Recently, there has been a resurgence in attention to sex differences in schizophrenia, reflecting the important contribution of these differences to the heterogeneity of schizophrenia phenomenology (for review, see references
1–4).
The majority of investigations have focused on sex differences in the epidemiology and clinical expression of schizophrenia. These studies have observed a consistent two- to three-fold increase in the incidence of schizophrenia in men versus women when the DSM-IV criterion of age at onset before 45 years is adopted
(5). Indeed, as early as 1896, Kraepelin noted that “men appear to be three times more likely to suffer from the illness”
(6). Men with schizophrenia also exhibit more negative symptoms than do women
(7) and experience an earlier age at onset (of about 3–5 years) that is consistent across different cultures
(8), definitions of psychosis onset
(9), and diagnostic tools
(10).
Another large set of studies has addressed sex differences in schizophrenia in relation to neuroanatomical deficits. The consensus from these studies, despite the use of different brain imaging methodologies, is that male subjects with schizophrenia exhibit greater structural brain abnormalities than do female schizophrenia subjects
(11). Key neuroanatomical abnormalities include larger ventricle-brain ratios
(12), reduced gray matter volume—particularly in temporolimbic structures
(13), and a lack of the normal asymmetry shown by healthy male subjects
(14). Male schizophrenia patients also display a greater rate of minor physical anomalies and neurological soft signs than do female patients
(15).
By comparison, a smaller number of studies have examined sex differences in schizophrenia in relation to brain function. In general, men show a greater deviation than do women across neuropsychological tests of cognitive function
(16) as well as a reduction in regional cerebral blood flow
(17). To date, very few investigations of sex differences in schizophrenia have been published that have used high-temporal-resolution measures such as EEG
(18,
19). In a previous investigation, Turetsky et al.
(20) reported significant sex differences in the event-related potentials of patients with schizophrenia. Results revealed a dissociation by sex in the P300 event-related potential: while male subjects exhibited a reduced P300 in the right parietal region, female subjects showed a greater deficit in the anterior (frontal) region.
Given the dearth of neurophysiological investigation into sex differences in schizophrenia, we focused for the first time in this study on sex-related variation in functional brain connectivity. We employed a measure of high-frequency synchronous brain activity—40-Hz gamma phase synchrony
(21)—extracted from the EEG recordings. Disturbances of functional integration have been recognized as a fundamental characteristic of schizophrenia since the earliest attempts to describe this disorder in terms of “loss of the inner unity” and a disturbance in the “intrapsychic coordination” of mental activities
(22). Previous models of schizophrenia have postulated that the abnormal temporal integration of brain networks underlies the core failure of cognitive coordination (or “cognitive dysmetria”) in this disorder
(23,
24).
Synchronous gamma activity has been observed in animal and human studies, and across microscopic and macroscopic scales of recording, using both depth-electrode and scalp-recorded EEG (for a review, see reference
25). It has been proposed that in-phase synchronous gamma activity may be a mechanism involved in the “binding problem.” This refers to the manner in which the brain is able to integrate or bind together the diverse neuronal activities relating to a single stimulus among the vast array of parallel processing occurring at any given time
(26).
In-phase synchronous gamma oscillations in scalp-recorded EEG activity are distinct from the power (or amplitude) of this activity. Our preliminary investigation of sex differences in patients with chronic schizophrenia focused on gamma power
(27). Schizophrenia patients as a group exhibited decreased gamma power frontally and in the left hemisphere, consistent with previous reports
(28–
30). However, no sex differences in gamma power were observed. It was postulated that the measure of power may fail to reveal more specific sex-related variation in functional connectivity or that the sample may have comprised a more severely ill spectrum of women with chronic schizophrenia. To address these issues, this study examined sex differences in gamma phase synchrony in patients with first-episode schizophrenia as well as those with chronic schizophrenia.
Studies of brain structure in healthy subjects may provide some basis from which to speculate about the normal pattern of sex differences in gamma phase synchrony. Of particular relevance are neuroanatomical and cytoarchitectural morphometry studies demonstrating greater inter- and intrahemispheric connectivity in healthy women compared with men
(31,
32). Furthermore, healthy women have been shown to exhibit greater EEG power and coherence than do men
(33). Therefore, we predicted that healthy women would show greater functional connectivity than men, reflected in greater intra- and interhemispheric gamma phase synchrony. While both chronic and first-episode schizophrenia patients would show an abnormal pattern of gamma phase synchrony, women with first-episode schizophrenia would exhibit relatively greater functional connectivity in terms of gamma phase synchrony.
Method
Subjects
Forty patients with chronic schizophrenia (20 men [mean age=33.9, SD=7.9] and 20 women [mean age=34.4, SD=8.5]) and 24 subjects in their first episode of schizophrenia (12 men [mean age=19.0, SD=2.5] and 12 women [mean age=19.7, SD=4.2]) took part. Diagnosis of schizophrenia was confirmed by using section G (schizophrenia and psychotic disorders) of the Composite International Diagnostic Interview and consensus of three psychiatrists (two independent from the study), according to DSM-IV and ICD-10 criteria. The additional criterion for the first-episode schizophrenia group was first presentation to health services with psychotic symptoms that warranted such a diagnosis. Confirmatory information for the first-episode schizophrenia diagnosis was provided by interviews with family and case managers (including case notes).
An equal number of healthy comparison subjects were matched in age (within 3 years) and sex to subjects in each of the schizophrenia groups. For the healthy subjects matched to the chronic schizophrenia group, the mean age was 34.1 years (SD=9.1) for the men and 34.1 years (SD=9.3) for the women. For the healthy subjects matched to the first-episode group, the mean age was 19.8 years (SD=3.6) for the men and 19.9 years (SD=3.2) for the women.
Exclusion criteria for all subjects were left-handedness
(34), neurological disorder or head injury, mental retardation, and meeting DSM-IV criteria for drug dependence, assessed by using Composite International Diagnostic Interview section M and the Westmead Hospital Clinical Information Base
(35). The healthy subjects were also screened for history of a psychiatric illness (in themselves or in a first-degree relative) or treatment with psychiatric medication. IQ equivalence for all subjects (patients and healthy subjects) was greater than 85 as assessed by the Wide Range Achievement Test
(36) and was therefore within the normal range.
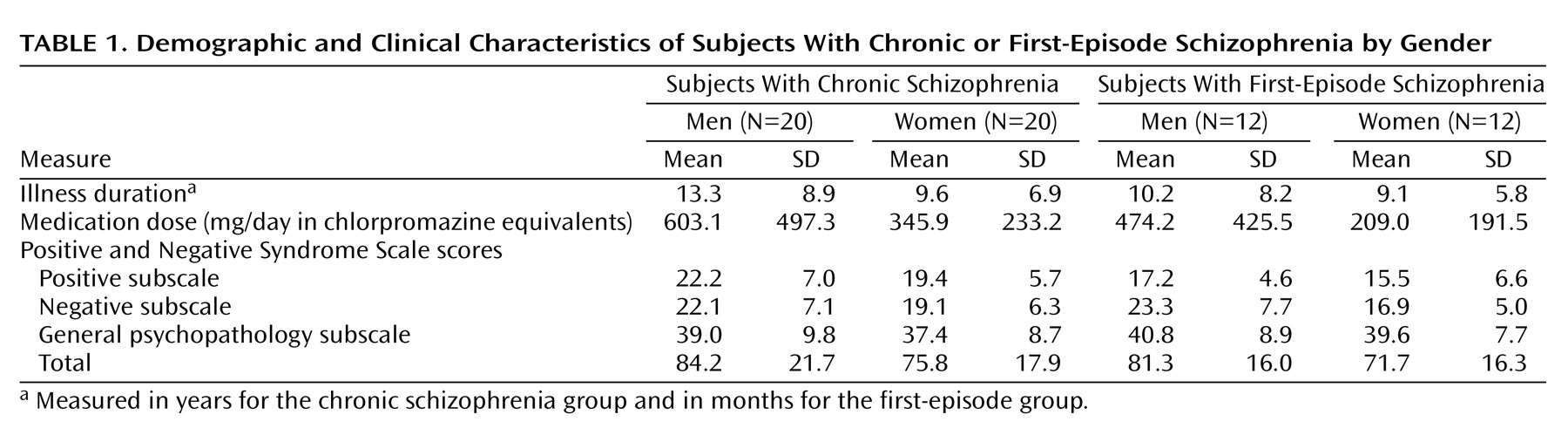
Table 1 summarizes the clinical data, including medication dosage, duration of illness, and symptom ratings for both the chronic and first-episode schizophrenia groups. Schizophrenia symptoms were rated with the Positive and Negative Syndrome Scale
(37). Medication dosage was measured in terms of chlorpromazine equivalents
(38). Because male subjects in the chronic schizophrenia group were receiving higher medication doses than were the female subjects (t=2.04, df=38, p<0.05), dosage was controlled in the focal analyses. Male subjects in the first-episode schizophrenia group exhibited more severe negative symptoms than did female subjects (t=5.88, df=22, p<0.03), which was also controlled in the focal analyses.
All subjects were asked to refrain from smoking or drinking caffeinated beverages for at least 3 hours before testing. After a complete description of the study to the subjects, written informed consent was obtained in accordance with National Health and Medical Research Council guidelines.
Experimental Task
EEG data were acquired using a standard auditory “oddball” paradigm. Stereo headphones conveyed regular tones at a constant interval of 1.3 seconds to both ears. Eighty-five percent were 1000-Hz “background” (task-irrelevant) and 15% were “target” (task-relevant) tones of 1500 Hz. All tones were presented at 80 dB above the subject’s auditory threshold (determined before recording). Target tones were randomly intermixed with backgrounds, with the only constraint being that two targets were never presented in succession. Subjects were instructed to respond to the target tones as quickly and as accurately as possible by pressing two reaction time buttons with the index finger of each hand (to counterbalance motor effects).
EEG Data Acquisition
All subjects were seated in a sound- and light-attenuated room. An electrode cap
(39) was used to acquire data from Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, and O2 scalp sites. Linked earlobes served as the reference. Horizontal eye movement potentials were recorded by using two electrodes placed 1 cm lateral to the outer canthus of each eye. Vertical eye movement potentials were recorded by using two electrodes placed on the middle of the supraorbital and infraorbital regions of the left eye. Skin resistance at each site was <5 kΩ. A continuous acquisition system was employed, and data were electro-oculogram-corrected offline
(40). The sampling rate was 250 Hz. A low-pass filter was applied to the signals before digitization. The cutoff for this filter was 50 Hz, with attenuation being 40 dB/decade above 50 Hz. In addition, a 50-Hz notch filter was applied to eliminate interference from the 50-Hz alternating current main power supply.
Each subject was instructed to keep their eyes open and to look at a colored dot in the center of the screen in order to minimize eye movements during presentation of tone stimuli. The recording session continued until 40 correctly identified target epochs were acquired.
Gamma Data Reduction
Only correctly identified target epochs for which a button press was obtained within 1 second of the target tone (total of 40) were scored. For each target epoch, from each recording site, a 64-sample Welch window was moved along sample by sample, starting with the center of the Welch window at 500 msec before the stimulus (–500 msec) and ending with the center of the Welch window at 750 msec after the stimulus. Linear trends were first removed by subtracting the line of best fit over all samples centered at stimulus presentation. At each sample position, gamma frequency was computed by means of fast Fourier transformation
(41). Frequency bins of 3.91 Hz ranged within a narrow band of gamma activity from 37.1 to 41.0 Hz, which encompasses the focal frequency of 40 Hz and were centered at 39.1 Hz
(42). Phase synchronicity was then estimated at each time point by taking the phase estimates across each site of interest at a given time and computing the circular variance of these phase estimates
(43). This yielded a single-scale estimate of the extent of phase locking across these sites for each point in time. The result was a time series of gamma phase synchronicity across the sites in question. Five such time series were derived for each epoch, one for global (all sites) synchronicity and four regional synchronicity waveforms: frontal (Fp1, Fp2, Fz, F3, F4, F7, and F8), posterior (O1, O2, T5, T6, P3, P4, and PZ), left hemisphere (Fp1, F3, F7, C3, T3, T5, P3, and O1), and right hemisphere (Fp2, F4, F8, C4, T4, T6, P4, and O2). The time series of gamma phase synchrony was indexed at each time point in terms of magnitude (an inverted normalized measure that ranges between 0 and 1, with 1 reflecting greater synchrony) and the latency corresponding to each measure of magnitude (in milliseconds). Phase synchrony differs from coherence estimates in that it may be derived using multiple sites (rather than pairs), and it indexes the synchrony in the phase of activity across these sites rather than the simple correlation in activity.
Magnitude and latency of gamma phase synchrony were averaged across the 40 target epochs for each subject at each time point. This yielded an averaged target gamma phase synchrony waveform for each region of interest (global, frontal, posterior, left hemisphere, and right hemisphere). Averaged gamma phase synchrony waveforms were smoothed with a 15-sample running average, following the previously published procedure
(42).
Previous studies have revealed two peaks in the averaged gamma phase synchrony time series in response to target stimuli, including an early response maximal (within the latency window –150 to 150 msec, or “early gamma”) and a late poststimulus response maximal (in the latency window 200 to 450 msec, or “late gamma”). In this study, we analyzed peak magnitude and latency of gamma phase synchrony in relation to sex differences in schizophrenia compared with healthy subjects.
Statistical Analysis
Prior to statistical analysis, all outliers were removed from the data and replaced with the group mean for the relevant electrode site. Values more than one and a half times the interquartile range above the upper or below the lower quartiles were considered to be outliers. The groups were not statistically different on percentage of missing data.
Mixed-design multivariate analyses of variance (MANOVAs) were undertaken to analyze between-group differences in peak magnitude and latency of both early and late gamma phase synchrony in response to target stimuli. To examine global gamma phase synchrony, group (schizophrenia versus healthy subjects) was the between-group factor, and sex was the within-subject factor. For regional gamma phase synchrony, region (frontal versus posterior or left versus right hemisphere) was included as a second within-subject, repeated-measures factor. Interactions with sex were explored further using within-group MANOVAs (within schizophrenia and healthy groups) with sex as the between-subject factor.
Multivariate analyses of covariance were also conducted to examine the possible confounding effects of medication and symptoms on significant effects.
While each analysis was undertaken in relation to explicit predictions, we nonetheless employed a strict alpha level of 0.01, given that analyses of gamma phase synchrony were conducted for five different regional groupings.
Results
Gamma Phase Synchrony in Chronic Schizophrenia
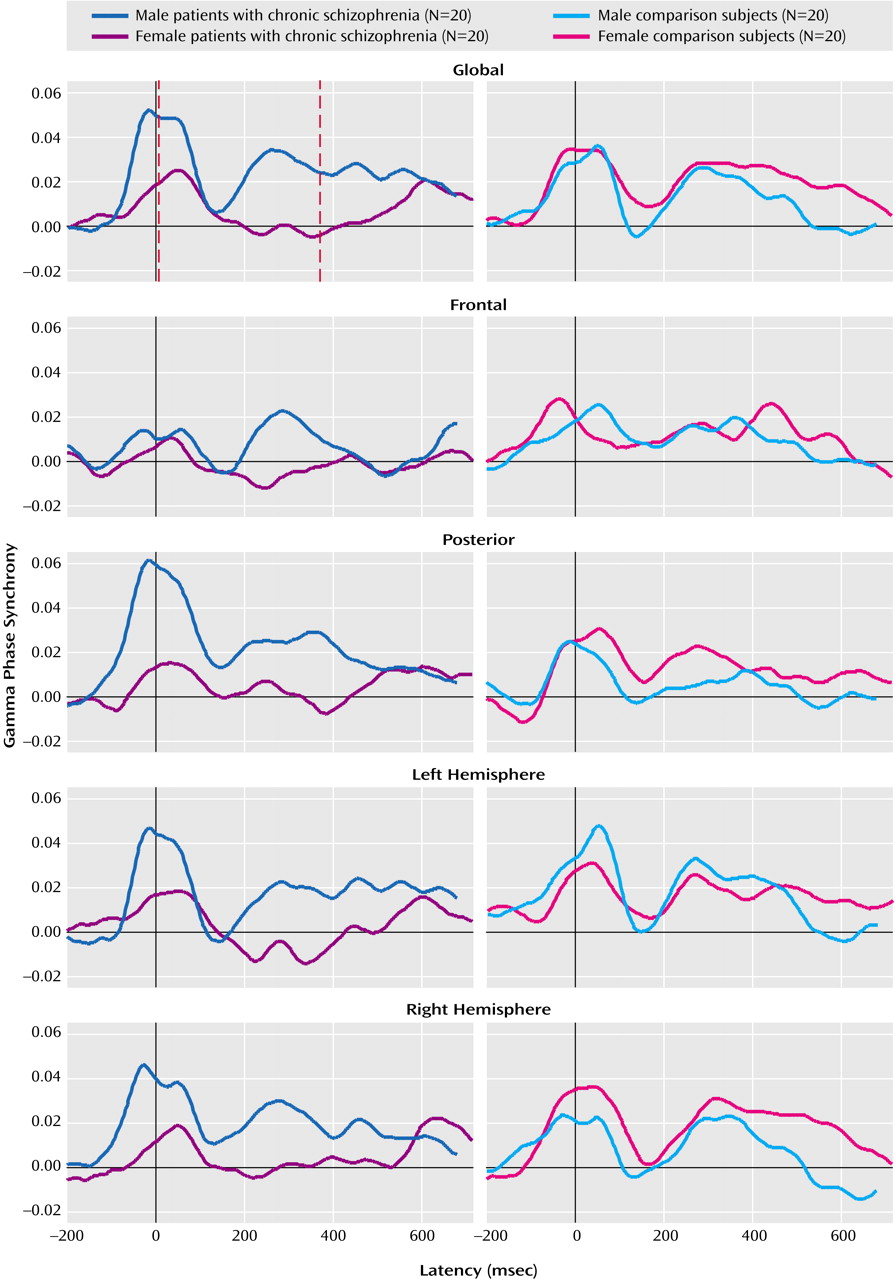
Figure 1 presents the averaged regional waveform (including peak magnitude and latency, marked by red lines) for both early and late gamma phase synchrony for male and female chronic schizophrenia patients and their matched healthy comparison subjects in response to target stimuli.
A significant group-by-sex interaction was revealed for the magnitude of global late gamma phase synchrony (F=17.51, df=1, 76, p<0.0001). This interaction primarily was due to the reduction of gamma phase synchrony in female relative to male subjects in the chronic schizophrenia group (F=29.74, df=1, 38, p<0.0001). There were no significant effects involving sex or group for early global gamma phase synchrony.
There were no significant interactions involving group or sex for the frontal or posterior gradient of early or late gamma phase synchrony.
Analyses revealed a significant group-by-laterality interaction for early gamma phase synchrony (F=9.14, df=1, 76, p<0.004) that was due to reduced left hemisphere gamma phase synchrony in chronic schizophrenia patients relative to healthy subjects (F=9.52, df=1, 76, p<0.004), but a lack of group differences for the right hemisphere. For late gamma phase synchrony, there was a significant sex-by-laterality interaction (F=7.09, df=1, 76, p<0.01) that was due to the generally greater reduction for women relative to men in left hemisphere gamma phase synchrony (F=14.27, df=1, 76, p<0.001) but not right hemisphere gamma phase synchrony.
There were no significant interactions between group and sex for the laterality of early or late gamma phase synchrony magnitude or latency.
Gamma Phase Synchrony in First-Episode Schizophrenia
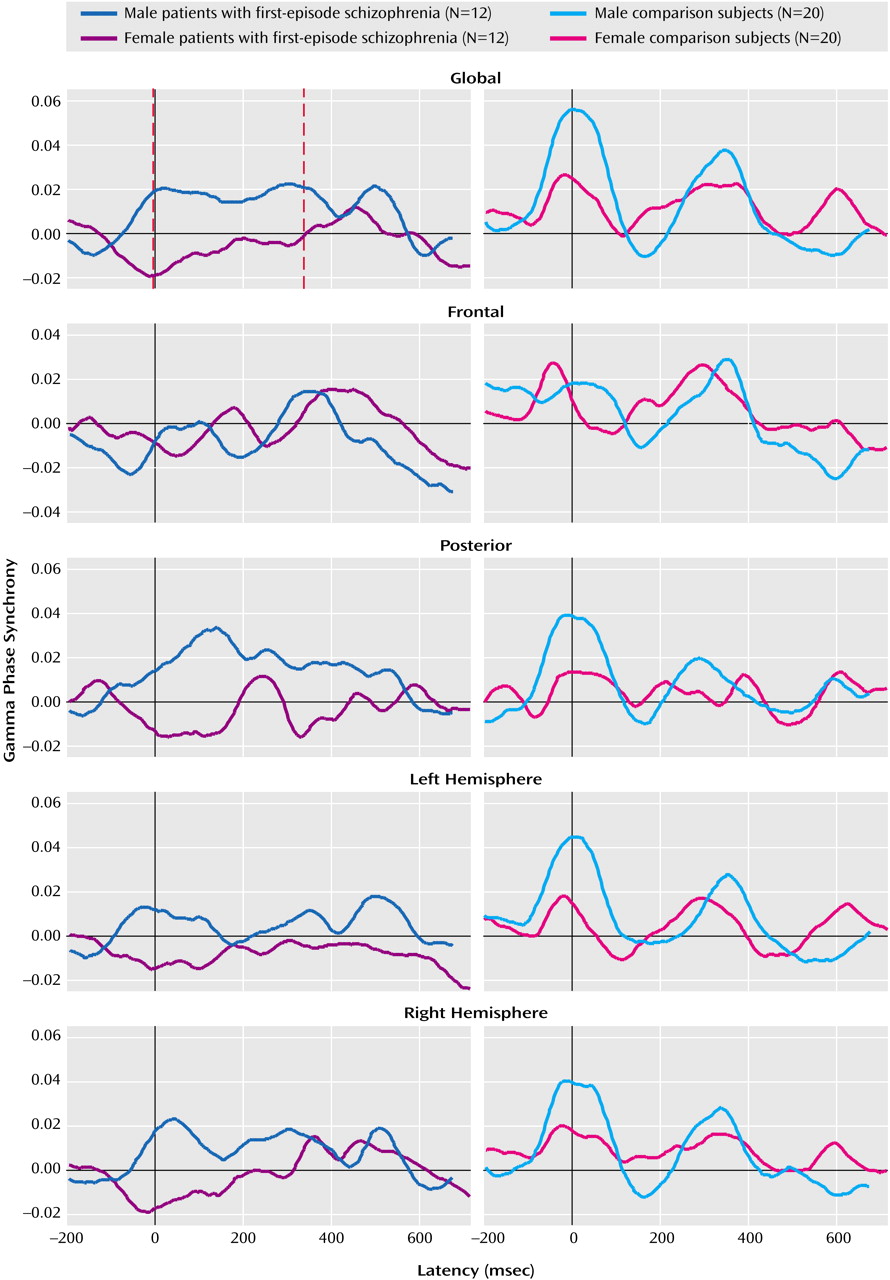
Figure 2 presents the averaged regional waveform (including peak magnitude and latency, marked by red lines) for both early and late gamma phase synchrony for male and female first-episode schizophrenia patients and their matched comparison subjects in response to target stimuli.
A significant group-by-sex interaction effect was revealed for the peak latency of early global gamma phase synchrony (F=6.68, df=1, 44, p<0.01). This interaction was due to faster latency in female versus male first-episode schizophrenia patients (F=7.80, df=1, 22, p<0.01) not present in the healthy subjects.
There were no interactions with group and sex for late global gamma phase synchrony.
A significant group-by-region interaction was revealed for the early gamma phase synchrony latency across the frontal and posterior gradient (F=8.56, df=1, 44, p<0.006). Latency of frontal synchrony was delayed relative to posterior synchrony in first-episode schizophrenia patients (F=15.56, df=1, 46, p<0.001), whereas there was no difference in healthy subjects. This interaction may have accounted for the significant main effect for group (F=8.23, df=1, 44, p<0.007), which revealed the generally delayed early gamma phase synchrony in the frontal region of first-episode schizophrenia patients relative to healthy subjects (F=8.87, df=1, 46, p<0.006). There were no interactions with group and sex for late global gamma phase synchrony.
A significant group main effect was found for the magnitude of early gamma phase synchrony (F=16.73, df=1, 44, p<0.001) with first-episode schizophrenia patients displaying reduced magnitude within both the left (F=9.58, df=1, 46, p<0.004) and right (F=11.57, df=1, 46, p<0.002) hemispheres relative to the healthy group. There was also a significant main effect for group (F=16.72, df=1, 44, p<0.0001) that was due to delayed early gamma phase synchrony latency in first-episode schizophrenia patients relative to healthy subjects for both the right (F=7.34, df=1, 46, p<0.009) and left (F=14.43, df=1, 46, p<0.001) hemispheres. There were no interactions with group and sex for late global gamma phase synchrony.
Discussion
This was the first study to explore sex differences in patients with schizophrenia using a new high-temporal-resolution measure of functional connectivity, gamma phase synchrony. This study also sought to examine the effect of illness chronicity on sex differences in brain function by including both chronic and first-episode schizophrenia patients. Consistent with predictions, both patient groups showed marked deficits in functional connectivity relative to healthy subjects. With regard to sex, female first-episode schizophrenia subjects demonstrated relatively enhanced synchronization as predicted. However, female chronic schizophrenia subjects exhibited even more severe impairments than male chronic schizophrenia subjects. These findings may be considered in terms of both illness chronicity and the temporal course of gamma phase synchrony.
It has been previously reported that early gamma phase synchrony may play a role in feed-forward anticipation of stimulus processing and the integration of sensory features. Late gamma phase synchrony, on the other hand, may reflect aspects of poststimulus discrimination and context processing
(44). As a group, both chronic schizophrenia and first-episode schizophrenia patients exhibited marked disturbances in both early and late gamma phase synchrony. Specifically, chronic schizophrenia patients displayed a global reduction in functional connectivity, with particularly pronounced deficits in the left hemisphere, while first-episode schizophrenia patients exhibited a similar disturbance in left hemisphere gamma phase synchrony in addition to deficits in frontal connectivity. This widespread pattern of disconnectivity is consistent with previous studies demonstrating deficits in cognitive coordination of both early stimulus integration and the later processes of selective attention and context
(23,
45). Furthermore, the pattern of hypofrontality and left hemisphere disconnectivity also adds weight to structural and functional investigations that suggest these regions are central to the neurodevelopment of schizophrenia
(46–
48).
In the first-episode schizophrenia group, faster early synchronization was observed in female relative to male subjects, suggesting that they have a greater adaptive capacity. Given the role of early gamma phase synchrony
(49), this capacity may reflect an attempt to broaden the span of apprehension for incoming stimuli in an attempt to regulate a fundamental deficit in information processing. This is similar to observations of an extended span of apprehension and enhanced perceptual grouping
(50), previously attributed to overactivity, which seem to suggest pathophysiologic rather than structural abnormality
(51). While these studies have not considered sex differences, we might speculate that the onset of schizophrenia in women is preferentially associated with a distinctive pathophysiologic process rather than neuroanatomical impairment. This suggestion is consistent with findings of greater anatomical abnormalities in male schizophrenia subjects
(11).
By contrast, the relatively enhanced speed of integration was not seen in female chronic schizophrenia subjects. Indeed, female chronic schizophrenia subjects by comparison showed a greater deficit in global late gamma phase synchrony than their male counterparts. In view of the function of late gamma phase synchrony, we may speculate that the departure in the pattern of synchronization according to chronicity in women with schizophrenia reflects the developmental progression of a pathophysiologic process. Pronounced deficits in context-related integration may reflect a particularly virulent progression of the pathophysiologic course, and associated cognitive impairment, in those women with schizophrenia unable to compensate for or adapt effectively to their illness. Supporting this view are reports of a consistently earlier age at onset in men and the corresponding evidence that suggests that chronic schizophrenia in women represents a particularly severe spectrum of the illness
(1).
The differences in early versus late gamma phase synchrony observed in women with first-episode relative to chronic schizophrenia accounted largely for the total difference between the first-episode and chronic schizophrenia groups. That is, as a whole, first-episode schizophrenia patients exhibited more pronounced deficits in early gamma phase synchrony, whereas deficits in the chronic schizophrenia patients were most apparent for late gamma phase synchrony. The development of more “entrenched” impairments in stimulus discrimination and context in chronic schizophrenia is consistent with reports of greater deficits in executive functioning in this group
(52). The consistently greater contribution of the female patients to this pattern of chronicity highlights an important sex-specific finding that requires further exploration in a larger sample of male and female subjects. It is important to note that a reduction in the gamma phase synchrony of the older group of healthy women was also found, further emphasizing that the process underlying impaired synchrony in women may have a basis in otherwise healthy physiologic development.
In conclusion, our study provides the first preliminary evidence that suggests schizophrenia disturbances in functional brain integration are related to sex and to illness chronicity. Current literature is attributing an increasingly significant role to gonadal hormones, particularly estrogen, in brain function. Therefore in view of this evidence
(53), future investigations of gamma phase synchrony might also seek to consider additional variables such as menstrual cycle fluctuations indexed by precise blood specimen measures.