Most schizophrenia patients have a deficit in auditory sensory gating
(1–
3). This deficit in P50 auditory gating, which appears to be mediated by the α-7 nicotinic receptor, is not improved by conventional antipsychotic treatment
(2). Nicotine briefly improves P50 auditory gating in schizophrenia patients, probably because the α-7 nicotinic receptor desensitizes rapidly
(4). Another way to enhance cholinergic transmission in this pathway is to use ondansetron, an antagonist of the serotonin type 3 (5-HT
3) receptor. The 5-HT
3 receptor tonically inhibits the release of acetylcholine
(5). In rats, 5-HT
3 antagonists cause release of acetylcholine, which acts at the α-7 nicotinic receptor and improves P50 auditory gating in the hippocampus
(6). The 5-HT
3 receptors also inhibit GABA
B inhibitory interneurons. Therefore, 5-HT
3 blockade would be expected to potentiate the action of GABA
B interneurons.
Ondansetron also facilitates release of a variety of neurotransmitters—not only acetylcholine but also norepinephrine and dopamine. Excessive catecholaminergic release exacerbates psychoses and disrupts P50 sensory gating
(3). Ancillary catecholaminergic blockade may be required to permit ondansetron’s positive effects on acetylcholine release to be therapeutically useful. Its effects on P50 sensory gating have not been studied previously. This study examined the effects of ondansetron, a highly selective 5-HT
3 antagonist, on the P50 auditory evoked potential.
Method
Eight stable schizophrenia patients (four men and four women, mean age=41.5 years, SD=5.9) were studied on two occasions, 1 week apart. Six were taking conventional antipsychotic medications; one was also taking a low dose of olanzapine, and one subject was taking only olanzapine. All had impaired baseline P50 auditory gating. All gave informed consent before participation in the study.
Subjects were given a single dose of ondansetron (16 mg) or placebo in a double-blind, placebo-controlled, randomized and balanced crossover design. Serial measurements of the P50 evoked potential were done at baseline and 1 hour, 2 hours, and 3 hours after receipt of placebo or ondansetron. Subjects were assessed with the Positive and Negative Syndrome Scale
(7) and the Brief Psychiatric Rating Scale (BPRS)
(8).
Details of the paired-click recording paradigm have been described previously
(9). The P50 potential was identified and measured by using a previously described computer algorithm
(9). The amplitude of the P50 test wave was divided by the amplitude of the P50 conditioning wave, expressed as a percentage: the P50 ratio. Subjects were given no special instructions concerning the clicks they were hearing.
Both parametric and nonparametric methods were used to evaluate the data. An a priori comparison was made for the P50 ratio at 2 hours posttreatment, which corresponds to peak plasma ondansetron levels for oral ondansetron in most subjects (per the United States Pharmacopeia Drug Information [USP DI], 2001) by Student’s t test (two-tailed). Other t tests were Bonferroni corrected.
Results
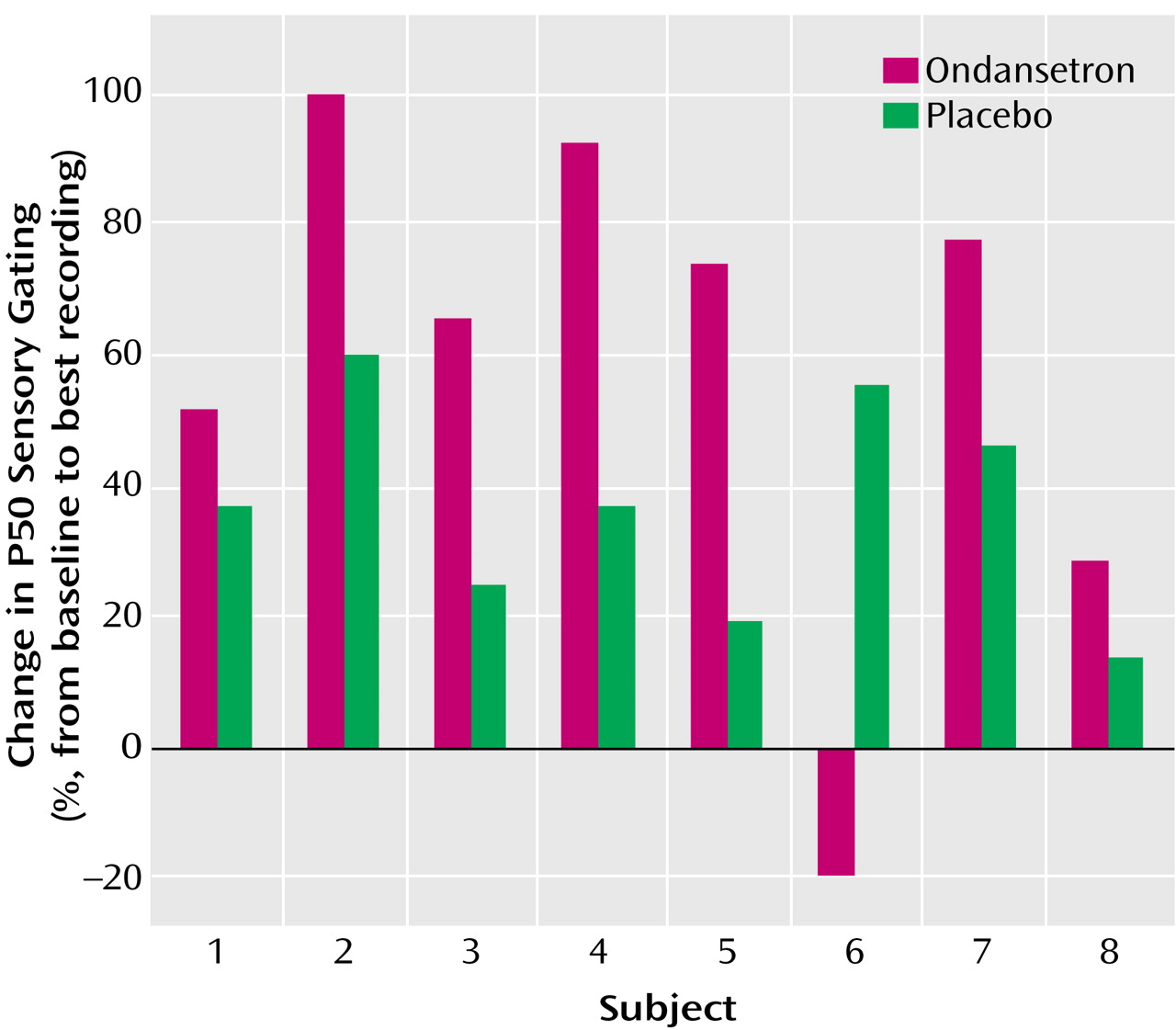
Ondansetron significantly enhanced P50 auditory gating compared with placebo (mixed effects, type III, analysis of variance [ANOVA]: F=14.54, df=1, 55, p=0.0003) (
Figure 1). A planned separate variance Student’s t test at 2 hours was significant (ondansetron: mean=41.4%, SD=39.7%; placebo: mean=80.2%, SD=21.3% [t=–2.43, df=10.7, p<0.04]). The effect of ondansetron tended to persist beyond the 2-hour time point to 3 hours (ondansetron: mean=51.9%, SD=43.1%; placebo: mean=87.3%, SD=14.8% [separate variance t test: t=–2.2, df=8.6, p<0.06]).
Test amplitude decreased with ondansetron treatment (mixed effects ANOVA: F=5.97, df=1, 56, p=0.02). There was no effect on either conditioning amplitude or latency, consistent with prior normalization with antipsychotic treatment
(2).
Although all subjects but one had a definite decrease in P50 ratio with ondansetron (
Figure 1), they did not all experience the greatest effect at 2 hours posttreatment. This is consistent with a range of peak plasma concentration and clinical effect of 1–3 hours posttreatment (USP DI, 2001). The single nonresponder was the individual treated solely with olanzapine.
There were no significant effects on BPRS total score, positive or negative symptoms, or anxiety.
Discussion
P50 auditory gating was significantly improved by ondansetron, as evidenced by the decreased P50 ratios of the schizophrenia patients. These results did not occur with placebo. The decrease (improvement) in P50 ratio and significant decrease of the test amplitude, without associated changes in the conditioning amplitude, parallel results seen in longer-term clozapine studies
(10) and more briefly in nicotine studies in schizophrenia patients
(4).
Sirota et al.
(11) found that combining ondansetron with a conventional antipsychotic reduced psychotic symptoms. The Sirota et al. study and ours may both have demonstrated positive effects with ondansetron because the patients were not required to be antipsychotic free, and adequate associated catecholaminergic blockade was therefore maintained. All but one of the patients in this study were taking typical antipsychotics. The subject who was only taking olanzapine was also the only subject who did not demonstrate transient improvement on sensory gating with ondansetron.
The results of this experiment would seem to support a greater role for the usefulness of 5-HT3 receptor blockade combined with catecholaminergic blockade in restoring P50 auditory gating. However, this experiment, by limiting the study to a single 5-HT3 receptor antagonist, does not lend itself to broad generalizations about other possible effective combinations of receptor antagonism.
A similar limitation is that this study only followed a small group of stable outpatients. The results cannot be applied to other populations without further study. Furthermore, the possibility of a dose response has not been addressed. However, to our knowledge, ours is the only study to date to use a double-blind, placebo-controlled design and demonstrate that selective 5-HT3 blockade added to an antipsychotic regimen significantly enhanced P50 auditory gating in schizophrenia patients.