Two articles published in this issue of the
Journal are of considerable interest to clinicians treating individuals with schizophrenia. Gründer and colleagues
(1) provide findings from a positron emission tomography study examining dopamine D
2 receptor occupancy that illustrate the unique pharmacology of the novel antipsychotic aripiprazole. The report by Mohamed and colleagues
(2) makes use of data from the CATIE study to illuminate the complex interaction of factors that determine quality of life and disability in patients with schizophrenia.
Gründer and colleagues report that a majority of patients treated with aripiprazole at typical clinical doses have blood concentrations high enough to saturate D
2 receptors in all brain regions studied; in some patients, the D
2 receptors remained virtually fully occupied for as long as a week after discontinuation of aripiprazole. These findings reflect aripiprazole’s unique profile as a high-affinity D
2 partial agonist with an extremely long elimination half-life. Traditional D
2 antagonists achieve antipsychotic efficacy by occupying 60% or more of D
2 receptors, thereby reducing overall D
2 activity by roughly the same amount as the percentage of receptors occupied. In contrast, as a partial agonist at the D
2 receptor, aripiprazole in theory would need to occupy a higher percentage of D
2 receptors to achieve a similar overall reduction in D
2 activity. In fact, results from a previous study suggested that the therapeutic threshold for aripiprazole may be greater than 80% occupancy of D
2 receptors
(3) . By modestly activating D
2 receptors in the striatum, aripiprazole largely prevents classic neurological side effects
(4) . Gründer and colleagues demonstrate that, unlike other atypical agents, this sparing of extrapyramidal side effects by aripiprazole is not the result of preferential binding to temporal-limbic dopamine receptors. It is the nature of partial agonists that these side effects are avoided even at high, saturating doses
(5) as observed by Gründer and colleagues.
Early in their development, D
2 partial agonists were expected to improve negative symptoms; however, most head-to-head trials failed to find an advantage for negative symptoms compared to full D
2 antagonists. Early concerns about whether a partial agonist would diminish dopamine transmission sufficiently to achieve adequate antipsychotic efficacy were allayed by studies of aripiprazole that suggested efficacy comparable to full antagonists, although antipsychotic efficacy of the partial agonist bifeprunox appears to be less robust than D
2 full antagonists, possibly reflecting a higher degree of intrinsic D
2 activation
(6) . Because aripiprazole was not available for inclusion in the CATIE trial, our understanding of the relative efficacy of aripiprazole compared to other agents is limited. Whereas neurological side effects are uncommon with aripiprazole, clinical consequences of dopamine receptor activation, such as insomnia, agitation, and nausea, may occur in some patients. These side effects can occur at relatively low doses and generally resolve as tolerance develops within 2–3 weeks. In sum, the mean level of D
2 occupancy observed by Gründer and colleagues is well in excess of levels typically associated with optimal treatment with full antagonists but may be necessary for full antipsychotic response and does not appear to carry a burden of parkinsonian side effects owing to the nature of a partial agonist.
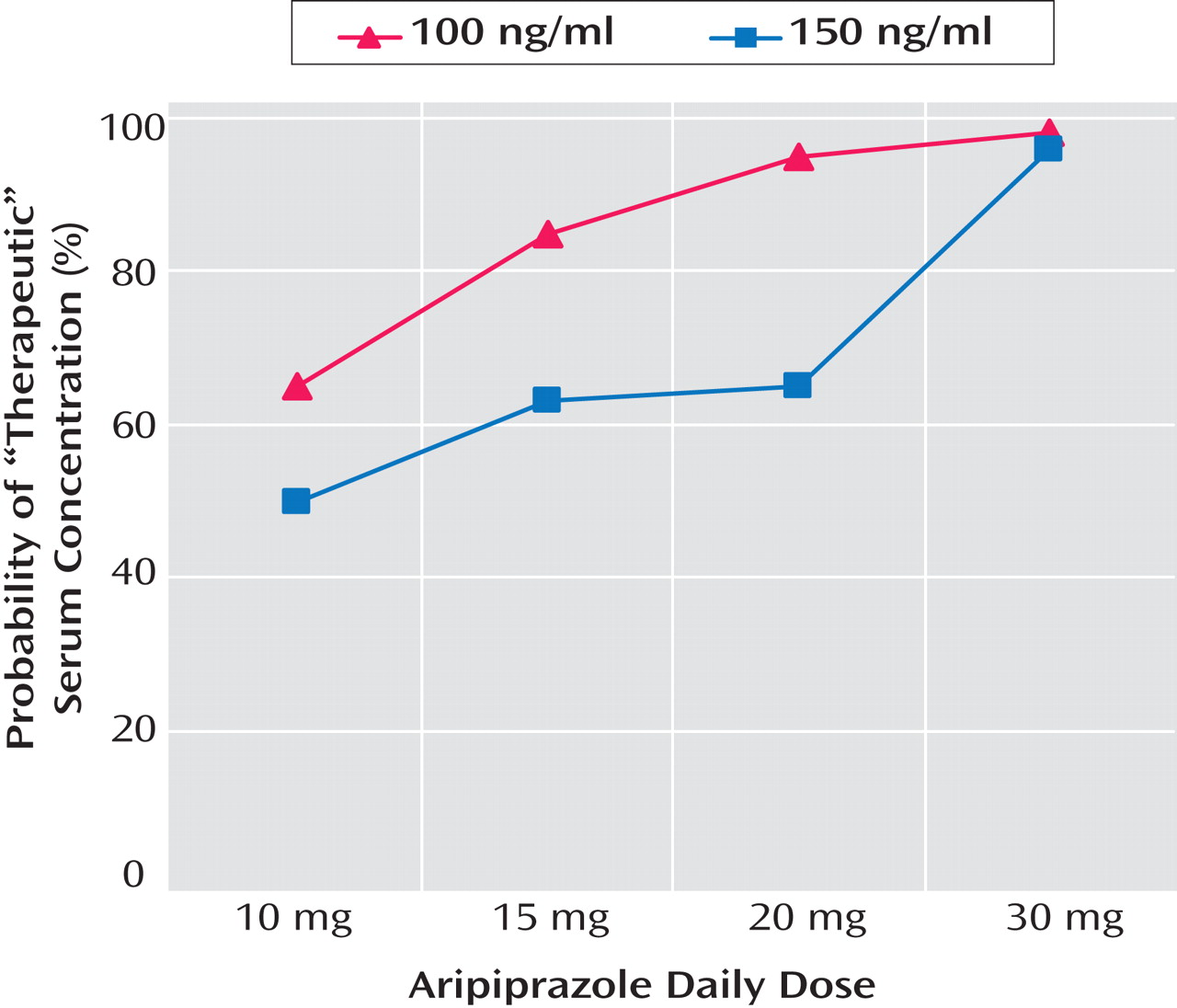
The low propensity of aripiprazole to produce neurological side effects may in part account for the extremely high blood levels of aripiprazole that Gründer and colleagues noted in some of the flexibly treated patients. Partial agonism at the D
2 receptor can eliminate the telltale side effects that, with other antipsychotics, indicate the upper limit of a therapeutic window. Although fixed-dose trials have not demonstrated additional therapeutic benefit with aripiprazole doses above 15 or 20 mg/day, no evidence for an upper limit to a therapeutic window has emerged. Gründer et al. found that some aripiprazole-treated patients had D
2 occupancy below 80% and serum concentrations below a level associated with D
2 receptor saturation. Given the very large variability in serum concentrations and D
2 occupancy and the weak correlation between dose and serum concentration, further work using fixed-dose trials is needed to examine the predictive power of serum concentrations to guide dosing. If a serum concentration of either 100 or 150 ng/ml is validated as a therapeutic threshold, the data reported by Gründer and colleagues would suggest that a dose of either 20 or 30 mg/day, depending on the serum concentration selected as a threshold, would ensure adequate D
2 occupancy for most patients (
Figure 1 provided by Gründer and colleagues), although levels would be far in excess of the therapeutic threshold in many patients.
Another factor that may contribute to the very high blood levels observed by Gründer and colleagues is aripiprazole’s long half-life. A 72-hour half-life presents to clinicians both challenges and potential advantages. When treatment is initiated with aripipazole or when the dose is changed, it takes about five half-lives or roughly 2 weeks before steady state concentrations are achieved. Clinicians prescribing antipsychotics are accustomed to titrating antipsychotic doses at intervals as short as every couple of weeks in response to symptom response or side effects. Aripiprazole’s very slow buildup of blood levels means that dose increases may occur before the full clinical effect of the current dose is evident. This can result in premature and unnecessary dose escalation, although higher-than-necessary dosing usually is not associated with toxicity. While dose titration may be challenging, the tolerability of high blood levels and the slow elimination of aripiprazole can result in therapeutic levels of D 2 blockade for as long as a week after treatment discontinuation, as reported by Gründer and colleagues. This suggests that aripiprazole may represent an intermediate option between short-acting oral antipsychotics and depot preparations. For the many schizophrenia patients whose adherence is inconsistent, missed doses of aripiprazole will not significantly lower D 2 receptor occupancy and hence jeopardize efficacy. Dosing intervals less frequent than daily may be feasible with aripiprazole and would be welcome under clinical circumstances in which supervision of medication taking cannot be provided on a daily basis.
The analysis of predictors of functional impairment among subjects in the CATIE study reported by Mohamed and colleagues provides valuable insights into a topic that has become increasingly important in the evaluation of treatment outcomes in schizophrenia. The dramatic success of chlorpromazine and later antipsychotics in controlling psychotic symptoms allowed the release of many schizophrenia patients from long-term hospitalization but surprised clinicians by the disappointing lack of functional recovery. Several investigators subsequently identified the negative symptoms as a source of this disability; for example, Heinrichs et al. originally developed the Quality of Life Scale to measure symptoms of the deficit syndrome
(7) . More recently, Green reported the widely cited observation that cognition is a primary determinant of functioning in schizophrenia
(8) . This finding has been well replicated, although the relative contributions to disability of symptoms versus cognition have varied between studies
(9) . Cognitive performance is generally viewed as an indicator of the capacity to function, whereas symptoms influence the extent to which functioning skills are actually employed. Negative symptoms, particularly apathy, seem to be obvious determinants of functional performance; other symptoms such as paranoia, disorganization, and depression would also be expected to contribute to social and vocational disability. In a recent study of 78 older schizophrenia patients, Bowie and colleagues
(10) found that cognitive performance predicted functional capacity or “competence” measured in a laboratory setting, which, in turn, predicted objective ratings of functioning in the community. Negative symptoms and depression contributed to ratings of social performance independent of cognitive scores or functional capacity.
Although the theory behind the attribution of disease-related deficits to community functioning may seem relatively straightforward, the demonstration of such relationships is complicated by the difficulty in measuring community functioning. A wide range of quality of life scales have been developed that differ both in their focus on attitudes and behaviors and in the degree to which they rely on subjective self-report versus more objective ratings by caregivers or other informants
(11) . The Quality of Life Scale employed by Mohamed and colleagues is completed by a trained rater based largely on interview with the patient and, compared to other quality of life scales, tends to produce scores that correlate well with ratings of negative symptoms
(12) . Subjective assessments of a patient’s satisfaction with his or her life situation or treatment are of great importance in assessing the appropriateness of care and are reliable predictors of treatment adherence. However, self-report of functional status has been demonstrated to correlate poorly with assessments made by direct observation
(9) .
Mohamed and colleagues provide a sophisticated analysis of data from the CATIE study, taking advantage of this large, representative sample from which so much has already been learned about the psychiatric, social, and medical status of schizophrenia patients in this country. The authors modified the PANSS score in an attempt to reduce overlap between symptom ratings and the Heinrichs-Carpenter Quality of Life Score. The reliance upon a rater interview for assessment of community function represents an improvement upon strictly self-report instruments but falls short of the validity of measurements provided by direct observation. In their mixed-model regression analysis, with control for potentially confounding factors, the authors establish that both cognitive performance and symptoms contribute to disability in schizophrenia; in the CATIE sample, with this method of functional assessment, the contribution of symptoms was greater than that of cognitive performance.
The important message for clinicians is that community functioning is determined by a complex interaction between capacity for functioning (reflected by cognitive performance) and limitations upon an individual’s realization of this capacity that are often due to negative, depressive, or other symptoms, although poverty, stigma, health issues, and medication side effects can all contribute as well. Monitoring of patients’ satisfaction with the quality of their life is essential to enhancing treatment compliance and in identifying potentially meaningful interventions. The improvement of vocational and social functioning can be enhanced with psychosocial interventions; these approaches may benefit in the future in combination with drugs currently in development that target cognitive deficits and negative symptoms.