Two articles in this issue of the Journal highlight complementary approaches toward testing the common hypothesis that schizophrenia is associated with frontal lobe dysfunction and disconnectivity.
Yoon et al.
(1) used functional magnetic resonance imaging (fMRI) to measure brain activity in first-episode schizophrenia patients and healthy comparison subjects during the AX continuous performance task (
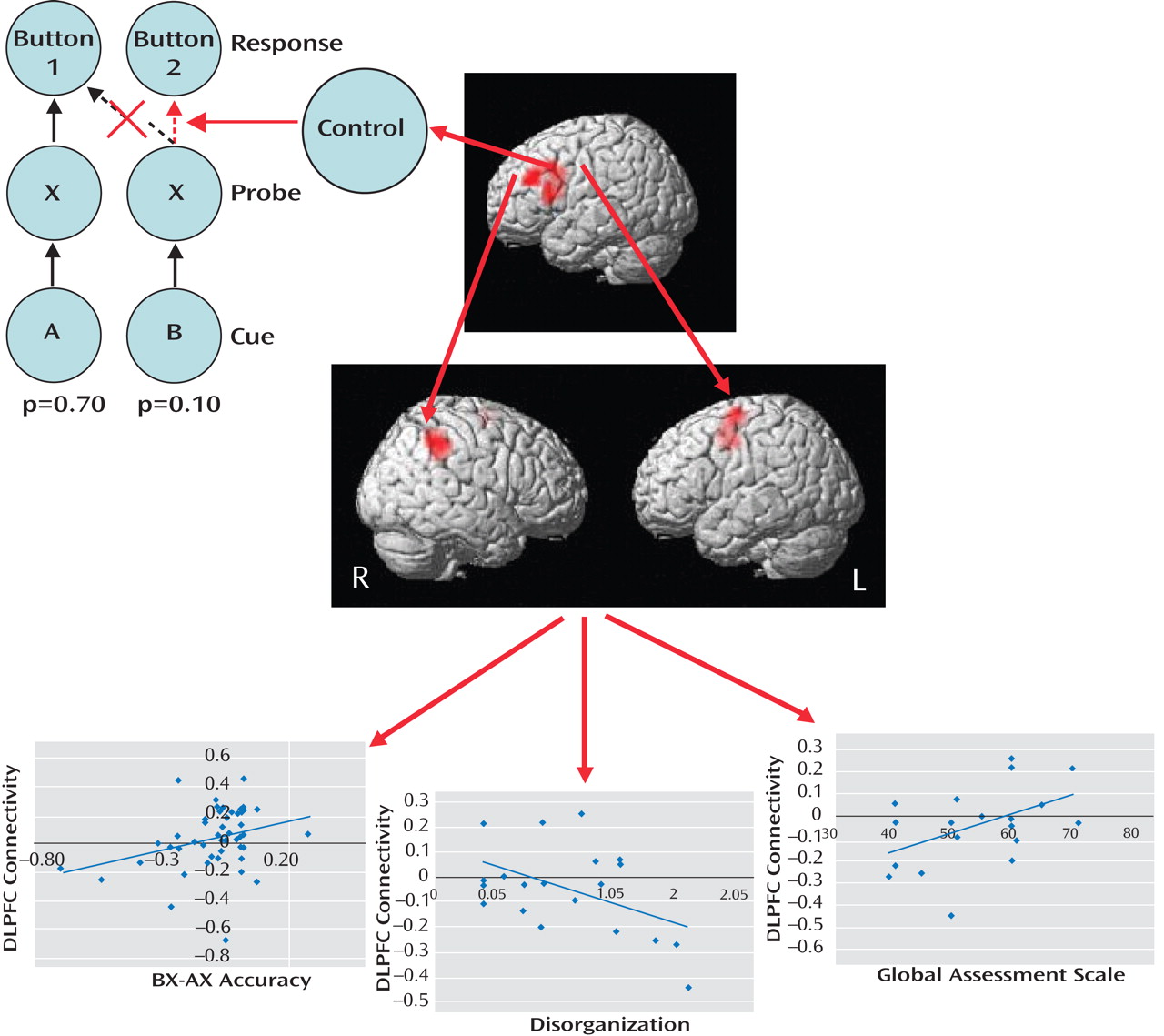
Figure 1 ). In each trial of this task, subjects view a cue letter followed by a probe letter, to which they must respond by pressing one of two buttons. Subjects press button 1 when the probe is X but only if cued by A. If X is cued by any other letter (designated collectively as “B”) or if a non-X probe (designated collectively as “Y”) is presented, subjects press button 2. Since AX trials are highly probable (p=0.70), two prepotent tendencies are generated: One is to
prepare to press button 1 following each A cue, and the other is
to press button 1 following each X probe. Inhibition of these prepotent response tendencies is costly, with subjects being slower and more inaccurate during AY and BX trials than AX and BY trials. While both groups in the Yoon et al. study showed these effects, patients made more errors than healthy comparison subjects during BX trials, indicating deficient use of the cue-related context, as well as during AX trials, suggesting lapses in vigilance. In terms of brain activity, B cues relative to A cues increased activation of the left dorsolateral prefrontal cortex in comparison subjects, which was presumably the result of recruitment of cognitive control processes to inhibit the prepotent button 1 response. Patients showed less dorsolateral prefrontal cortex activation, which is consistent with deficiencies in context maintenance and cognitive control during BX trials. Functional connectivity analysis revealed that in comparison subjects, dorsolateral prefrontal cortex activity following B cues, relative to A cues, correlated significantly with activity in the right inferior parietal lobule and left premotor cortex. This enhanced dorsolateral prefrontal cortex connectivity following B cues was diminished in patients, particularly among those with poorer performance, greater disorganization, and poorer global functioning.
Ferrarelli et al.
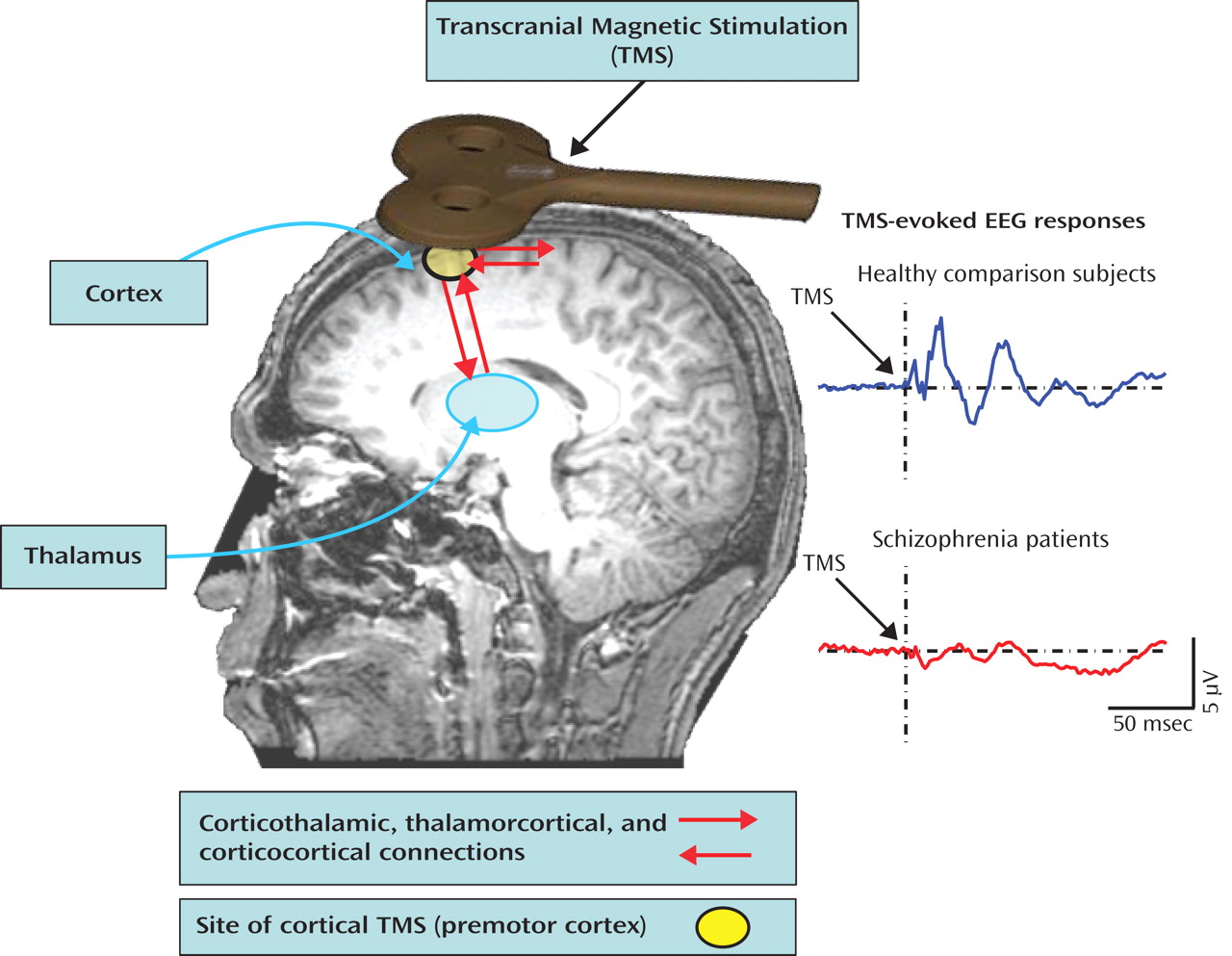
(2) used electroencephalography (EEG) to measure brain activity in response to transcranial magnetic stimulation (TMS) of the right premotor cortex (
Figure 2 ). This produced two successive evoked potential peaks between 12 and 100 msec over frontocentral electrodes that were significantly smaller in schizophrenia patients relative to healthy comparison subjects. These evoked oscillations were in the gamma band (30–50 Hz)—a ubiquitous oscillatory frequency subserving communication between distributed neuronal assemblies during sensation, perception, and higher cognitive processes. In the Ferrarelli et al. study, time-frequency analysis of single-trial EEG epochs showed that the evoked potential amplitude reductions in patients resulted from diminution of both the power and phase synchronization of gamma oscillations across trials. In addition, EEG source analysis examining cortical current density over the first 100 msec following the TMS pulse showed that, in comparison subjects, the focus of neural activity propagated from the right premotor stimulation site to the left premotor and bilateral sensorimotor regions. Patients failed to show this propagation, suggesting deficient connectivity between premotor and adjacent brain regions.
The
cognitive challenge approach taken by Yoon et al. can be characterized as “top down” in that subjects performed a cognitive control task involving inhibition of prepotent responses. The explicit engagement of cognitive functions that are deficient in schizophrenia patients is a strength of this approach, capturing the most illness-relevant cognitive states during the assessment of dorsolateral prefrontal cortex function. However, the resulting patient performance deficits potentially confound the interpretation of the brain data: If patients are doing something different than comparison subjects during the task, their pattern of brain activity is bound to be different. Even though Yoon et al. analyzed only correct trials, it could be argued that the
performance profile in patients indicates that they were performing the task differently than comparison subjects. A related confound is the possibility that performance deficits in patients and corresponding brain activation abnormalities result from patients’ lapses of general attention or deficits in motivation rather than from impairment in prefrontally mediated cognitive control processes per se. Finally, complex cognitive tasks often engage multiple cognitive processes, introducing uncertainty about whether a comparison between conditions is “process pure.” For example, while the contrast between the B and A cue is presumed to isolate control processes related to overcoming prepotency, the B cue is less probable than the A cue. Low probability stimuli presented in a stream of frequent stimuli can evoke an involuntary orienting of attention and, if task-relevant, can also engage top-down attentional resources related to target detection. Both of these responses to deviance elicit the P300 event-related brain potential
(3), which is reduced in amplitude in schizophrenia
(4) and is generated from distributed regions, including the dorsolateral prefrontal cortex and inferior parietal lobule
(5), regions implicated in the Yoon et al. study. Thus, while cognitive challenge paradigms possess criterion-related validity by virtue of their direct focus on cognitive performance deficits in schizophrenia, interpretation of the brain data they generate can be complicated.
The
minimal task approach taken by Ferrarelli et al. is decidedly “bottom up” in that direct TMS of the frontal cortex obviated the need to engage cognitive processes. The strength of this approach is that it eliminates the differences in performance, task strategy, general attention, and motivation that potentially confound patient-control comparisons in cognitive challenge paradigms. Although TMS also bypasses the sensory channels, eliminating peripheral sensory pathology as the basis for abnormal cortical responses, the approach is otherwise similar to passive sensory stimulation paradigms such as auditory
(6) and visual
(7) steady-state driving, in which stimuli repeat rapidly at a specific frequency, driving the cortical EEG activity at that frequency. Prepulse inhibition
(8), paired-click P50 suppression
(9), and mismatch negativity
(10) are other passive electrophysiological paradigms that have revealed functional brain abnormalities in schizophrenia. Similarly in fMRI, resting state studies of schizophrenia have shown dysfunction of “default mode” networks
(11) . Only slightly above
minimal tasks, on the continuum toward
cognitive challenge approaches, are simple tasks that engage cognitive processes without unmasking performance deficits. For example, simple “oddball” target detection tasks have revealed cortical dysfunction in schizophrenia using both event-related brain potentials
(3) and fMRI
(4) . Minimal task approaches show that cognitive challenges exposing cognitive deficits are not necessary to reveal brain dysfunction in schizophrenia.
However, minimal task approaches are not without limitations. First, they generally lack face and ecological validity and often have limited criterion-related validity from the standpoint of sensitivity to clinically relevant cognitive deficits. Second, they do not necessarily eliminate confounding patient-control differences in cognitive states. In the absence of task demands, subjects engage in their own idiosyncratic thought processes, introducing variation in arousal, emotional states, and attention. Methods that passively probe brain function, including TMS, may evoke responses that are modulated by subjective state variations that differ between patients and comparison subjects, potentially confounding group comparisons. This was described by Sam Sutton
(12), who emphasized the use of task demands to minimize “subject option” in cognitive studies comparing psychiatric patients with comparison subjects.
Interestingly, both Yoon et al. and Ferrarelli et al. linked their findings to a mechanistic failure of neural oscillations possibly mediated by gamma-aminobutyric acid (GABA)-ergic deficits. However, whether the frontal lobe dysfunctions revealed by cognitive challenge and minimal task approaches arise from the same pathophysiological mechanisms must be determined from studies in which both approaches are applied to the same patients. Ultimately, the power of cognitive challenge and minimal task approaches is maximized by exploiting their differences, integrating insights derived from each, and exploring whether they are sensitive to the same or distinct underlying pathophysiological mechanisms in schizophrenia.