Isotretinoin (13-
cis-retinoic acid) is a retinoid that inhibits sebaceous gland functioning, keratinization, and inflammatory responses and is currently approved by the Food and Drug Administration (FDA) for the treatment of cystic acne
(1,
2). Isotretinoin has been prescribed to 2 million patients in the United States and over 8 million patients worldwide and is highly effective for acne. The exact mechanism of action of isotretinoin remains unknown.
In the last several years there has been controversy over the possible role of isotretinoin in the development of depression and suicide
(3–
6). Case reports in the literature describe depression that developed in conjunction with isotretinoin treatment, resolved with discontinuation of the medication, and in some cases returned when the medication was restarted
(7–
14). Estimates of the incidence of depression following treatment with isotretinoin include 1%
(14), 4%
(15), and 6%
(10). Other reports have noted suicidality, behavioral disturbances, and psychotic-type symptoms with isotretinoin treatment in addition to the typical symptoms of depression
(8,
12). Isotretinoin is chemically similar to the retinoid vitamin A, a fat-soluble vitamin stored in high concentrations in the liver. Vitamin A is converted after oxidation to retinoic acid, when it has biological effects. Arctic explorers who fed on polar bear liver developed symptoms of confusion and psychosis. Large doses of vitamin A can have a number of other neurological and mental effects, including fatigue, decreased interest, headache, and diplopia (double vision)
(16,
17). Published case reports of vitamin A toxicity include symptoms of aggression, personality changes, depression, poor concentration, tearfulness, psychotic symptoms, and guilty rumination
(17–
19) that resolved with discontinuation of vitamin A. Among reports to the World Health Organization and the FDA of adverse events associated with acne treatments, adverse events related to depression and suicide have been more common with isotretinoin than with other treatments for acne, such as antibiotics
(4,
11).
The relationship between isotretinoin treatment and depression and suicide, however, remains controversial. Although the manufacturer, on the basis of FDA guidelines, lists depression as a possible side effect, there is no consensus on a causal role for isotretinoin in the development of depression and suicide. The high incidence of depression in the general population makes it difficult to identify small increases specifically related to an additional factor, such as isotretinoin administration. One large epidemiological study did not demonstrate a significantly increased risk for suicide in patients treated with isotretinoin
(3). Some authors have argued that cases of depression associated with isotretinoin administration are merely coincidental
(20) or that isotretinoin actually leads to an improvement in anxiety and depression because of the clearing of disfiguring acne
(21). Studies have shown an improvement in feelings of general well-being or self-image
(22,
23) or in feelings of anxiety
(20,
24–28) among patients with cystic acne following isotretinoin administration, although the findings were more directly related to improvement in measures of patient satisfaction, rather than clinical symptoms of depression.
To establish a causal role of isotretinoin in the development of depression and suicide, it is critical to establish a plausible biological pathway. This requires that isotretinoin must enter the central nervous system (CNS) and have an effect on the functioning of brain areas and neurochemical systems that mediate depression. Retinoids have important effects on the developing brain in animal studies
(29,
30), and use of isotretinoin during pregnancy has long been known to result in CNS defects in newborns
(31). Multiple positron emission tomography (PET) and single photon emission computed tomography (SPECT) studies have shown low metabolism and/or blood flow at baseline in depressed subjects in the left
(32–
35) and bilateral
(36–
43) dorsolateral prefrontal cortex and medial prefrontal cortex/anterior cingulate
(34,
38,
41–48) or blunted activation with cognitive tasks in the anterior cingulate
(49,
50). Other PET and SPECT studies of patients with unipolar depression showed low metabolism and/or blood flow in the caudate
(36–
41,
51,
52), thalamus
(37), temporal cortex
(37,
38,
51,
53,
54), parietal cortex
(34,
40,
51), and left putamen
(37). Experimental induction of depression resulted in a specific decrease in metabolism in the orbitofrontal cortex (part of the prefrontal cortex)
(55,
56). The purpose of the current study was to assess the effects of isotretinoin treatment on brain functioning. We hypothesized that treatment with isotretinoin, but not antibiotic, would be associated with a decrease in orbitofrontal cortical brain metabolism as measured with [
18F]fluorodeoxyglucose (FDG) PET.
Method
Subjects
The study participants included 28 healthy men and women between the ages of 18 and 50 years with treatment-resistant acne, as defined by a failed 3-month antibiotic trial, who were seeking a second trial of an antibiotic or isotretinoin. Subjects were recruited by advertisement. They were not randomly assigned to treatment with isotretinoin or placebo; they had decided with their physicians to take either a second trial of an antibiotic or a trial of isotretinoin. Because of the side effects of isotretinoin (severe skin dryness) it was decided that it would not be possible to blind the subjects or the raters to treatment condition. Subjects with serious medical or neurological illness, organic mental disorder or current psychiatric illness according to the Structured Clinical Interview for DSM-IV
(57), premenstrual dysphoric disorder, current alcohol or substance abuse or dependence, retained metal that would prevent magnetic resonance imaging (MRI) scanning, a history of head trauma or loss of consciousness, a history of cerebral infectious disease, or dyslexia were excluded. Postmenopausal women were excluded.
This project was approved by the Emory University Human Investigation Committee. All subjects provided written informed consent for participation. The subjects were paid for their participation.
Each subject received a PET brain scan at baseline and again after 4 months of treatment with an antibiotic (N=15) or isotretinoin (N=13). The subjects were treated by their outpatient physicians with 1 mg/kg of isotretinoin or with an antibiotic in a standard 4-month course of treatment for acne. The antibiotics used included doxycycline (N=10), minocycline (N=2), tetracycline (N=2), and erythromycin (N=1). The subjects continued treatment until they completed the second PET scan.
Psychiatric diagnoses were established with the Structured Clinical Interview for DSM-IV, Patient Edition (SCID-P)
(57). There were no current psychiatric conditions in any subject according to the SCID-P. Two of the subjects in the antibiotic group had past psychiatric conditions, including a past history of major depression in one and a past history of bulimia and alcohol dependence in another. None of the isotretinoin subjects had a current or past psychiatric disorder. None of the subjects had current alcohol or substance abuse or dependence.
Behavioral Assessment
Symptoms of depression were measured by using the Hamilton Depression Rating Scale at baseline and every month after the initiation of treatment
(58). The severity of acne was measured with a clinician-administered acne questionnaire before and after treatment, on a scale of 0 (no acne) to 6 (very bad acne). The patients’ subjective evaluations of the severity of acne on the face and back and their feelings of depression related to their acne, on a scale of 0 (not at all) to 4 (very severe), were recorded before and after treatment by using the Skindex questionnaire
(59). The Skindex is a 16-item self-report questionnaire with questions about emotional, functional, and symptomatic aspects of acne that has been validated for use in acne patient populations. It was also administered before and after treatment. The patients were also evaluated during the course of treatment for symptoms related to treatment.
PET and MRI Scanning
Two PET scans of resting brain metabolism were performed 4 months apart, before and after treatment with isotretinoin or antibiotic. The PET scans took place at 11:00 a.m. The subjects were scanned with an ECAT EXACT 921 PET camera (CTI Molecular Imaging, Knoxville, Tenn.). The ECAT EXACT has an axial field of view of 16.2 cm, the total system sensitivity is 216 kcps/Ci per ml for a 20-cm cylinder phantom in two dimensions, and the approximate axial resolution is 5.0 mm
(60). Each subject was placed in a preparation room adjacent to the PET scanner room, and an intravenous line was inserted in the hand and warmed with a heating pad for measurement of arterialized venous blood samples. This method has been shown to yield metabolic values equivalent to those obtained by arterial line placement
(61). The subject then received an intravenous injection of 10 mCi (370 MBq) of FDG in a single bolus. Twenty-three arterialized venous blood samples were obtained at multiple time points after injection for measurements of radioactivity in the plasma, which were used for construction of a plasma time activity curve. Three blood samples were also obtained for measurement of plasma glucose concentrations. The subject was then placed in the scanner with his or her head held in a head holder to minimize patient motion. The head was positioned with the canthomeatal line parallel to the external laser light. Following positioning within the camera gantry, postinjection transmission data were collected by using rod windowing with three orbiting
67Ga/
68Ge rod sources
(60). These data were used to correct the emission data for attenuation due to overlying bone and soft tissue. The subject underwent emission scanning of the brain over the 40–60 minutes after injection with his or her eyes open in a dimly lit room. Brain and tissue measurements were used to estimate the cerebral glucose metabolic rate (in milligrams per minute per 100 milliliters)
(62,
63). In one patient blood samples could not be obtained, and this patient’s data were used only for the analysis of the ratio of regional metabolism to whole brain metabolism. A 20-cm cylindrical fluid-filled phantom with a known amount of radioactivity was scanned in order to obtain calibration factors for conversion of native pixel values into units of millicuries per milliliter.
MRI scans were obtained in all subjects for coregistration with the PET scans and determination of regions of interest from the MRI scans resliced to correspond to the PET slices. MRI scans in the same subjects were obtained on a 1.5-T Philips Gyroscan Intera device (Philips Medical Systems, Andover, Mass.). Axial images were acquired with a T1-weighted gradient echo three-dimensional sequence with TR=35 msec, TE=12 msec, flip angle=35°, number of excitations=2, matrix=256×256, field of view=22 cm, and slice thickness=3 mm.
Image Processing and Analysis
The PET and MRI scans were transferred to a workstation for analysis. A surface-matching algorithm and the ANALYZE software package (Mayo Clinic, Rochester, Minn.) were used for coregistration of images
(64). Brain surfaces from PET and MRI were matched by using this program. The MRI scan was resliced to correspond to the PET slices. Using this technique
(65), we have shown a registration error of 2.86 mm. Regions of interest were drawn on the resliced MRI scans by a blinded rater using specific criteria based on anatomical landmarks with a method that we have shown to be highly reliable
(66). Multiple brain regions were selected for analysis, including the temporal cortex, inferior, middle, and superior frontal gyri, superior portion of the dorsolateral prefrontal cortex, thalamus, putamen, caudate, occipital cortex, subcallosal gyrus, orbitofrontal cortex, anterior cingulate, postcentral gyrus, hippocampus, amygdala, and midbrain. These regions correspond to the regions measured in our prior studies of neural findings associated with a return of depressive symptoms induced by tryptophan depletion
(55) and alpha-methylparatyrosine
(56), since a primary aim of the current study was to replicate the brain findings of those prior studies. Global brain metabolism was calculated as the mean of brain tissue activity in all slices, including gray and white matter and the ventricular spaces.
Data Analysis
The brain regions were separated into those that were and were not hypothesized to change with isotretinoin. The region most consistently affected in our two prior studies of depression was the orbitofrontal cortex
(55,
56). This region has also been reported to be smaller in volume in depressed patients than in comparison subjects
(67,
68).
The data were analyzed to determine differences in the changes from pre- to posttreatment in regional brain metabolic rates between the isotretinoin and antibiotic treatment groups, by using repeated-measures analysis of variance with time (before and after treatment) as the repeated factor and treatment status (isotretinoin versus antibiotic) and hemisphere (left versus right) as factors in the analysis. The interaction between treatment status and time was examined in this model. Secondary analysis examined the ratio of regional to whole brain metabolism, and it added baseline global metabolism as a factor in the model. Bonferroni corrections were performed to correct for multiple comparisons (p=0.05/12=0.004).
Correlations between brain metabolism and behavioral variables were also examined by comparing the relationships between the changes, from before to after treatment, in scores on the Hamilton depression scale, clinician-administered acne questionnaire, Skindex, and acne severity self-report and the change in regional brain metabolism (mean of left and right orbitofrontal metabolism) in the isotretinoin and antibiotic treatment groups. Corrections were made for multiple comparisons (p=0.05/4=0.0125). Analyses also examined the correlation between baseline orbitofrontal brain metabolism and age, education, and behavioral factors related to self-assessment of acne severity, depression, and emotions related to acne, measured with the Skindex. Corrections were made for multiple comparisons (p=0.05/6=0.008).
The data were analyzed by using the SAS System for Windows V8 (SAS, Cary, N.C.).
Results
Recruitment and Demographic Factors of Subjects
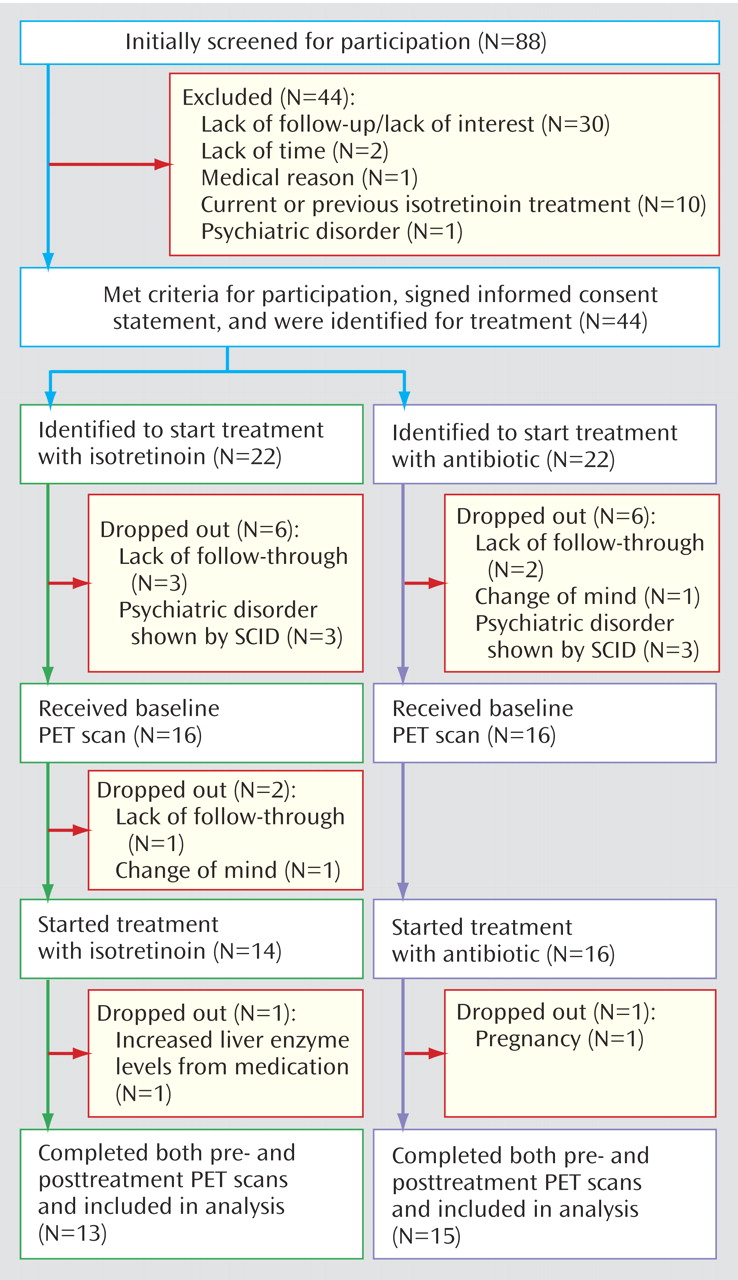
Eighty-eight subjects were initially screened for participation in the study. Of these, 44 met the criteria for participation according to the initial screening, signed informed consent statements, and were identified as acne patients who were beginning a second trial of antibiotic (N=22) or who were going to be treated with isotretinoin (N=22) (
Figure 1). Twenty-eight subjects completed participation in this protocol, including pre- and posttreatment imaging. Of these, 13 were treated with isotretinoin and 15 with antibiotics.
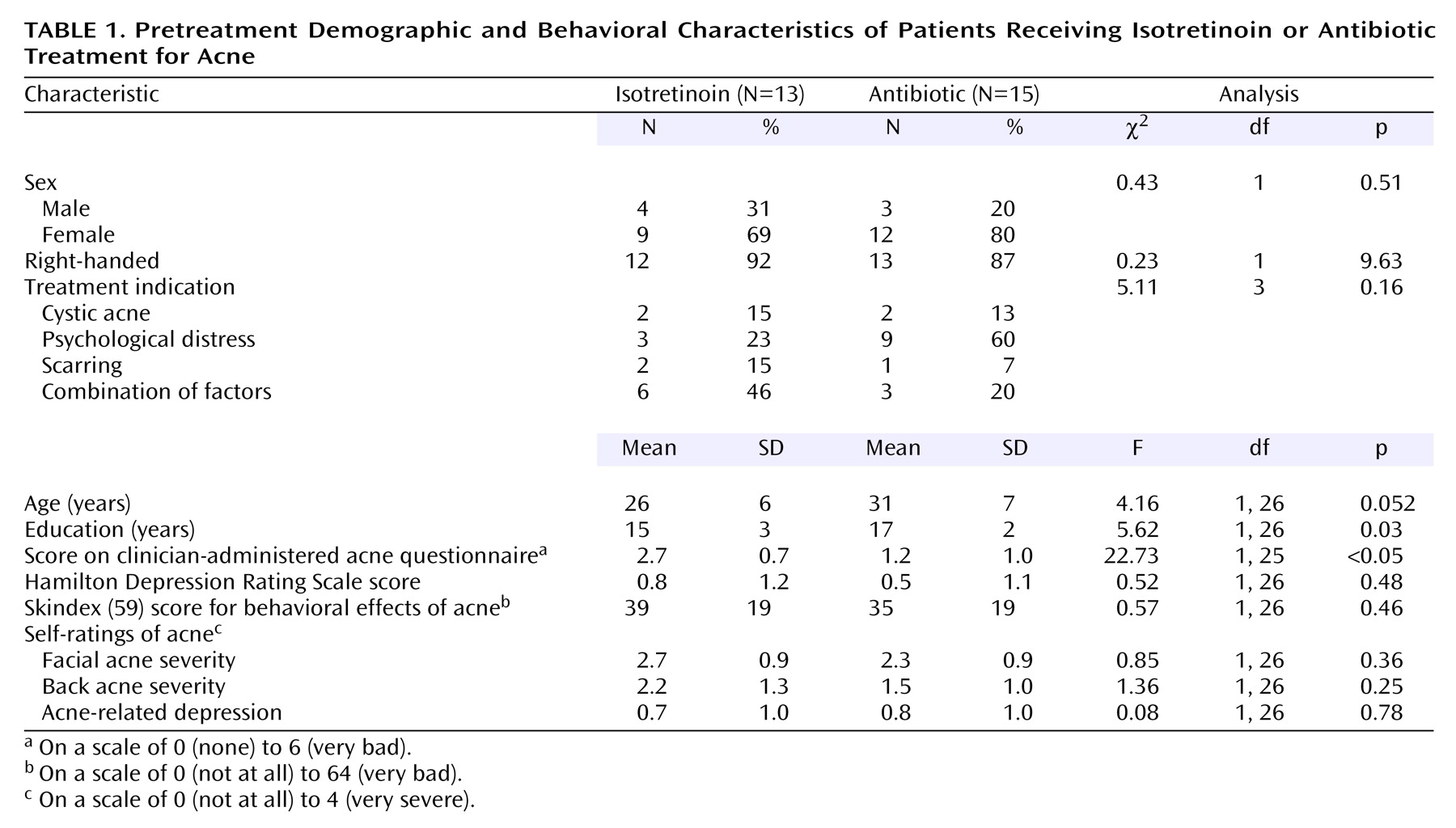
Demographic, behavioral, and acne-related variables related to the two treatment groups before the initiation of treatment are presented in
Table 1. The isotretinoin subjects had fewer years of education and were younger, but the latter difference was not statistically significant. They did not differ significantly from the antibiotic group in their reasons for receiving treatment (cystic acne, psychological distress, scarring, or a combination). According to the clinician ratings, the isotretinoin subjects had more severe pretreatment acne than the subjects receiving antibiotics. However, according to the self-ratings there were no differences in acne on the face or back or in feelings of depression related to the acne. There were no differences between the two groups in behavioral, emotional, and functional effects of acne as measured by the Skindex. There were also no differences in baseline depressive symptom levels as measured by the Hamilton depression scale.
Effects of Isotretinoin on Regional Cerebral Metabolism
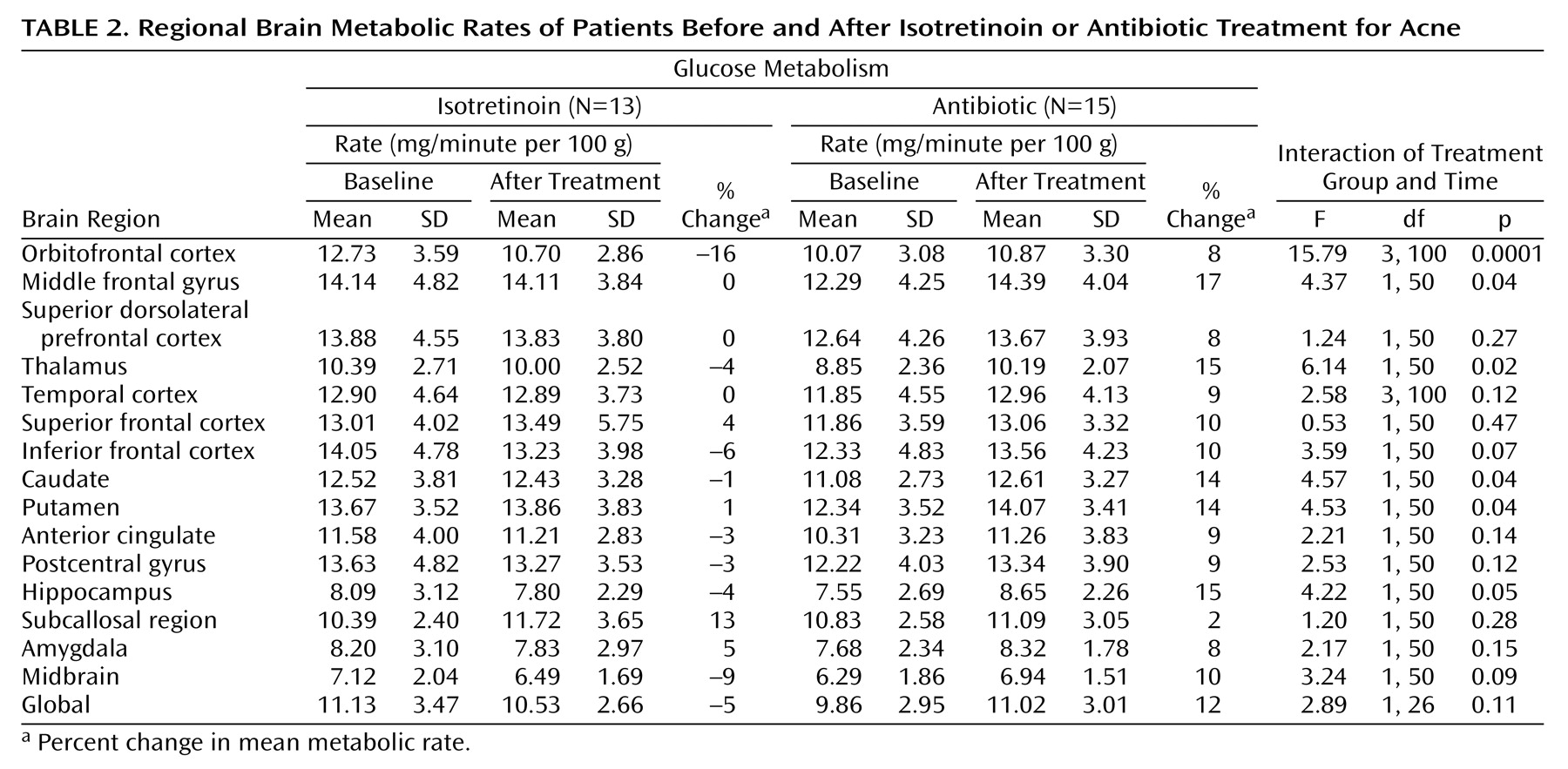
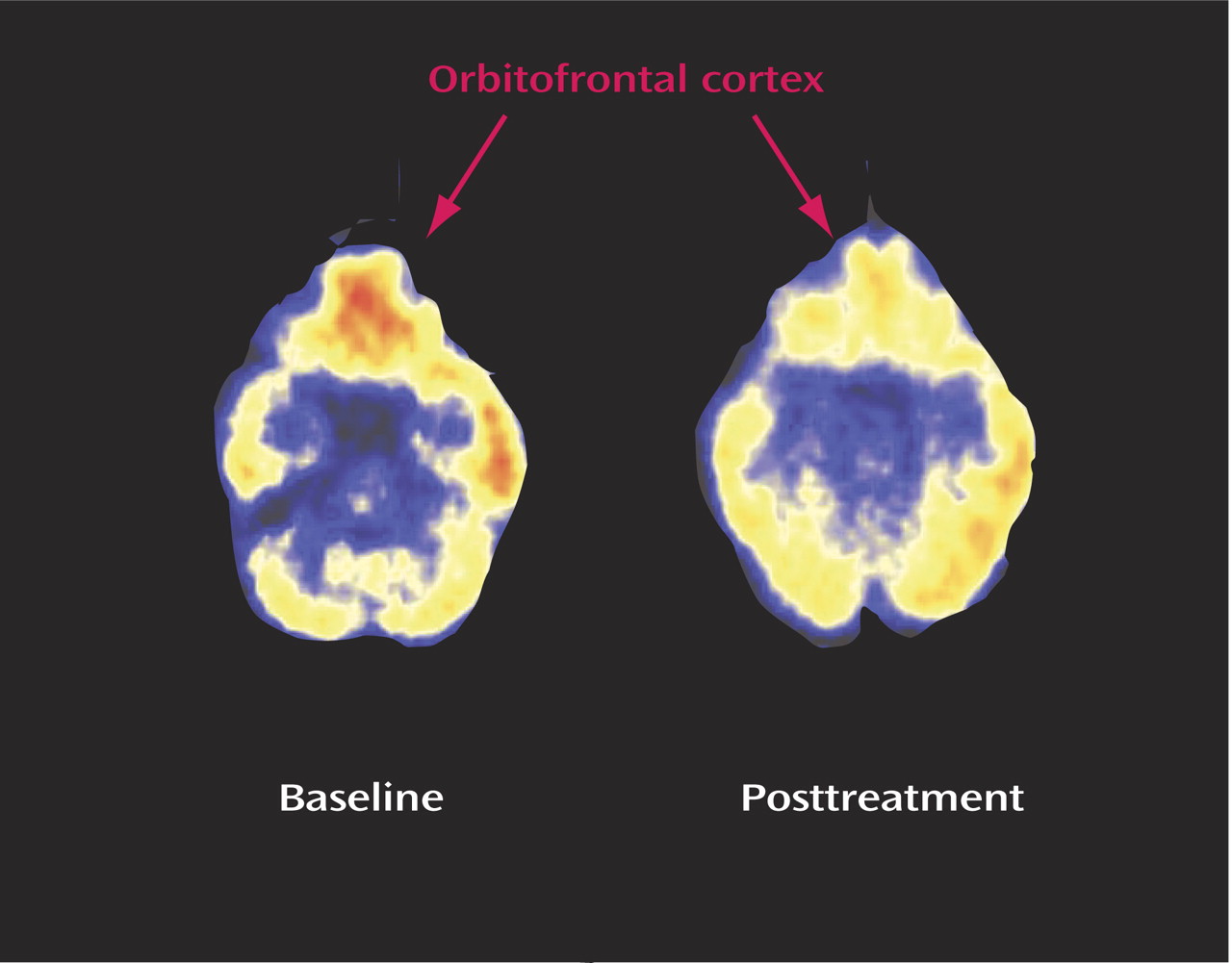
Administration of isotretinoin but not antibiotic was associated with decreased brain metabolism in the orbitofrontal cortex after 4 months of treatment (
Figure 2,
Figure 3). This effect was seen for both absolute metabolism (
Figure 2,
Table 2) and for the ratio of orbitofrontal to whole brain metabolism (F=4.64, df=1, 110, p<0.05). A secondary analysis included pretreatment whole brain metabolism in the model and also showed greater reductions in orbitofrontal metabolism after treatment in the isotretinoin group than in the antibiotic group (F=9.66, df=1, 104, p=0.002). The value for the interaction between treatment status (isotretinoin versus antibiotic) and time (before and after treatment) and the percentage change in the mean metabolic value with treatment are presented for each region in
Table 2. Differences in functioning between the groups at the p<0.05 level were also seen for the middle frontal gyrus, thalamus, hippocampus, caudate, and putamen. These differences were not significant after correction for multiple comparisons, however, and there were no differences after we corrected for whole brain metabolism by examining differences in the ratios of regional to whole brain values.
The mean pretreatment rate of metabolism in the orbitofrontal cortex was higher for patients in the isotretinoin group than for those in the antibiotic group (F=2.05, df=7, 107, p=0.03). This was not hypothesized a priori and was not significant after correction for multiple comparisons.
Relationship Between Behavior and Brain Metabolism
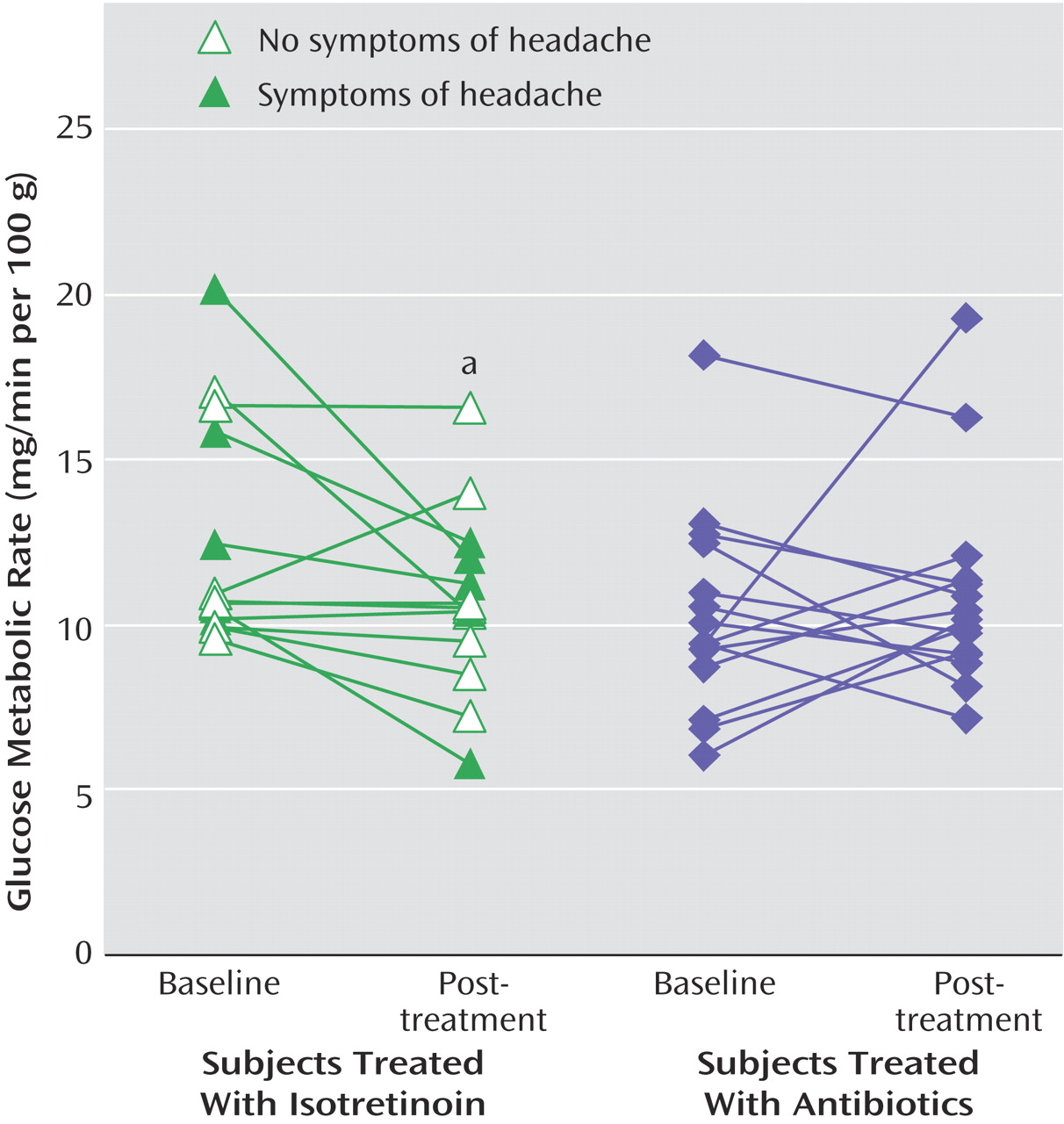
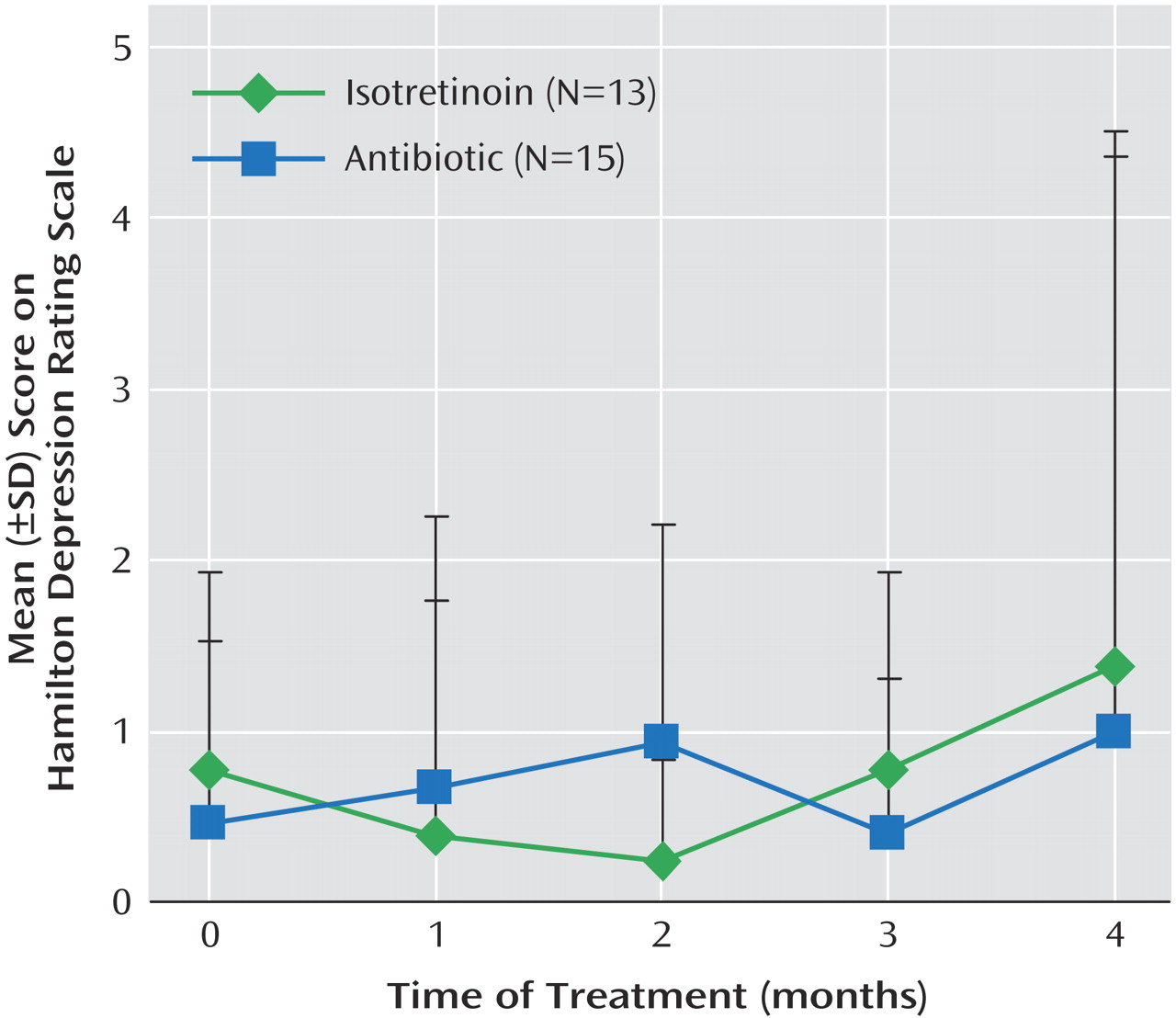
Five patients treated with isotretinoin had symptoms of headache. These patients also had subtle changes in irritability and/or mood as assessed by self, family, or the research staff. These subjects all had decreases in brain metabolism with isotretinoin administration (
Figure 2). A representative subject is shown in
Figure 3. However, these subjects did not show clinically significant depression as assessed with the Hamilton depression scale (
Figure 4). One subject in the isotretinoin group and one in the antibiotic group had a clinically significant increase in depression as measured by the Hamilton scale (greater than 9-point increase); however, there were no significant increases in Hamilton depression scores in the groups as a whole and no significant differences between groups.
Patients in the isotretinoin group had more severe acne as rated by clinician assessment at baseline (F=18.80, df=1, 25, p<0.05), and they had a greater improvement with isotretinoin treatment, as indicated by less severe acne according to the clinician-administered questionnaire after treatment than before treatment, than did patients receiving antibiotics (F=22.73, df=1, 25, p<0.05). There was also a greater improvement with isotretinoin in self-reported acne (F=2.62, df=4, 88, p<0.05). There were no differences in change in feelings of “depression related to acne” between the groups. There was no relationship in either the isotretinoin or antibiotic group between baseline orbitofrontal cortical metabolism and depression as measured with the Hamilton scale, self-reported or clinician-assessed acne severity as measured with the analogue scales, feelings of depression related to acne as measured with the analogue scale or the Skindex, effects of acne on work functioning as measured by the Skindex, or overall psychological effects of acne as measured with the Skindex. The decrease in orbitofrontal cortical metabolism with treatment in the isotretinoin group was correlated with a single item of the Skindex at baseline, effect of acne on ability to work (r=–0.67, N=9, p=0.03). This correlation was not significant after correction for multiple comparisons. There was no relationship between “worrying about skin condition” as measured with the Skindex and orbitofrontal metabolism at baseline or with treatment. There was no correlation between acne-related depression at baseline and decrease in orbitofrontal cortical metabolism with isotretinoin.
There was no correlation between age or education and baseline orbitofrontal cortical metabolism or change in metabolism with treatment.
Discussion
A 4-month treatment trial with isotretinoin was associated with a decrease in brain functioning in the orbitofrontal cortex, a brain region implicated in depression. These changes were not seen after a similar course of treatment with an antibiotic. After correction for differences in whole brain metabolism, this effect was specific to the orbitofrontal cortex. The greatest magnitude of decrease was observed in subjects who developed symptoms of headache during the course of treatment with isotretinoin. Isotretinoin was not associated, however, with any changes in depressive symptom severity as measured with the Hamilton depression scale.
Isotretinoin has a variety of effects on brain neurochemical systems
(69–
71). Retinoids modulate gene expression in the brain in a broad spectrum and have effects on several neurochemical systems, including the dopamine system, which has been hypothesized to play a role in dysregulation of mood and emotion
(70). High levels of the enzyme involved in retinoid synthesis, aldehyde dehydrogenase, are found in mesostriatal and mesolimbic dopamine pathways
(72,
73). Dopamine mesocortical pathways involve release of dopamine transmitter in the orbitofrontal cortex and other parts of the prefrontal cortex. Isotretinoin may influence these pathways. Administration of retinoids causes changes in dopamine receptors
(74), while genetic mutations of retinoid receptors are associated with deficits in dopamine receptors as well as mesolimbic dopamine functioning
(75). Retinoids are associated with an inhibition of neurogenesis in the hippocampus
(76), a brain area with connections to prefrontal cortical areas, including the orbitofrontal cortex. Inhibition of neurogenesis in the hippocampus has been hypothesized to play a role in depression
(77–
80). Retinoids also have effects on brain trophic factors
(81). These findings have led to the hypothesis that retinoids play a role in the development of psychiatric disorders
(69,
82).
A number of limitations of the current study are worthy of mention. This was a pilot study designed to evaluate the possibility of an effect of isotretinoin on brain functioning. For this reason the study group was small, which may have contributed to the fact that we did not observe treatment-related changes in mood as assessed by the behavioral ratings in this study. Some patients, however, complained of headache with isotretinoin, and these patients exhibited greater decreases in orbitofrontal brain metabolism during isotretinoin treatment. Because of the costs of isotretinoin we were unable to pay for this medication for all subjects. Therefore, we were unable to randomly assign subjects to treatment with isotretinoin or antibiotic and were unable to control which antibiotic the subjects were taking. We therefore recruited subjects who were preparing to undergo a second treatment course with an antibiotic or to switch to isotretinoin on the basis of a decision made in conjunction with the subject’s own physician. This method likely contributed to the fact that the isotretinoin subjects had more severe acne at baseline. The isotretinoin group also had less education. We examined a variety of demographic factors, including age, education, psychological distress, and self- and clinician assessments of acne severity, and found no relationships with baseline orbitofrontal metabolism or change in orbitofrontal functioning with treatment. An unexpected finding was a pattern of greater baseline functioning in the orbitofrontal cortex in the isotretinoin group than in the antibiotic group. One might question whether factors in the isotretinoin group, such as increased worrying related to more severe acne, might have contributed to differences in brain functioning. Obsessive-compulsive disorder has been associated with higher orbitofrontal metabolism
(83). However, we did not observe any differences in depression, psychological distress, or even self-rating of acne severity between the two groups before treatment. We also did not find a correlation between orbitofrontal metabolism at baseline or change with treatment and the item related to “worrying about skin condition” on the Skindex. Antibiotics act by inhibiting bacterial protein synthesis. Although they can pass the blood-brain barrier, they are not known to have effects on brain functioning. The most common side effects of antibiotics are nausea, vomiting, and diarrhea
(84), and psychiatric and neurological side effects are much more common in acne patients treated with isotretinoin than those treated with antibiotics. For this reason it is unlikely that effects of antibiotics on brain functioning would account for the results of the current study. We excluded subjects with a history of mental illness. This may have involved exclusion of subjects who were prone to the development of depression and may limit the generalizability of the findings. In summary, a randomized, placebo-controlled study would provide more definitive results than the current study.
To our knowledge, this is the first study of the effects of isotretinoin on human brain functioning. The findings suggest that isotretinoin may affect brain functioning, providing a possible biological mechanism by which isotretinoin treatment could lead to depression in a minority of vulnerable acne patients. Future studies using randomized designs to evaluate the effects of isotretinoin on brain functioning are warranted.