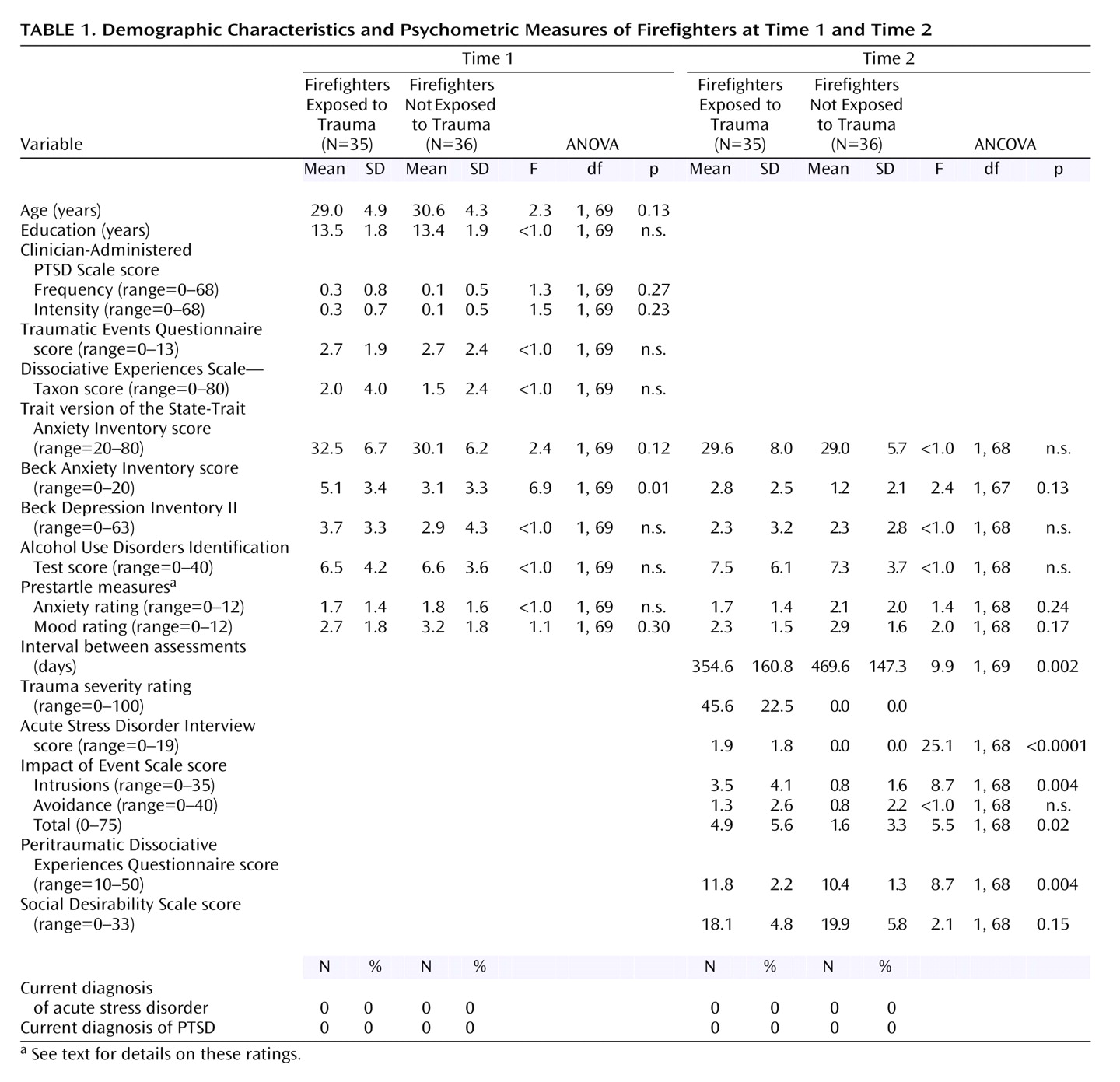
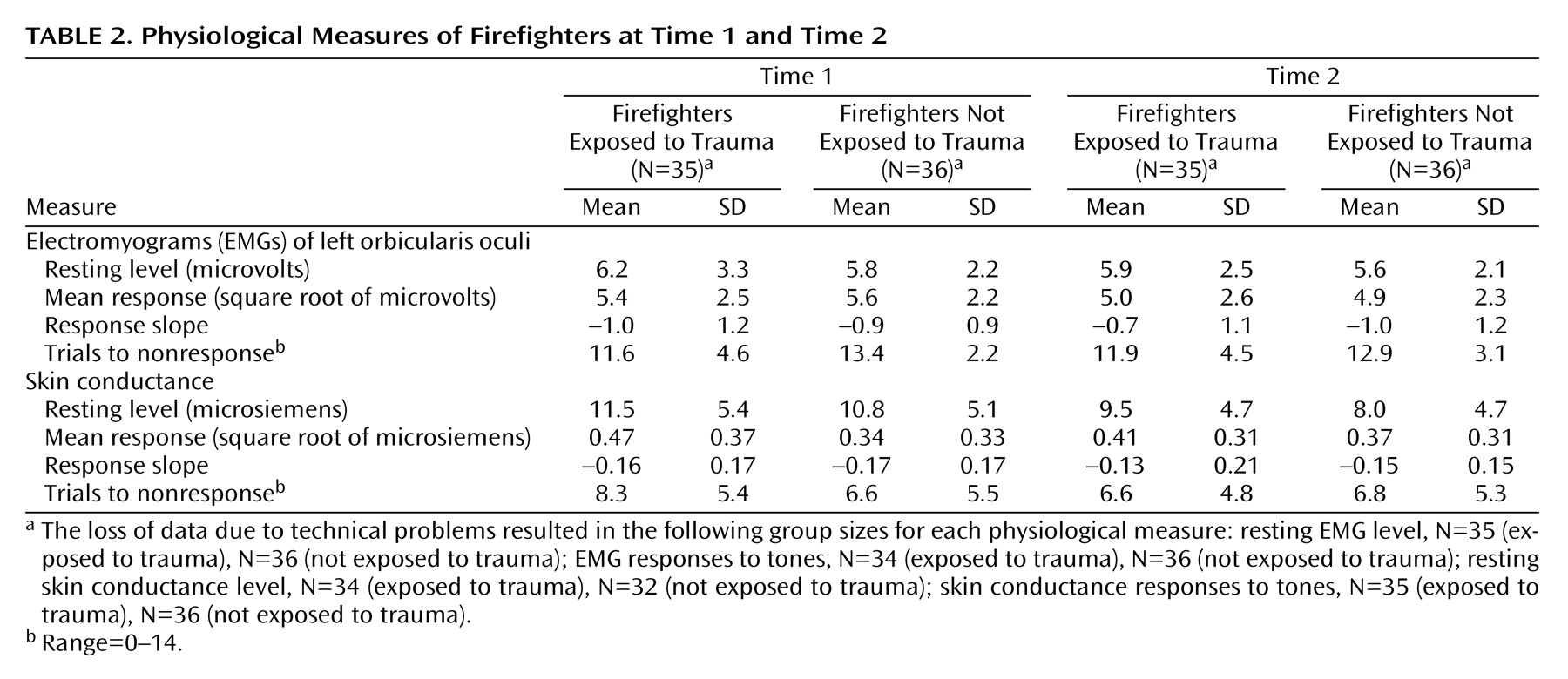
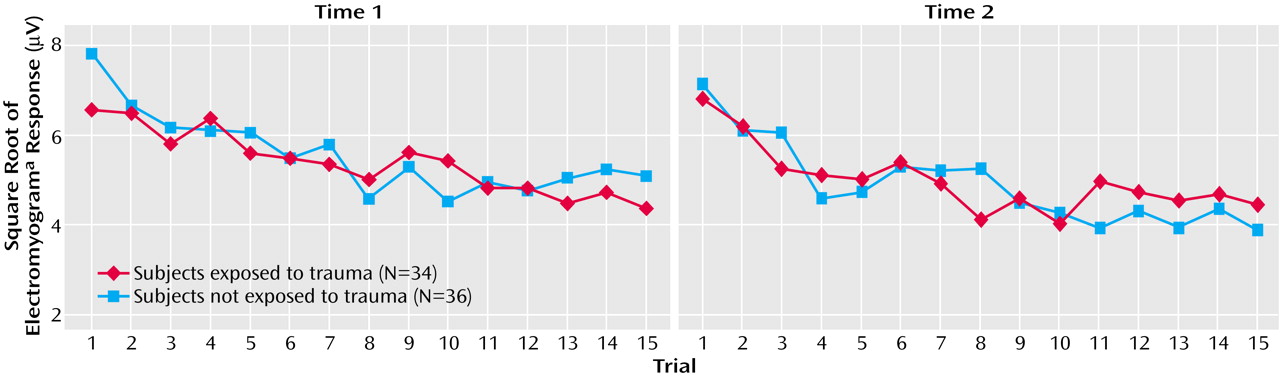
Prediction of Posttrauma Psychophysiology
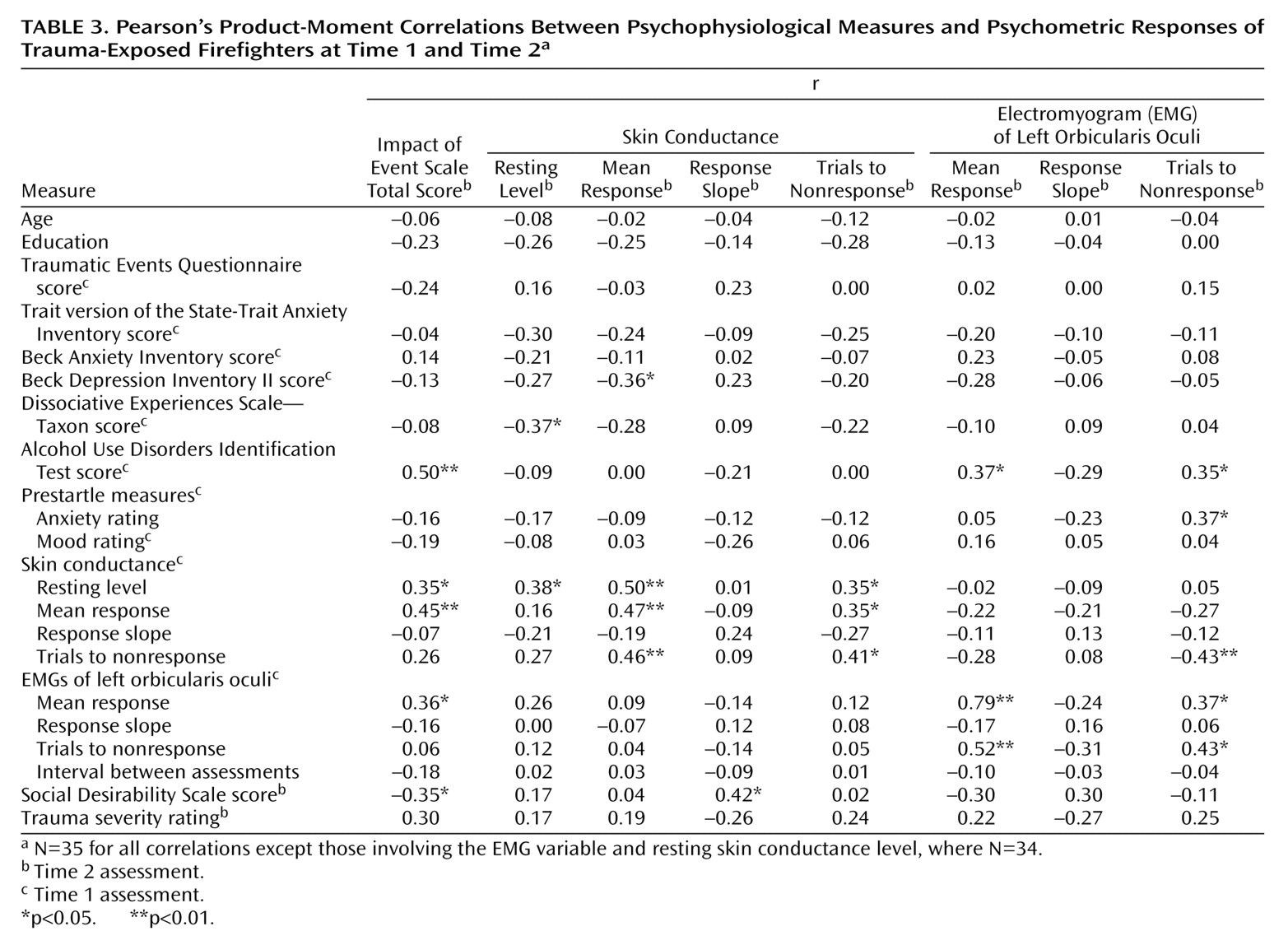
A series of stepwise multiple regression analyses were performed to identify the time 1 predictors of the time 2 psychophysiology measures. Pearson’s product-moment correlations, shown in
Table 3, were calculated for the trauma-exposed group between selected time 2 psychophysiology-dependent variables and time 1 psychometric and psychophysiological variables to determine a subset of potential predictors for the regressions. To predict each time 2 psychophysiological measure, the psychometric and physiological time 1 variable that correlated the highest with the dependent variable was entered with assessment interval into the regression equation. This approach for restricting the battery of potential predictors for regression analyses has been employed in previous prospective research
(42).
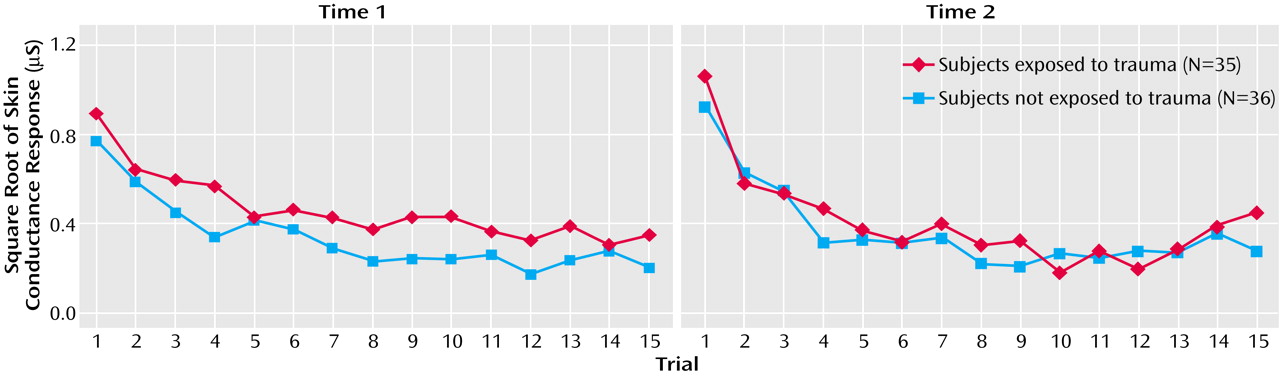
To predict time 2 baseline skin conductance level in the trauma-exposed group, time 1 resting skin conductance level, Dissociative Experiences Scale—Taxon score, and assessment interval were entered into the regression. Resting skin conductance level explained 12% of the variance in time 2 skin conductance level (β=0.38, SE=0.13, t=2.4, df=32, p<0.05), and Dissociative Experiences Scale—Taxon score accounted for a further 10% (β=–0.36, SE=0.02, t=–2.3, df=31, p<0.05). To predict time 2 skin conductance mean response, the time 1 variables entered into the regression equation were resting skin conductance level, Beck Depression Inventory II score, and the interval between assessments. Of these variables, resting skin conductance level accounted for 22% of the variance (β=0.50, SE=0.01, t=3.2, df=32, p<0.005). The subset of time 1 variables entered into the regression equation to predict time 2 number of skin conductance trials to nonresponse was trials to skin conductance nonresponse, years of education, and the interval between assessments. Time 1 number of trials to skin conductance nonresponse was the only significant predictor and explained 14% of the variance (β=0.41, SE=0.14, t=2.6, df=32, p<0.05).
To predict time 2 mean EMG response in the trauma-exposed group, the time 1 variables entered into the regression equation were mean EMG response, Alcohol Use Disorders Identification Test score, and the assessment interval. Of these variables, mean EMG response was the only significant predictor, explaining 61% of the variance (β=0.79, SE=0.11, t=7.3, df=32, p<0.001). To predict time 2 number of EMG trials to nonresponse, the time 1 variables entered into the regression were the number of EMG trials to nonresponse, prestartle anxiety rating, and the assessment interval. Time 1 number of trials to EMG nonresponse was the only significant predictor and accounted for 16% of the variance (β=0.43, SE=0.16, t=2.7, df=32, p<0.05).
An identical series of multiple regression analyses was performed for the non-trauma-exposed group, using the same predictors employed for the trauma-exposed group analyses. Time 1 number of trials to skin conductance nonresponse was the only significant predictor of time 2 trials to skin conductance nonresponse, explaining 18% of the variance (β=0.45, SE=0.15, t=3.0, df=34, p<0.01). The only significant predictor of time 2 mean EMG response was time 1 mean EMG response, which accounted for 44% of the variance (β=0.68, SE=0.13, t=5.4, df=34, p<0.001). In the non-trauma-exposed group, there were no significant predictors of time 2 resting skin conductance level, mean skin conductance response, or trials to EMG nonresponse from the time 1 variables entered into the regression equations.