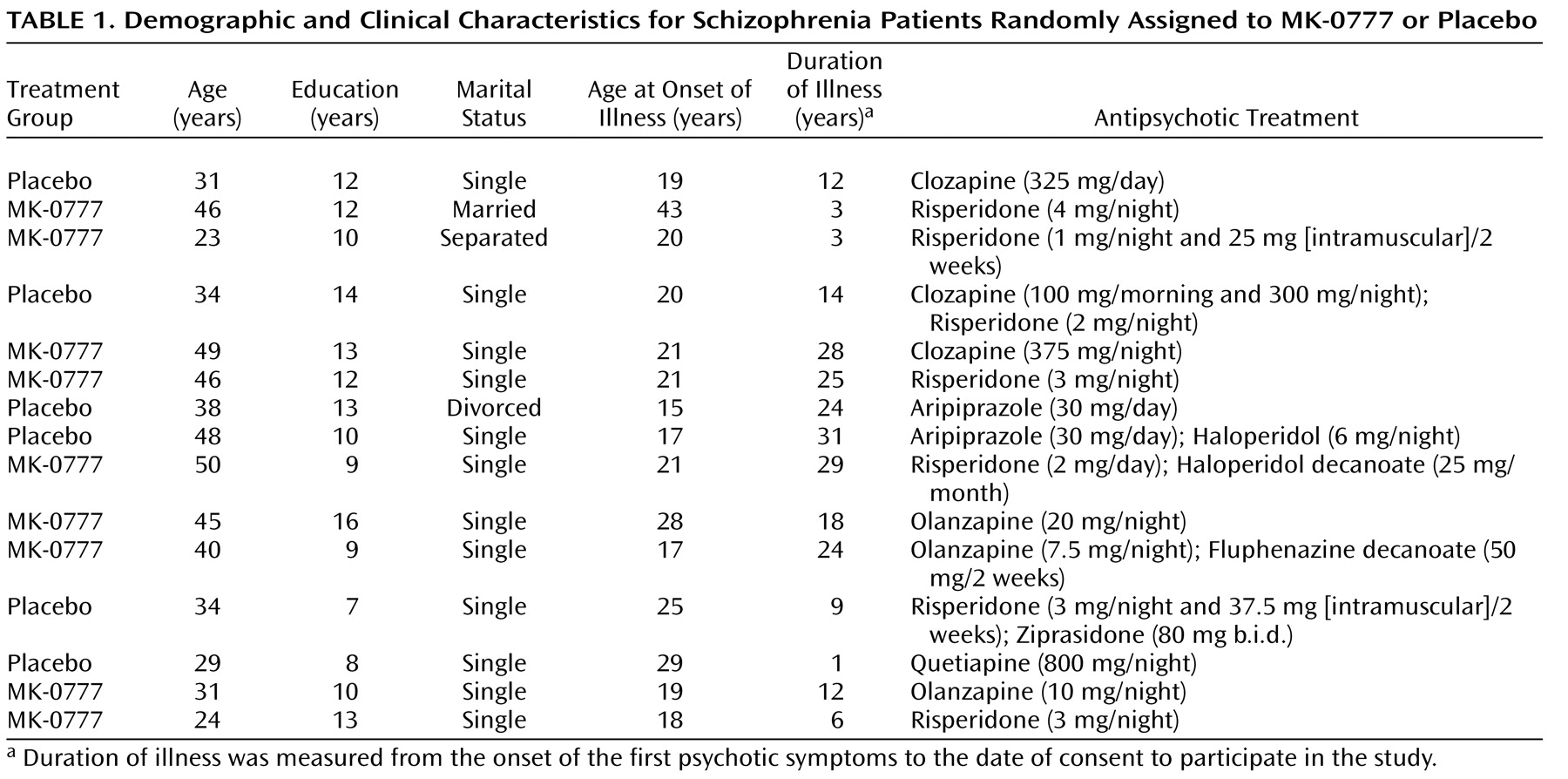
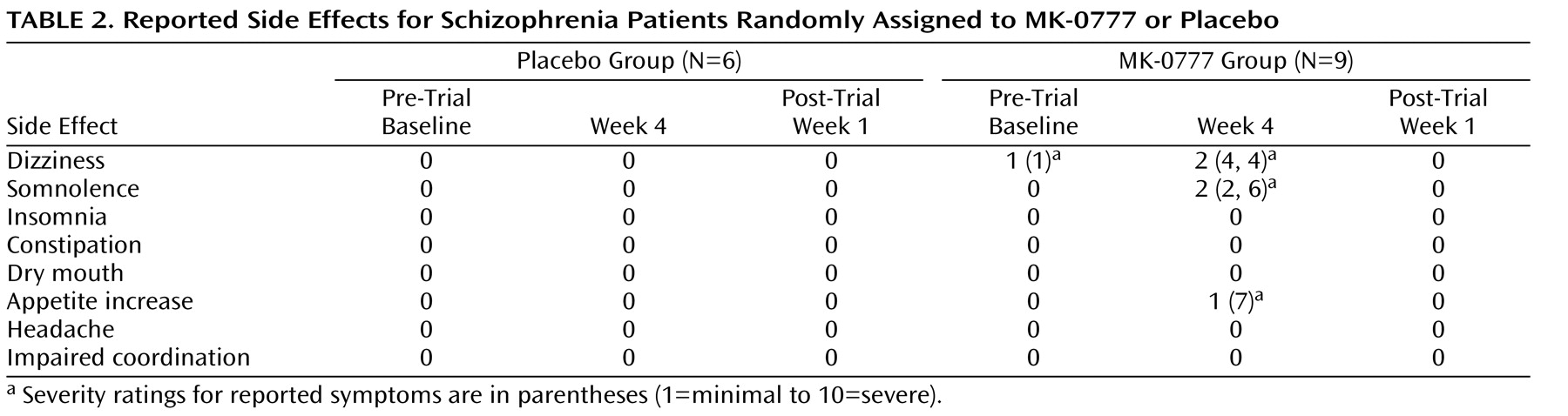
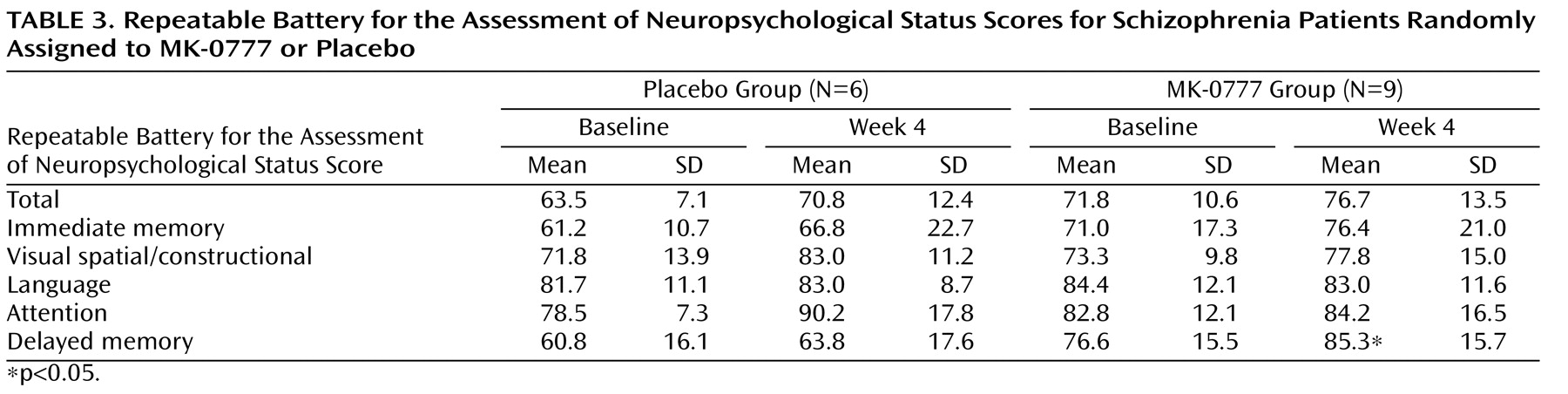
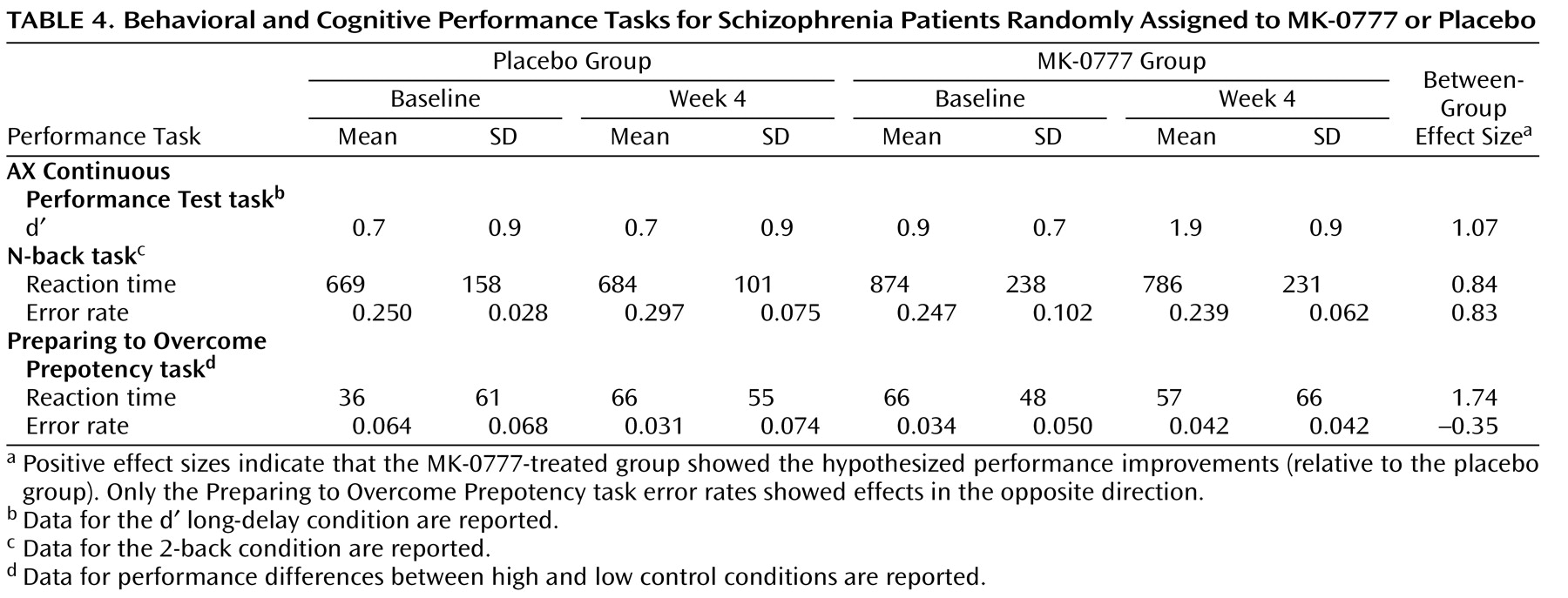
In individuals with schizophrenia, a characteristic pattern of cognitive deficits occurs with high frequency, is relatively stable over time, is independent of psychosis, and is the best predictor of long-term functional outcome
(1 –
3) . Thus, the development of effective treatments for cognitive deficits remains a major goal of schizophrenia research
(4) .
In nonhuman primates, activity of gamma-aminobutyric acid (GABA) neurons in the dorsolateral prefrontal cortex is essential for normal working memory function
(9), which suggests that altered GABA neurotransmission in the dorsolateral prefrontal cortex could contribute to the cognitive impairments in schizophrenia patients. Consistent with this hypothesis, postmortem studies have repeatedly found reduced levels of mRNA for the 67 kilodalton isoform of glutamic acid decarboxylase (GAD
67 )—the major determinant of GABA levels—in the dorsolateral prefrontal cortex of individuals with schizophrenia
(10 –
14) . The affected GABA neurons include those that express the calcium binding protein parvalbumin. In contrast, the estimated 50% of GABA neurons that express the calcium binding protein calretinin appear to be unaffected
(15) . Parvalbumin-positive neurons include the chandelier subclass; the axon terminals of these neurons synapse exclusively on the axon initial segments of pyramidal neurons. In the dorsolateral prefrontal cortex of individuals with schizophrenia, GABA plasma membrane transporter-1 (GAT-1) immunoreactivity is reduced in chandelier axon terminals
(16), whereas in the postsynaptic axon initial segments of pyramidal neurons immunoreactivity for the α
2 subunit of the GABA type A (GABA
A ) receptor is markedly increased
(17) . Together, these findings suggest that reduced expression of GAD
67 mRNA in protein parvalbumin-positive chandelier neurons results in decreased GABA synthesis and release. These changes appear to be accompanied by compensatory, but insufficient, responses that include decreased presynaptic GABA reuptake and upregulated postsynaptic GABA
A receptors
(18) .
Reduced GABA signaling from chandelier cells to pyramidal neurons could contribute to working memory dysfunction via a pathophysiological mechanism involving protein parvalbumin-positive GABA neurons. Parvalbumin-positive GABA neurons regulate the synchronized oscillatory activity of cortical pyramidal neurons in the gamma band range (30–80 Hz)
(19) . Gamma band oscillations in the human dorsolateral prefrontal cortex increase in proportion to working memory load
(20), and frontal lobe gamma band oscillations were reduced during a delay-dependent cognitive control task among individuals with schizophrenia
(21) . Consequently, a deficit in the synchronization of pyramidal cell activity, resulting from altered regulation by parvalbumin-positive GABA neurons, is hypothesized to contribute to both reduced gamma band oscillations and impaired dorsolateral prefrontal cortex-dependent cognitive performance in schizophrenia patients
(18) .
This hypothesis suggests that a positive allosteric modulator (i.e., a drug that increases chloride ion flow through GABA
A receptors only when GABA is bound to the receptor) with selective activity at GABA
A receptors containing the α
2 subunit might have beneficial effects in schizophrenia patients. By augmenting GABA neurotransmission from chandelier neurons, such a medication is predicted to enhance the synchronization of pyramidal neuron activity at gamma band frequencies and thereby improve the function of dorsolateral prefrontal cortex circuitry in individuals with schizophrenia
(22) . In contrast, the adverse cognitive effects and sedation associated with the benzodiazepines currently available (which are attributable to their activity at GABA
A receptors containing α
1 and α
5 subunits) are likely to mask the hypothesized cognitive benefits associated with α
2 selectivity
(23) . As a proof-of-concept test of this hypothesis, we conducted a randomized double-blind, placebo-controlled trial to determine whether the Merck compound MK-0777, a benzodiazepine-like agent with selective activity at GABA
A receptors containing α
2 or α
3 subunits, would improve prefrontal-mediated cognitive functions and gamma oscillations in schizophrenia patients.
Discussion
The present study was designed as an initial test of the hypothesis that enhancing GABA signaling through GABA
A receptors containing an α
2 subunit improves the cognitive impairments associated with dorsolateral prefrontal cortex dysfunction in individuals with schizophrenia. Although the study drug was well-tolerated, delayed memory was the only domain of neuropsychological function that showed improvement on the Repeatable Battery for the Assessment of Neuropsychological Status. However, evidence of improvement in the MK-0777-treated group was found on each of three cognitive tests that tap cognitive processes mediated by the circuitry of the dorsolateral prefrontal cortex and that are known to be impaired in schizophrenia patients. Furthermore, this improvement was accompanied by increased frontal gamma band power. Thus, the results of this preliminary study provide suggestive evidence supporting the hypotheses that 1) reduced GABA neurotransmission through GABA
A receptors containing an α
2 subunit is associated with impaired frontal gamma oscillations and cognitive performance
(18), and 2) selective positive modulation of α
2 containing GABA
A receptors can lead to improvement in these measures
(22) .
However, there are several limitations to the study. The “experimental medicine probe” design was known at the outset to be limited in statistical power as a result of the small sample size. Perhaps of equal importance to the sample size limitation is the question of whether the Repeatable Battery for the Assessment of Neuropsychological Status has the appropriate sensitivity for a short-term study designed to test the ability of a drug to normalize the pathophysiology linking the neurobiological and cognitive alterations in schizophrenia. Neuropsychological test batteries, such as the Repeatable Battery for the Assessment of Neuropsychological Status and NIMH’s MATRICS initiative (i.e., Measurement and Treatment Research to Improve Cognition in Schizophrenia), are advantageous for larger clinical trials because their psychometric properties, such as test-retest reliability and floor and ceiling effects, are thoroughly characterized. However, practice effects have been observed with such batteries
(33) . Indeed, the total and subtest scores for the Repeatable Battery for the Assessment of Neuropsychological Status improved in both the placebo and active-treatment groups in the present study, which might have obscured a real drug effect. Perhaps more importantly, a global summary score from these test batteries measures the engagement of multiple complex cognitive systems. If a drug has its effect on one specific cognitive system (e.g., prefrontal-dependent cognitive control functions regulating working memory and response inhibition), then more fine-grained cognitive measures that target those specific functions (e.g., the N-back task and AX Continuous Performance Test) may provide a more sensitive approach to measuring drug effects
(34) . The moderate to large effect sizes seen with these tasks in our data suggest that this might be the case for the MK-0777 compound. Furthermore, the improvement in delayed memory in the MK-0777-treated subjects supports the hypothesis that the information value of early-phase clinical trials is likely to be improved by the inclusion of selective probes that tap specific cognitive processes.
The randomized assignment of subjects resulted in between-group differences in baseline measures of clinical symptoms (BPRS) and neuropsychological function (Repeatable Battery for the Assessment of Neuropsychological Status). Whether these differences blunted or heightened the magnitude of the effect of MK-0777 can only be resolved by larger trials in which the randomization is more likely to result in subject groups with equivalent baseline performance levels. It is also important to note that the inclusion criterion of a baseline Repeatable Battery for the Assessment of Neuropsychological Status score <90 excluded subjects with more modest cognitive impairments who might be more likely to benefit from pharmacological interventions
(35) .
In addition to the target and compound, this treatment approach is novel in that the therapeutic intervention was designed to augment a compensatory response (i.e., increased density of α
2 containing GABA
A receptors) as opposed to correcting a detrimental molecular abnormality. That is, the reduced expression of GAD
67 mRNA in protein parvalbumin-positive chandelier neurons has been interpreted to result in decreased GABA synthesis and release and to induce compensatory responses
(18) . For example, the reduced levels of GAT-1 in chandelier axon terminals in schizophrenia
(16) are predicted to enhance GABA neurotransmission, since the blockade of GABA reuptake prolongs the duration of inhibitory postsynaptic currents when synapses located close to each other are activated synchronously
(36) . The prolongation of inhibitory postsynaptic currents increases the probability of inhibitory postsynaptic current summation, which increases the efficacy of inhibitory postsynaptic current trains. Similarly, the upregulation of the postsynaptic GABA
A receptors containing α
2 subunits in schizophrenia is predicted to increase the efficacy of the GABA that is released from chandelier neurons. However, the presence of cognitive impairments in individuals with schizophrenia indicates that these neuroplastic changes are insufficient as compensatory responses. Thus, as suggested by the results of the present study, pharmacological augmentation of these compensatory responses might be of therapeutic value.
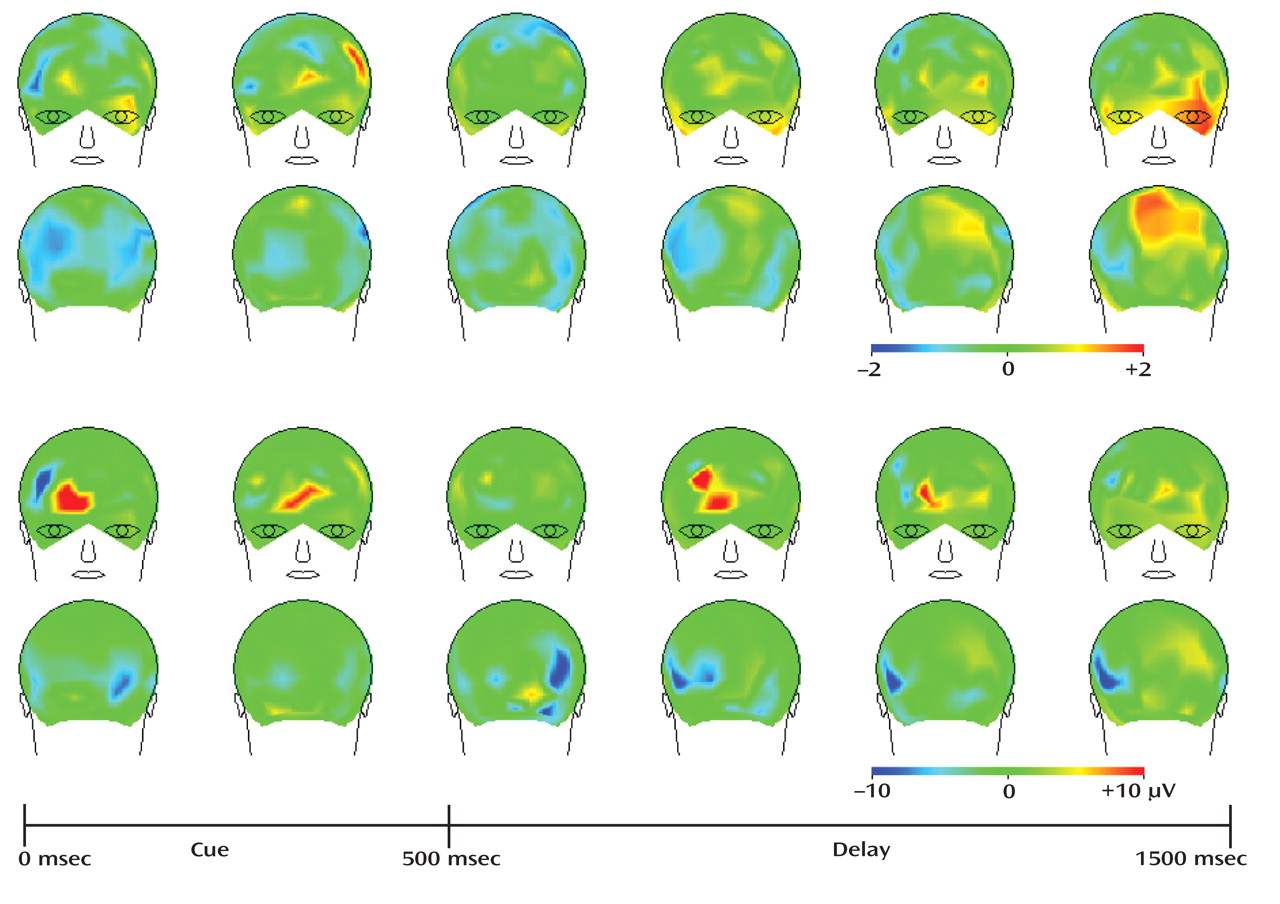
Synchronization of cortical neuronal activity at gamma frequencies appears to be important for a number of perceptual and higher cognitive processes, such as working memory
(20,
37) . The precise mechanisms by which gamma oscillations support perception and cognition have been the subject of much theorizing, but both empirical
(38,
39) and theoretical
(40,
41) evidence suggest that synchronous oscillations serve as a mechanism by which cortical circuits can coordinate activity to support cognitive functions. For example, appropriate subsets of neurons can engage in such oscillations to represent specific information, such as location in a spatial working memory task
(38,
39), and intracranial recordings in the human dorsolateral prefrontal cortex have demonstrated that gamma band power increases in proportion to working memory load
(20) . We previously reported that a cognitive control task was associated with increased induced gamma activity over prefrontal cortical areas in healthy comparison subjects but not in schizophrenia patients
(21) . The disturbances in gamma activity in patients also correlated with disorganization symptoms, similar to previous findings demonstrating that the degree of impaired prefrontal activation (as assessed by fMRI) in a similar cognitive control task predicted clinical ratings of disorganization
(29) . These findings indicate that disturbances in induced gamma oscillations might underlie the dorsolateral prefrontal cortex-mediated cognitive deficits in schizophrenia. Although the sample size was small and clearly requires replication and extension, the present findings, which suggest that there is increased gamma band power and improved cognitive performance following MK-0777 treatment, support this interpretation.
The potential clinical value of augmenting GABA neurotransmission via α
2 containing receptors awaits the results of larger trials of the MK-0777 compound as well as future trials of other compounds currently in development that possess greater potency and selectivity for α
2 containing GABA
A receptors. In addition to an adjuvant treatment for cognitively impaired individuals with chronic schizophrenia, such compounds offer potential promise for early intervention for high-risk individuals. The presence of cognitive impairments before the onset of psychosis in such individuals suggests that the target clinical features and underlying molecular circuitry alterations are present. Furthermore, the potential for anxiolytic effects with positive modulation at α
2 containing GABA
A receptors
(42) raises the possibility that such agents could also attenuate the increased stress responsivity that frequently precedes the onset of psychosis.