Information-processing problems comprise core deficits in schizophrenia. Observations of semantic memory impairments in these patients
(1) do not conclusively pinpoint where these problems occur (e.g., at representation or retrieval). We examined this issue using 1) the “distance” effect: judgments about stimuli further apart in size are easier to make than about those whose sizes are more similar
(2); and 2) the “congruency” effect: processing congruent information is more efficient than processing incongruent information (e.g., a Stroop paradigm). Congruency effect findings have been inconsistent in schizophrenia
(3).
We capitalized on the distance and congruency effects by employing a framework within which we could specify the nature of anomalies in knowledge representation and processing. If knowledge organization is unsystematic in patients, responses will not be lawful, as predicted by the distance effect, and problems in cognitive control will result in greater interference from incongruent stimuli. Also, since decisions involving words are harder than images (words first require a conversion to their visual analogs
[4]), a combination of general slowing and an extra processing step should disproportionately slow patients’ responses to words.
Method
Twenty-four patients (20 men; mean age=32.5 years, SD=10.5) with a DSM-IV diagnosis of schizophrenia or schizoaffective disorder and 24 normal healthy volunteers (10 men; mean age=31.3 years, SD=8.9) participated in the study. After the procedure had been fully explained, written informed consent was obtained before testing.
The patients were taking neuroleptic medication (mean illness duration=9.8 years, SD=7.7). Intellectual function was assessed with the Wide-Range Achievement Test—Revised (WRAT-R) reading section
(5) and a short form of the WAIS-R (6). The patients were matched with the comparison subjects for age (p>0.50) and WRAT-R score (p>0.10) but had lower WAIS-R scores (mean=89.0, SD=9.1, and mean=110.7, SD=9.0, respectively) (t=8.26, df=46, p<0.00001).
We compiled a set of line drawings
(7) and words whose relative size was determined by 20 healthy volunteers in a pilot study. The participants in the present experiment decided as quickly and accurately as possible, by pressing one of two buttons, the relative size of two stimuli displayed on a computer monitor, either words (“word” condition) or images (“image-same” and “image-different” conditions). The prompt was, “Is the stimulus on the bottom larger or smaller in the real world than the stimulus on the top?” In the image-different condition, the images were congruent if real-world size and presentation size were consistent (small “bee,” large “sailboat”) and incongruent if the two dimensions were not consistent (large “bee,” small “sailboat”). Each trial consisted of the appearance of an asterisk (for 1,000 msec), then a blank screen (for 250 msec), then the stimulus pair, which remained until a response was given (or for a maximum of 6,000 msec). Before the next trial, there was another blank screen (for 250 msec).
The magnitude of the real-world distance (size difference) between the two stimuli was systematically varied. Each stimulus was rank ordered from smallest to largest, with the ordinal distance of a stimulus pair defined as the difference in ranking. Each distance between stimuli (1 through 8) was tested 16 times per condition, for a total of 128 trials.
In the image-same condition, the stimuli were images drawn to be equivalent in physical size (visual vertical and horizontal angles=6.30°). In the image-different condition, the stimuli were two images depicted in different sizes: small (visual vertical and horizontal angles=5.15°) and large (visual vertical and horizontal angles=11.42°). Half the trials were congruent. In the word condition, the stimuli were two words.
Data were analyzed with repeated-measures analysis of variance. Only correct responses were included in the reaction time analyses. Median and average reaction times yielded equivalent results; thus only median reaction times are reported.
Results
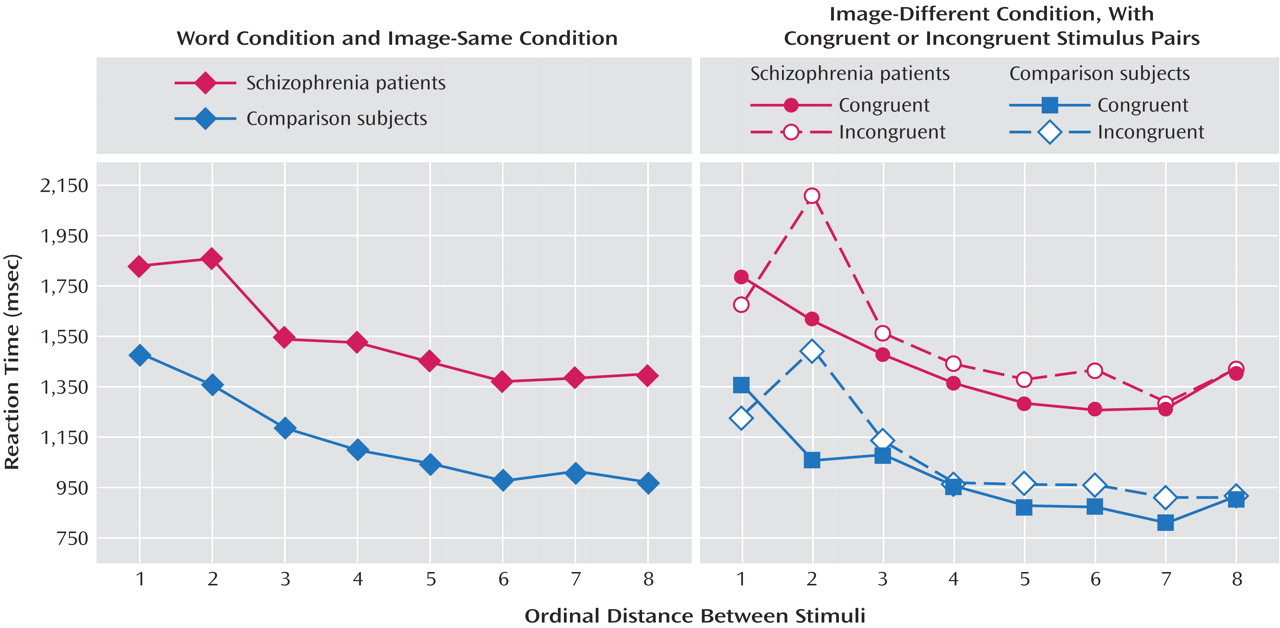
First, examination of the word and image-same conditions revealed a distance effect; a larger size difference correlated with faster judgments (F=79.14, df=7, 308, p<0.001). Although the patients were slower than the comparison subjects (mean=1,544 msec, SD=195, versus mean=1,141 msec, SD=189) (F=17.98, df=1, 44, p<0.001), there was no group-by-distance interaction (F=1.38, df=7, 308, p>0.10) (
Figure 1).
Second, congruent information was processed more rapidly than incongruent information (image-different condition) (mean=1,211 msec, SD=290, versus mean=1,304 msec, SD=333) (F=12.91, df=1, 44, p<0.001). As in the word and image-same conditions, there were significant effects of distance (F=53.35, df=7, 308, p<0.00001) and group (F=19.69, df=1, 44, p<0.0001) (mean=1,486 msec, SD=227, versus mean=1,029 msec, SD=190, for patients and comparison subjects, respectively) but no significant interactions (group-by-congruency: F=0.18, df=1, 44, p>0.50; group-by-distance: F=1.42, df=7, 308, p>0.10; group-by-congruency-by-distance: F=0.24, df=7, 308, p>0.50) (
Figure 1). This lack of a group-by-congruency interaction was
not attributable to the patients’ overall slowness (additional analyses with percentage change is available from the authors).
Third, words were processed slower than images (mean=1,558 msec, SD=378, versus mean=1,127 msec, SD=196) (F=182.27, df=1, 44, p<0.001). Of interest, there was a group-by-condition (words, images) interaction because patients were disproportionately slow when making decisions about words (F=37.36, df=1, 44, p<0.001).
Accuracy data for all three conditions were consistent with reaction times. Some results were nearly significant.
Discussion
This study capitalized on robust “distance” and “congruency” effects. Each condition produced the expected outcomes: slower responses for judgments involving a smaller size difference, incongruent trials, and decisions about words as compared to images. This pattern was similar in both groups, implying that patients display normal knowledge representation and manipulation in a task requiring cognitive control.
The preserved distance effect in patients indicates successful accessing of distance, a critical semantic feature. Although distance is only one feature in the semantic system, there is no particular reason to assume feature-specific deficits in schizophrenia. Our interpretation of this normal knowledge representation is consistent with previous studies
(8) (but see reference
1).
The congruency effect has been explored frequently in schizophrenia by using either the Stroop
(3) or flanker paradigms with inconsistent results, likely because of methodological differences. Although the current stimuli are quite different from those in traditional paradigms, we nonetheless found highly significant congruency effects, implying that semantic judgment can provide considerable conflict.
In terms of brain regions, the anterior cingulate cortex is reliably engaged in tasks requiring conflict monitoring
(9). In semantic tasks, the selection between conflicting responses has been found to activate the left inferior frontal gyrus
(10). To our knowledge, semantic tasks focusing on conflict have not been applied to functional neuroimaging paradigms in schizophrenia, although it has been shown that the neurodevelopment, morphometry, and function of the anterior cingulate cortex are abnormal in schizophrenia
(11).
Finally, the patients were disproportionately slow when processing words as compared to images, possibly attributable to extra slowing at each information-processing stage rather than being stimulus specific.
Our data provide evidence that some aspects of semantic knowledge are represented normally in schizophrenia and, crucially, that patients can exert cognitive control over this information in a systematic and lawful manner to “override” incongruent information. These findings usefully constrain theories about the way patients with schizophrenia understand and manipulate semantic information to make sense of the world.