Twelve unmedicated outpatients with PTSD (10 women and two men; mean age=44 years, SD=9) and a matched group of 11 never traumatized, healthy comparison subjects (10 women and one man; mean age=43, SD=8) participated in the study. PTSD status and severity were determined with the Clinician-Administered PTSD Scale. A minimal Clinician-Administered PTSD Scale score of 50 was required for inclusion (mean score=79, SD=19.4). Five PTSD subjects had suffered prepubertal sexual abuse, three had suffered childhood physical/emotional abuse, two had suffered sexual assault as adults, and two had experienced other severe traumatic events as adults. The Structured Clinical Interview for DSM-IV
(8) evaluated the presence of concurrent and lifetime DSM-IV axis I disorders. Patients with current or past comorbid diagnoses of anxiety or major depressive disorder were included, provided that diagnosis of PTSD preceded the comorbid condition. Other DSM-IV axis I disorders precluded participation. Two patients suffered from concurrent major depressive disorder, one had generalized anxiety, and another had specific phobia. Three patients previously suffered from major depressive disorder. Two had been previously treated with antidepressants, and four had received benzodiazepines. Depression and anxiety symptoms were assessed with the Montgomery-Åsberg Depression Rating Scale
(9) (mean=16.2, SD=8.3) and the Hamilton Anxiety Rating Scale
(10) (mean=10.3, SD=3.7).
All participants were free of medical illness or major head trauma and had not been treated with psychotropic drugs in the 3 weeks preceding scanning. Subjects provided written informed consent, as approved by the NIMH Institutional Review Board.
A 120-minute PET study of 5HT1AR binding was acquired by using a GE Advance PET scanner in three-dimensional mode (35 contiguous slices, 4.25 mm plane separation; reconstructed resolution=7 mm full width at half maximum in all planes), bolus intravenous injection of 8 mCi of high specific activity [
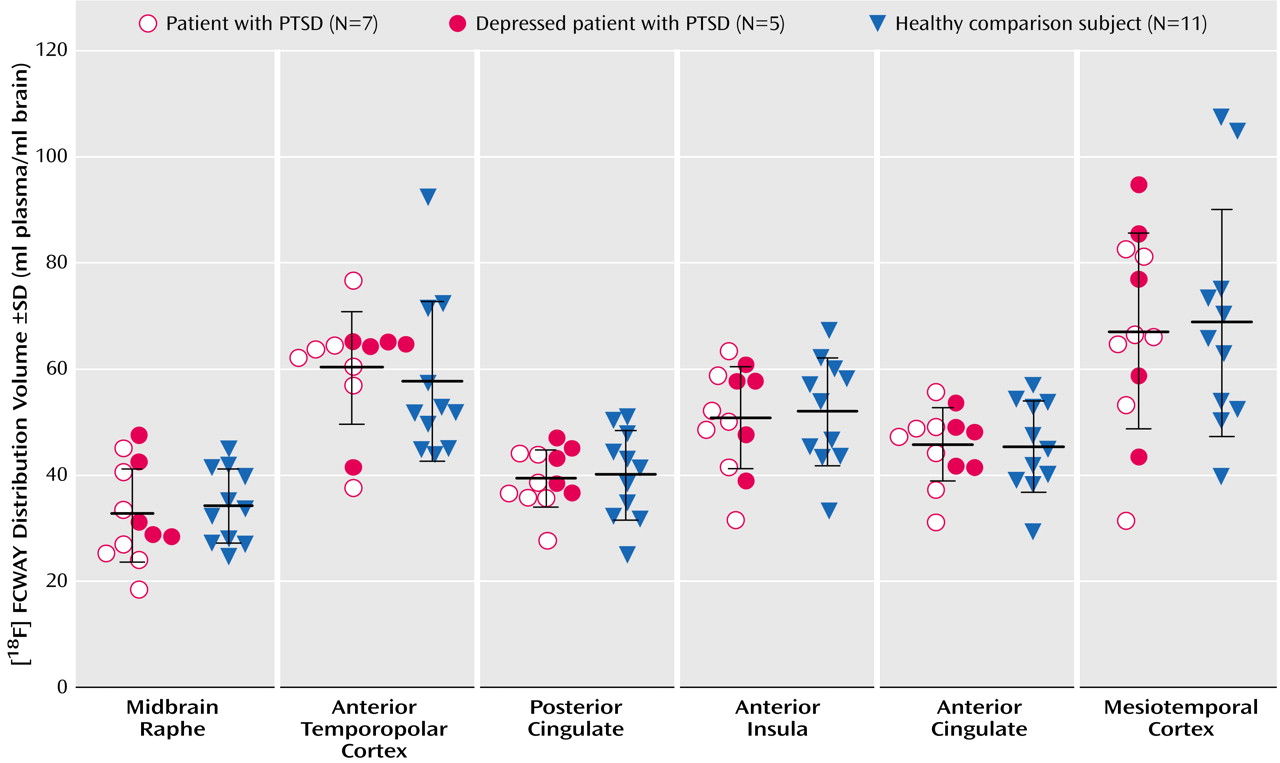
18F]FCWAY, and arterial blood sampling. Magnetic resonance images, obtained for each subject using a 3.0-T GE Signa Scanner and a three-dimensional MPRAGE sequence (TE=2.982 msec, TR=7.5 msec, inversion time=725 msec, voxel size=0.9×0.9×1.2 mm), were coregistered to the PET images to provide an anatomical framework for analysis and to perform partial volume correction of the PET images. Regional 5HT1AR binding was measured in regions of interest defined a priori and transferred to the coregistered PET images using MEDx software (Sensor Systems, Sterling, Va.). The regions of interest were localized to brain structures that contain abundant 5HT1AR concentrations: anterior cingulate cortex, posterior cingulate cortex, anterior insula, mesiotemporal cortex (hippocampus plus amygdala), anterior temporopolar cortex, and midbrain raphe. To correct data for free and nonspecifically bound radiotracer, a reference tissue region of interest was defined in the cerebellum, which is devoid of 5HT1AR (for details of region of interest placement see Neumeister et al.
[5]). The regional [
18F]FCWAY distribution volume (ml plasma/ml brain), corrected for plasma protein binding, and K
1, the delivery rate of [
18F]FCWAY from plasma to tissue (ml/min/min), were obtained by using quantitative tracer kinetic modeling. The mean distribution volume values were compared between groups by using unpaired t tests. The specificity for receptor-specific binding was assessed post hoc by comparing regional binding potentials as (DV
ROI/DV
cerebellum – 1) to factor out the influence of free and nonspecifically bound radiotracer.