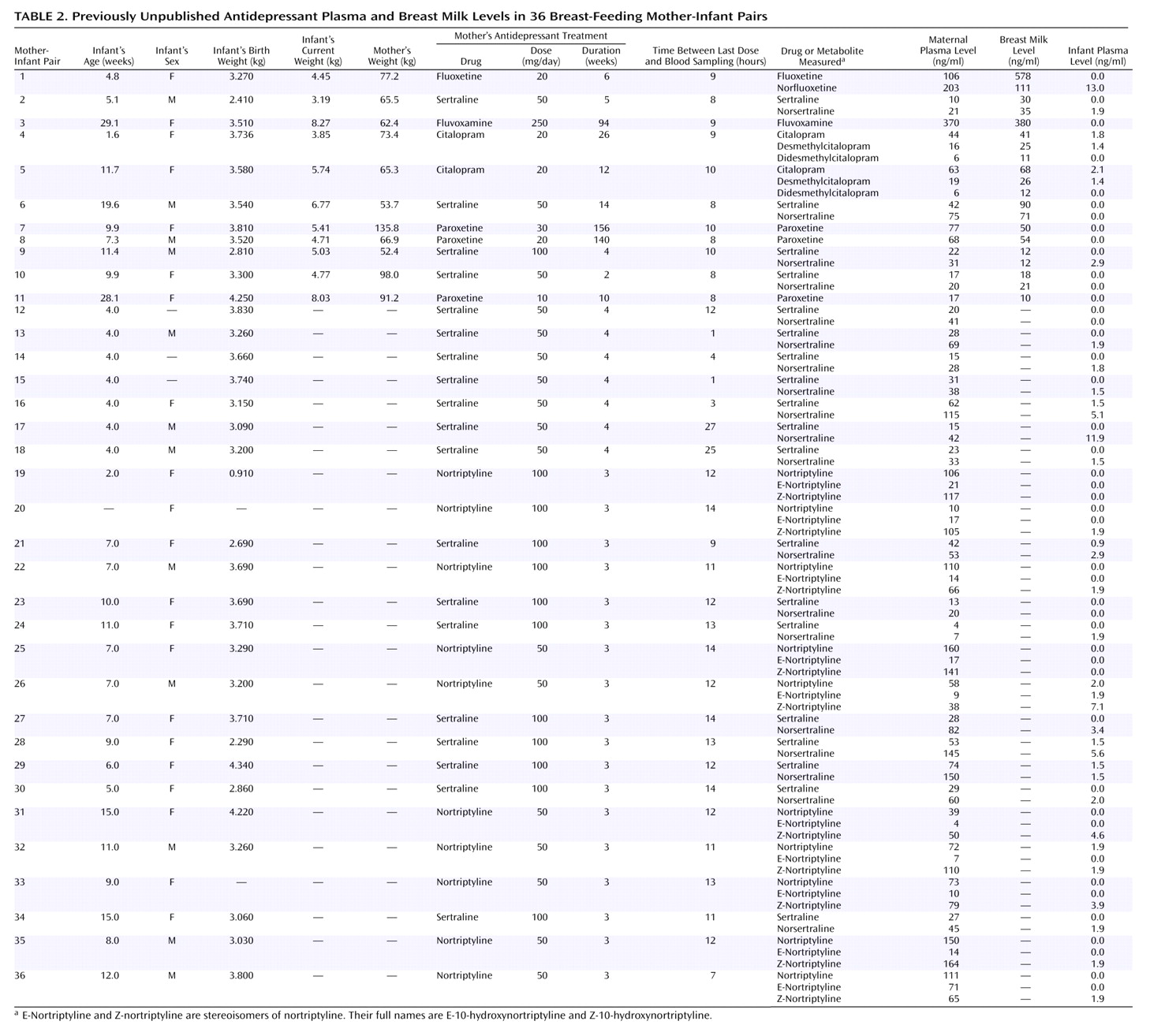
In addition to the published cases, data were also available from 36 unpublished cases (
Table 2). Dr. Weissman collected maternal blood, breast milk, and infant blood from 11 mother-infant pairs in which the mothers were taking five different antidepressants. Dr. Wisner collected maternal and infant blood from 15 mother-infant pairs; the mothers in five pairs were taking sertraline and the mothers in 10 pairs were taking nortriptyline as part of ongoing clinical trials. In these studies, after complete description of the study to the subjects, written informed consent was obtained. Blood was collected from mothers and infants by venipuncture. For breast milk samples, the entire output from one breast was collected while the infant nursed at the other breast.
The blood samples were immediately centrifuged, and the plasma was frozen at –70°C. The milk samples were immediately frozen as well. All samples were sent to the laboratory of James M. Perel, Ph.D., at the University of Pittsburgh Medical Center, for analysis.
Concentrations of the drugs and their major active metabolites were assayed with previously validated analytical methods that used high-performance liquid chromatography. The methods entailed solvent extraction from alkaline solution of 1.0-ml plasma samples, followed by back extraction into dilute acid. By using an automated sample processor, these extracts were injected onto a C-18 column and resolved with a phosphate buffer/acetonitrile mobile phase, and the levels were determined by using a variable-wavelength ultraviolet light detector and automatic integration. For fluvoxamine, the limit of quantitative detectability necessary to assay physiologically significant levels for infants was lower than the published range
(75). Adjustment of the wavelengths to optimize the signal-to-noise ratio, increasing the percentage of final extract injected, and careful chromatographic conditions permitted validation of all the methods at levels of quantification down to 1.5 to 2.0 ng/ml for the various compounds: sertraline
(71,
76), fluoxetine and citalopram
(77), and paroxetine (unpublished method of G. Rudolph and J. Perel, 2001; similar to preceding method). The level of detectability was about one-third the level of quantification. In cases where a full milliliter of infant plasma was not available, the levels of quantification and detectability were correspondingly compromised.
Milk samples were assayed by the method of standard addition, so that each sample served as its own matrix. A volume of 0.5 ml of milk was used per extraction tube instead of the 1.0 ml used in the plasma assays. Five 0.5-ml volumes for each milk sample, containing zero or various known amounts of the drug (and metabolites, as appropriate), were extracted and chromatographed as were the 1.0-ml plasma samples. The results were then graphically extrapolated to determine the correct values.
Statistical Analysis
For studies that had more than one value for breast milk levels for the same feeding (i.e., hind milk and fore milk levels or right and left breast levels), the values were averaged. For statistical analysis, levels reported as “not detected” were entered as zero and levels reported as detected but not quantifiable were entered as 0.1 ng/ml less than the limit of quantification. Sensitivity analyses were done by recoding “not detected” from “0” to half the limit of quantification and “detectable but not quantifiable levels” from 0.1 ng/ml below the limit of quantification to half the limit of quantification.
For some studies, several measurements per mother-infant pair were reported. To avoid undue influence from a given patient, we included only one measurement from a given source (maternal plasma, milk, or infant plasma) per subject. If there were several measurements from one source (e.g., milk), we chose the single measurement that corresponded in time to another measurement (e.g., plasma). Few infants had more than one plasma level measurement. For those who did, we used the first infant blood level obtained, since younger infants may metabolize the medication more slowly than older infants. For infants with multiple measurements and a history of prenatal exposure, we used the first measurement taken after the infant was older than five times the adult half-life of the parent drug.
Infants selected for study because they were symptomatic were analyzed separately from the others. Infants with prenatal exposure whose only measurements were taken within five half-lives of the parent drug were excluded from the primary analysis. This allowed us to examine data that reflected primarily breast-feeding drug exposure, rather than prenatal drug exposure. To compute correlations between mothers’ and infants’ levels, we used mother and infant levels from blood samples taken simultaneously.
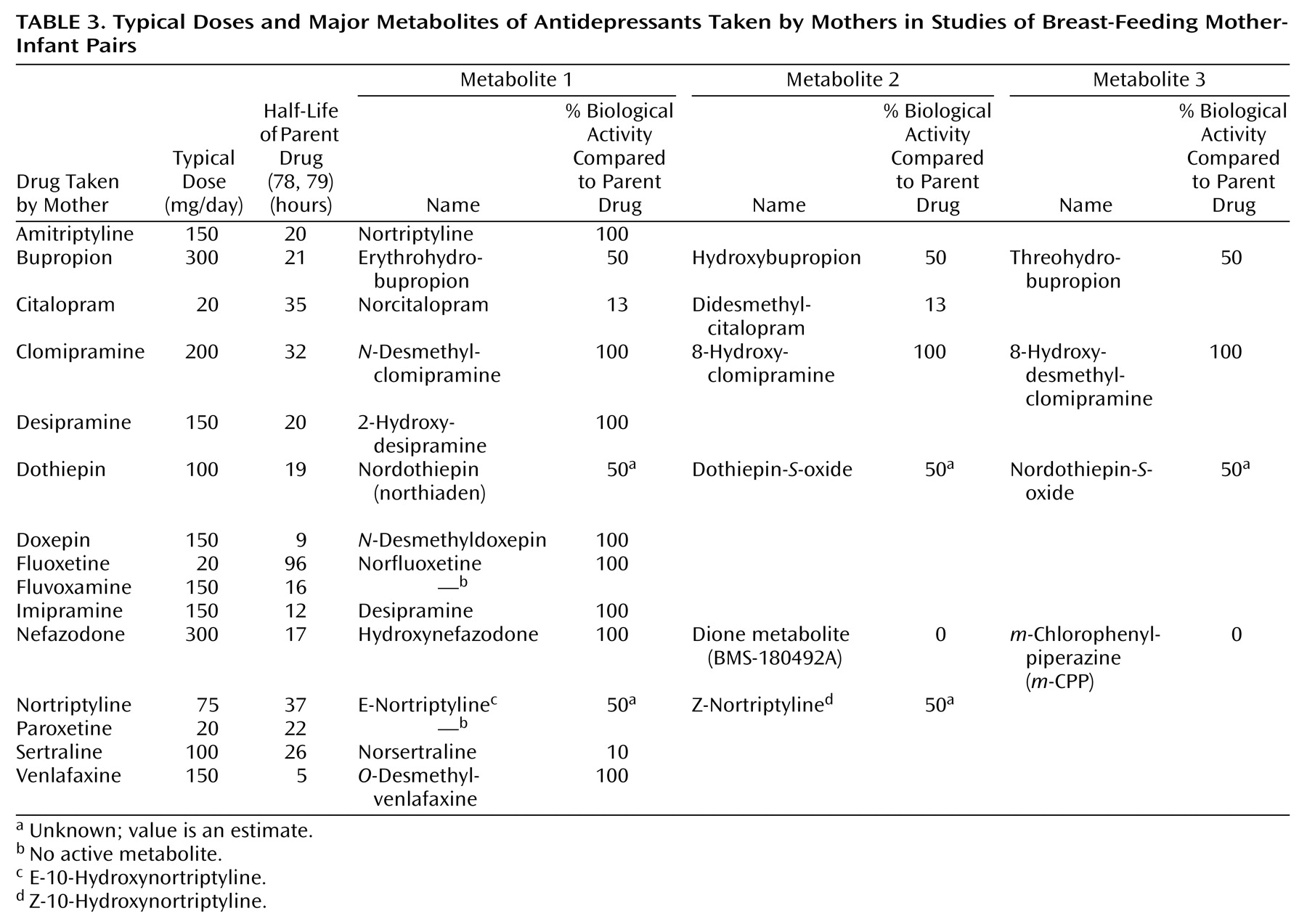
We included metabolite levels in some analyses by computing a weighted sum of the parent drug plus metabolite levels, using the metabolite’s biological activity relative to the parent drug as the weighting factor (
Table 3). However, because metabolites were often not measured, the analyses we will describe are based on parent drug levels alone except where otherwise stated.
A one-way analysis of variance (ANOVA) was used to compare infant plasma levels across medications. To standardize the infant plasma concentrations so that results with one drug could be compared to results with another, we divided each infant plasma level by the average maternal plasma level for each drug. We would have preferred to divide by the minimum therapeutic level for each drug, but minimum therapeutic levels have not been defined for antidepressants other than tricyclics.
Correlations of the milk level with the mother’s plasma level and of the infant’s plasma level with the mother’s dose and blood level were computed for each medication. Only correlations based on five or more observations are reported. Correlations for all subjects combined were adjusted for medication by using indicator variables.
Results
The complete database of published and unpublished cases included drug levels for 15 antidepressants and their major active metabolites (
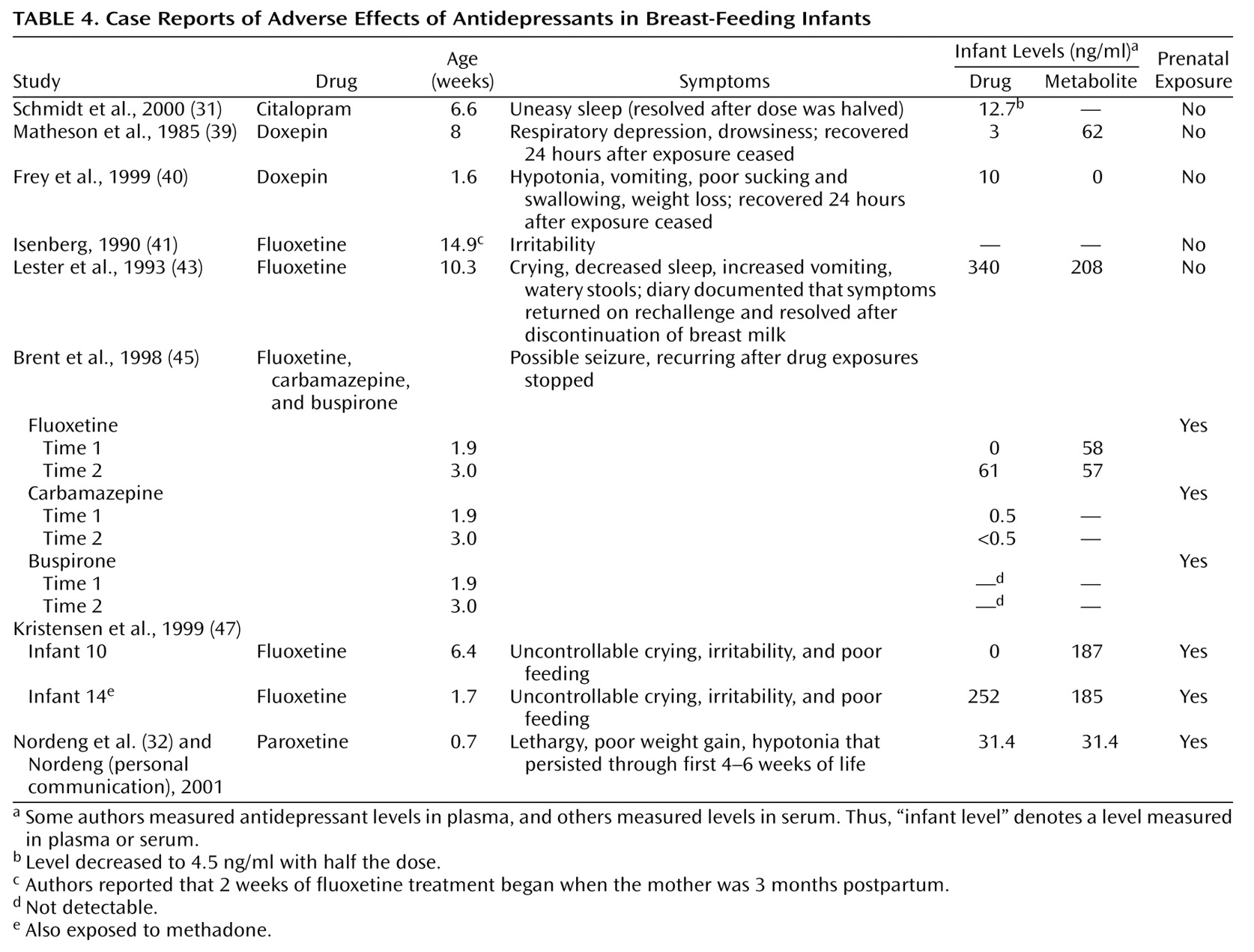
Table 3). The database contained 337 research cases, including measurements for 238 infants. An additional six cases that had been studied because of suspected adverse effects in the infant were classified as case reports
(39–
41,
43,
45,
47) (
Table 4).
These cases were excluded in the calculation of average blood or breast milk drug levels but were included in examination of predictors of breast milk and infant drug levels. Two of the infants in the research cases had also displayed adverse effects
(31,
32) (
Table 4), but these infants had not been selected for study on this basis and were included in all analyses.
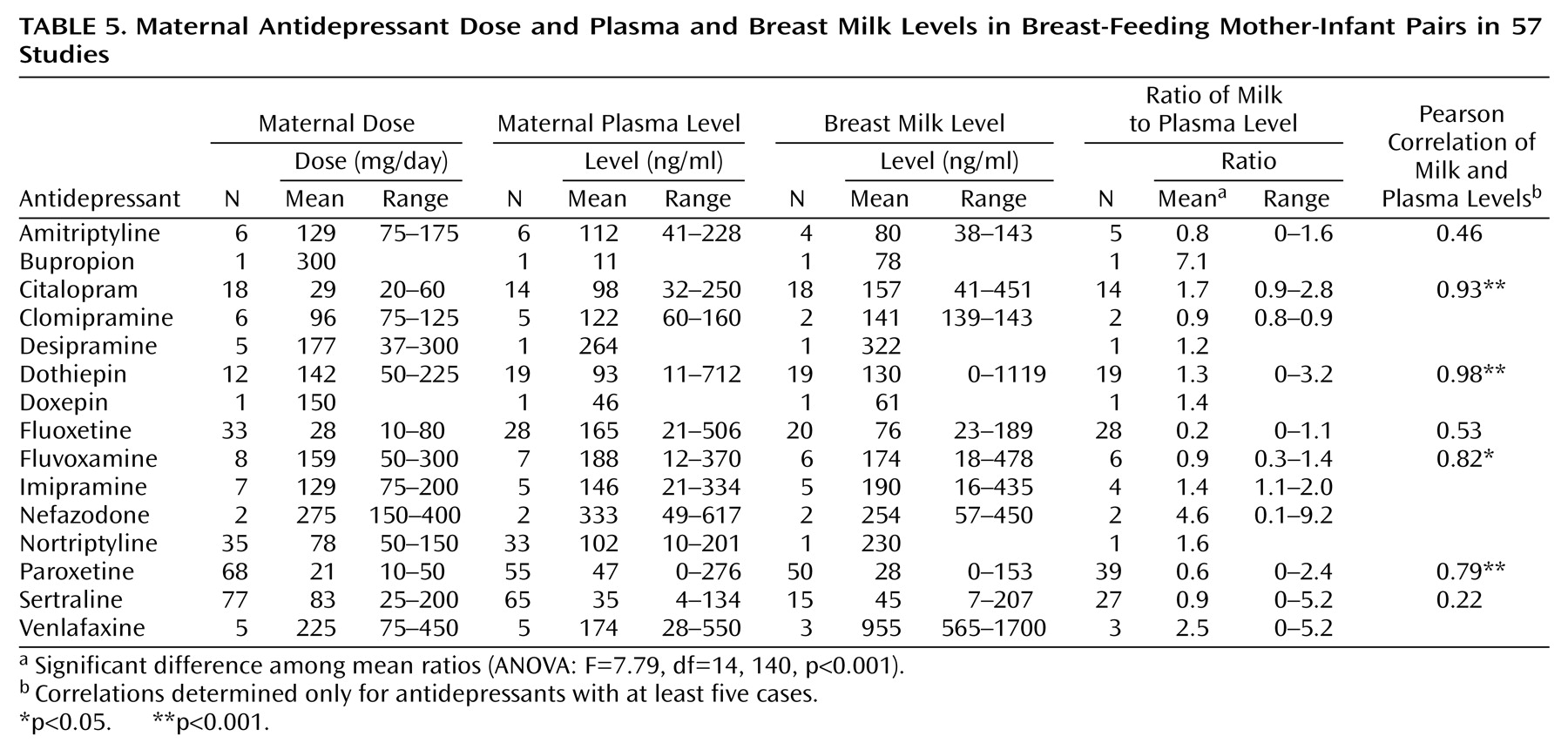
In addition to excluding the case reports, the analyses in
Table 5 and
Table 6 also excluded data from infants with prenatal exposure who were younger than five times the average adult half-life of the parent drug.
Table 5 presents the mean dose, maternal plasma level, breast milk level, and milk/plasma ratio for each drug. Drug levels were detectable in breast milk for all antidepressants studied. Pearson correlations between maternal plasma and breast milk levels were statistically significant for citalopram, dothiepin, fluvoxamine, and paroxetine. Despite large study groups, the correlation between maternal plasma and breast milk levels did not reach statistical significance for fluoxetine or sertraline.
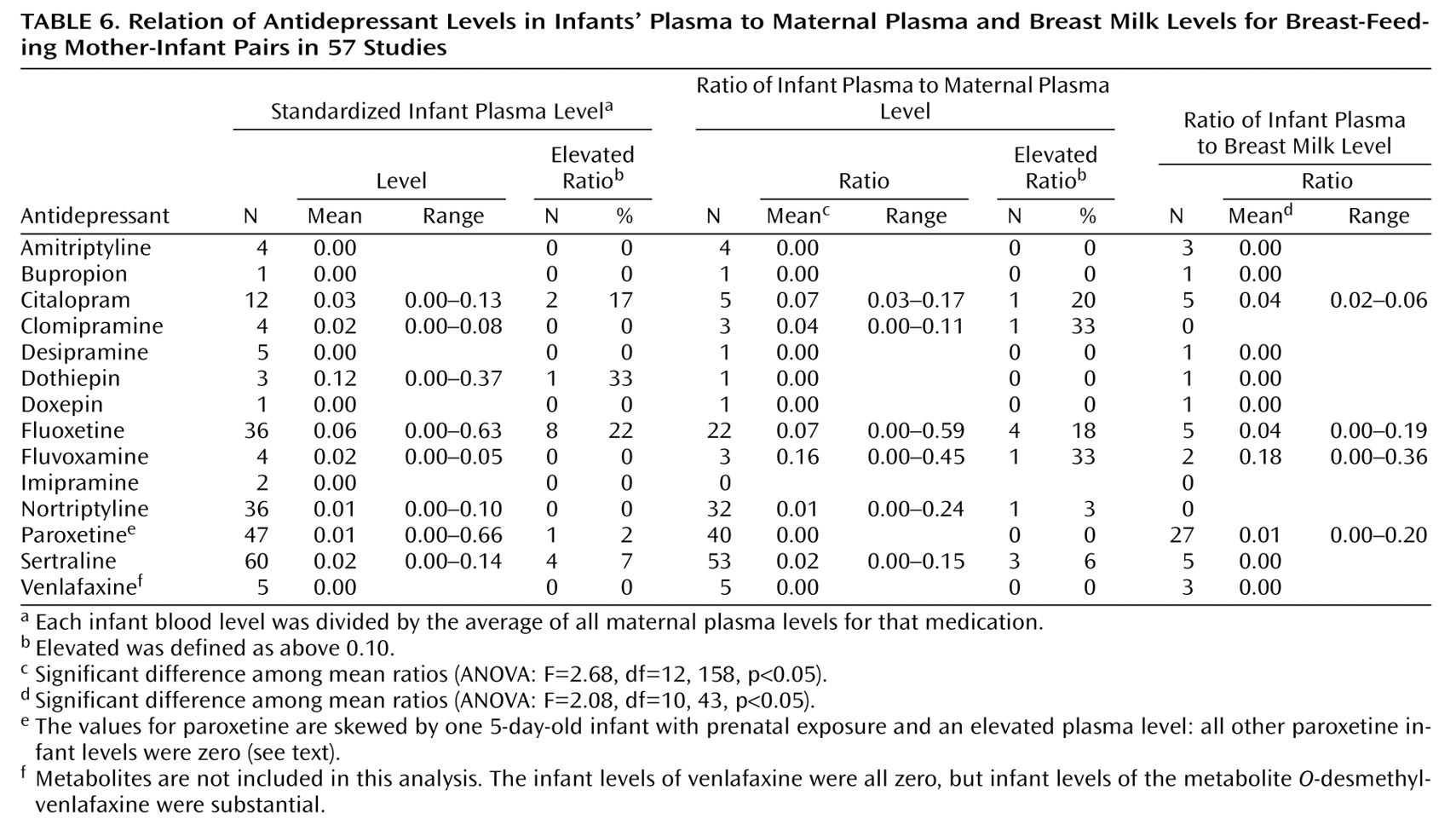
Table 6 compares the infant plasma levels with the maternal plasma and breast milk levels for each drug. The standardized infant plasma level for each drug (infant level divided by the average maternal plasma level for that drug) is given on the left side of the table. We defined elevated infant levels as those above 10% of the average maternal level. Other than dothiepin, which was studied in only three infants, fluoxetine produced the highest proportion of elevated infant levels and the highest mean standardized infant level (
Table 6). Citalopram also produced a relatively high percentage of elevated infant levels. Repeating the analysis of infant levels with the weighted sum of the metabolites included did not substantially change the results (data not shown). Of note, the only infant with an elevated paroxetine level, 31.4 ng/ml
(32), was 5 days old and had a history of prenatal exposure to paroxetine, which may have caused the high level. However, that infant’s mother also had an unusually high level of paroxetine in her breast milk. All other infant paroxetine levels were zero (limit of quantification, 4–5 ng/ml), and these infants included three with prenatal exposure who were 4.0–5.6 weeks old.
In the ratios of infant to maternal plasma levels for each individual mother-infant pair, fluoxetine and citalopram again produced relatively high proportions of elevated infant levels (
Table 6). Fluvoxamine and clomipramine each produced an elevated level in one infant out of the three studied. For venlafaxine, which had substantial infant metabolite levels, we repeated the analysis with the weighted sum of the metabolites. When the metabolites were included in the analysis, venlafaxine produced a mean standardized infant level of 0.06 ng/ml in the five infants studied, and one infant had an elevated plasma level. The infant-to-mother ratio for venlafaxine was 0.12, with elevated ratios for two of the five infants. These five infants all had substantial metabolite levels (16–225 ng/ml) despite having levels of the parent drug of zero. For the other drugs studied, including the metabolites produced no substantial changes in the analysis (data not shown).
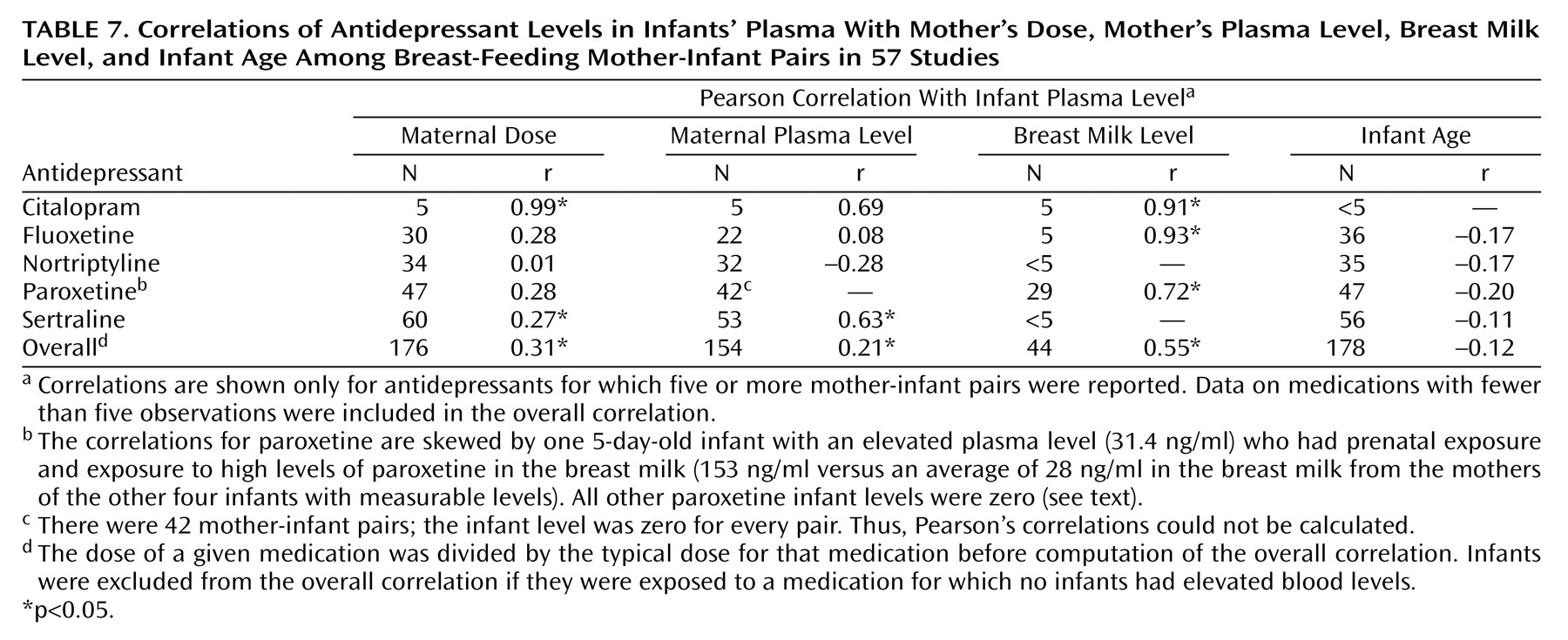
We analyzed the correlations of the infant plasma level to the mother’s dose, mother’s plasma level, breast milk level, and infant age for the five drugs for which five or more infant levels were reported (
Table 7). We found that the maternal dose was highly correlated with the infant plasma level for citalopram and was weakly correlated for sertraline. Maternal dose did not predict infant level for fluoxetine, nortriptyline, or paroxetine. The maternal plasma level was moderately correlated with the infant level for sertraline but not citalopram, fluoxetine, or nortriptyline. Maternal breast milk level was significantly correlated with infant plasma level for citalopram, fluoxetine, and paroxetine. However, the correlation between breast milk and infant plasma level for paroxetine is high because the 5-day-old infant
(32) with a paroxetine level of 31.4 ng/ml was exposed to unusually high levels of paroxetine in the breast milk (153 ng/ml versus an average of 28 ng/ml for the other infants with measurable levels). The level was zero for the other 24 infants. It is interesting that infant age was not correlated with infant plasma level for any drug. (Again, infants with recent prenatal exposure were excluded from this analysis.) Including the metabolites in the analysis produced no significant changes in the correlations (data not shown).
Of the 15 excluded infants (those with prenatal exposure who were younger than five times the average half-life of the parent drug), 12 had mothers taking fluoxetine and had measurable infant plasma levels. The mean plasma level for these 12 infants was 35.5 ng/ml, compared to a mean plasma level of 7.9 ng/ml for the 36 fluoxetine infants who were not excluded (t=2.52, df=46, p=0.03).
We also compared the mean infant fluoxetine plasma levels for infants with and without prenatal exposure who were older than five half-lives of the parent drug. The 22 older infants with prenatal exposure to fluoxetine had a mean level of 7.9 ng/ml, compared to 2.8 ng/ml for nine older infants with no prenatal exposure. This is not a statistically significant difference. Similar comparisons for paroxetine, citalopram, sertraline, and nortriptyline did not reveal any significant differences between infants with and without prenatal exposure who were older than 5 half-lives of the parent drug (data not shown).
Six infants originally selected for study because of symptoms were excluded from the preceding analysis. Of these, four were exposed to fluoxetine and two to doxepin. The symptomatic infants exposed to citalopram
(31) and paroxetine
(32) were included in our analyses because they were studied prospectively and then found to have symptoms (
Table 4).
For 11 antidepressants we had both ratios of milk to maternal plasma levels and published information on percentage of free drug, which allowed us to calculate protein binding
(80,
81). For these 11 drugs, the ratio of milk to maternal plasma levels had a statistically significant negative association with the percentage of the drug bound to protein (r
s=–0.69, N=11, p=0.02).
Our methods of data extraction could have led to over- or underestimation of the infant plasma levels. Initially, we coded undetectable infant levels as zero, which could lead to underestimation, and we coded detectable but not quantifiable infant levels as 0.1 ng/ml less than the limit of detectability, which could lead to overestimation of infant levels. We therefore repeated the analyses in
Table 6 and
Table 7 under different conditions to assess the robustness of our results.
First, undetectable infant levels were recoded from zero to half the level of quantification. The standardized infant levels (
Table 6) were now elevated (>10% of the average maternal level) in one of four amitriptyline infants, the one bupropion infant, the one dothiepin infant, three of 36 nortriptyline infants (8%), and three of 47 paroxetine infants (6%). The proportion of infants with elevated standardized levels changed from 22% to 25% for fluoxetine. The correlations in
Table 7 changed slightly. Two correlations changed in statistical significance: the correlation between maternal dose and infant level for paroxetine became significant at r=0.35 (p<0.05), and the correlation between maternal dose and infant level for sertraline became nonsignificant at r=0.25.
We then recoded detectable but nonquantifiable infant levels, reducing them from 0.1 ng/ml less than the limit of quantification to half the limit of quantification. The standardized infant levels (
Table 6) were now elevated (>10% of the average maternal level) in six (17%) of 36 fluoxetine infants instead of 22% and in 0% of sertraline infants instead of 7%. The correlations in
Table 7 again changed slightly, but there were no changes in statistical significance.
Discussion
There are many factors potentially affecting breast milk and infant plasma levels of maternally ingested antidepressants. These include protein binding, CYP2D6 polymorphisms, lipophilicity, and the presence of other drugs (including herbs) ingested by the nursing mother. We found a strong negative relationship between antidepressant protein binding and potential transmission to the fetus that to our knowledge has not been addressed in previous studies. According to currently available data, breast-feeding infants exposed to nortriptyline, paroxetine, or sertraline seem unlikely to develop detectable or elevated plasma levels, while infants exposed to fluoxetine appear to be at higher risk of developing elevated levels, especially following prenatal exposure or if levels are high in the breast milk. Citalopram may produce elevated levels in some infants, especially if the maternal dose or breast milk level is high, but more data are needed before firm conclusions can be drawn. Although our research does not show that the elevated levels have consequences for infants, a conservative approach to management may be to prescribe only medications that do not appear in infants’ plasma.
Short-Term Effects of Antidepressant Exposure
Short-term but potentially serious adverse effects of breast-feeding exposure to antidepressants have been noted in individual case reports (
Table 4). Of note, the 10-week-old infant studied by Lester et al.
(43) had no history of prenatal exposure to fluoxetine yet developed plasma levels higher than the average maternal plasma level in our database. This measurement may have been spuriously high because it was obtained at a commercial laboratory rather than a research laboratory
(4), but the infant’s symptoms correlated with withdrawal and reexposure to the mother’s breast milk. The fluoxetine-exposed infant studied by Brent and Wisner
(45) had levels that increased between 2 and 3 weeks of age. These findings support our conclusion that fluoxetine is more likely than other antidepressants to accumulate in nursing infants.
The symptomatic infant with citalopram exposure reported by Schmidt et al.
(31) is interesting because the symptoms occurred in an infant with no history of prenatal exposure whose level was 13% of the average maternal plasma level in our database. This supports our classification of infant levels above 10% of the maternal level as of potential clinical significance.
Long-Term Effects of Antidepressant Exposure
This pooled analysis focused only on infant plasma and breast milk levels. However, plasma levels or even brain tissue levels may not accurately predict the biochemical effect of an antidepressant on the brain
(82,
83). Therefore, a low or undetectable infant plasma concentration by itself cannot reassure us that the antidepressant will have no effect on the rapidly developing brain. The larger question—whether chronic exposure to low doses of antidepressant medication poses long-term neurodevelopmental risks to the infant—remains unanswered. The limited information available from small controlled
(24,
69,
84) and uncontrolled
(46,
50) studies of asymptomatic infants is reassuring. Platelet serotonin uptake is not decreased in breast-fed infants exposed to sertraline; by inference, breast-feeding exposure to sertraline should therefore not affect serotonin metabolism in the infant brain
(18). However, Chambers et al.
(85) found a small growth deficit of 392 g at 6 months of age in infants exposed to fluoxetine through breast milk. Although antenatal exposure is different from breast-feeding exposure, the available data on antenatal exposure points toward little or no long-term effect on developmental outcomes
(86). Clearly, further work needs to be done before we can be confident that antidepressant exposure poses no important long-term neurodevelopmental risks to the nursing infant.
Limitations
This pooled analysis included all the currently available data we could find on antidepressant levels in breast-feeding mothers and infants, as well as data from 36 unpublished cases. Most data sets did not include maternal, breast milk,
and infant levels. Infant levels were especially lacking. Because the neonatal liver undergoes a marked increase in metabolic capacity during the first weeks of life, from very low to above the adult range
(87), the infant’s ability to metabolize the drug can be a much stronger determinant of plasma level than the amount of drug ingested through breast milk. Despite the correlations we have presented, the current knowledge is not sufficient to support monitoring of breast milk levels in order to estimate individual infant plasma levels, and researchers should not rely on breast milk levels alone to estimate the extent of exposure of the infant.
Other data elements that were not routinely reported include the maternal weight, time after a maternal dose, whether the infant had been exposed to the drug before birth, the limit of quantifiability of the assay, and the use of other medications, including herbs, that may affect metabolism. Variation in pharmacogenetics may also be important, but these studies are unlikely to be done in the near future, given the current invasive nature of these assays. The pharmacokinetics of drug transfer by breast milk can be understood only if the presence or absence of prenatal exposure is reported, since prenatal exposure provides a “loading dose” that far exceeds any exposure from breast milk.
Many reported breast milk levels are unreliable because of variations in milk collection procedures. In some studies, an unspecified aliquot from a given feeding was collected or only fore or hind milk levels were determined, and these may not accurately reflect the overall drug concentration ingested by the infant. Because antidepressants tend to be lipophilic, breast milk concentrations for some drugs may vary more than twofold between the watery fore milk and the fattier hind milk
(56,
64), while others vary very little
(35).
Other possible confounding factors include cigarette smoking, a known inducer of cytochromes CYP1A1 and CYP1A2
(88). Almost none of the reports indicated whether the mothers used cigarettes, which can potentially affect infant metabolism and maternal metabolism. Similarly, alcohol use was not reported.
The studies we reviewed primarily made contemporaneous measurements of maternal, breast milk, and infant drug levels. However, the peak level in breast milk may lag behind the maternal plasma peak by 2 or more hours and can be two to three times as high as the trough level
(39,
44,
47). Different drugs peak in breast milk at different intervals after a maternal dose, and this interval may vary between patients taking the same drug
(63,
69). The peak drug level in the infant would be expected sometime after the feeding that contains a peak or near-peak level in the milk. Thus, one-time measurements may not accurately reflect the extent of infant exposure to the drug. Using the calculated area under the curve overcomes this problem but requires obtaining multiple samples from the same patient during a dosing interval. We are not aware of any study of multiple infants that has determined the area under the curve of an infant’s blood level. Given the logistics involved and quantity of blood that would be required, it is unlikely this will be done.
Conclusions
According to currently available data, breast-feeding infants exposed to nortriptyline, paroxetine, or sertraline are unlikely to develop detectable or elevated plasma drug levels, while infants exposed to fluoxetine appear to be at higher risk of developing elevated levels, especially following prenatal exposure. Citalopram may produce elevated levels in some infants, but additional data are needed before firm conclusions can be drawn. Current knowledge does not support clinical monitoring of maternal plasma or breast milk antidepressant levels in an attempt to estimate individual infant plasma levels. Further research is needed before we can be confident that antidepressant exposure produces no significant long-term risks to the infant; however, on the basis of current knowledge, breast-feeding women may reasonably choose to use antidepressants. In order to clarify factors influencing infant exposure to antidepressants through breast milk, investigators in future studies must measure infant levels and report relevant information such as the history of prenatal exposure, infant age, infant weight, and concomitant use of other medications.
Infant drug levels are just one factor potentially affecting the infant. Given the paucity of published reports of infant drug levels, the lack of studies that consistently take into account the many factors potentially influencing drug levels, and the numbers of women who may benefit from antidepressants during the time that breast-feeding is recommended, larger studies are imperative in the future in order to be able to give nursing mothers the best possible advice concerning use of and potential effects of antidepressants.
Because rare, adverse effects may not have been picked up by the small numbers of case reports and published studies, nursing mothers choosing to use antidepressants should be alert to subtle changes or worrisome infant behaviors, such as irritability, poor feeding, or uneasy sleep, that may indicate adverse infant effects from maternally ingested medication. Infants born prematurely and those with impaired metabolite efficiency are likely to have higher levels and should be watched closely for side effects.