The pathogenesis of attention deficit hyperactivity disorder (ADHD) is thought to involve anatomical and functional disturbances in cortico-striato-thalamo-cortical circuits. These circuits traverse portions of the frontal cortex and basal ganglia that support the learning of emotional responses to reinforcing stimuli, the regulation of attentional resources, and the programming of simple and complex motor behaviors. This pathophysiological model for ADHD is based as much on what is known of the neural bases of these processes from animal models (
1) and neurobiological studies of healthy humans as it is on direct experimental evidence from youth who have ADHD (
2). Indeed, findings from brain imaging studies of children with ADHD have been highly variable, with the preponderance of the largest and methodologically most rigorous studies indicating the presence of reduced volumes of the cerebral cortex (
3), particularly of the lateral prefrontal cortex (
4,
5), and reduced volumes of one or more basal ganglia nuclei (
6–
8) (caudate, putamen, or globus pallidus). With one exception (
9), these studies have not examined the local volumes of basal ganglia nuclei to determine which portions of those nuclei, and by implication which portions of the cortico-striato-thalamo-cortical pathways, are involved in the pathogenesis of ADHD. None of these studies has yet reported the presence of significant effects of stimulant medications on basal ganglia morphology, despite the fact that stimulant medications are among the most robustly and most predictably helpful medications available for any neuropsychiatric illness.
Herein, we present a magnetic resonance imaging (MRI) study of the three basal ganglia nuclei—caudate, putamen, and globus pallidus—in children with ADHD (N=47) and healthy comparison subjects (N=57). We examined the conventional volumes of the basal ganglia and their detailed surface morphologic features. We also hypothesized that these measures would differ between youth with ADHD and comparison youth and that the morphological features of basal ganglia nuclei in ADHD youth treated with stimulants would differ from patients not taking stimulants.
Method
Further details of the recruitment, behavioral assessments, MRI pulse sequence, and image processing are described in the data supplement accompanying the online version of this article.
Participants
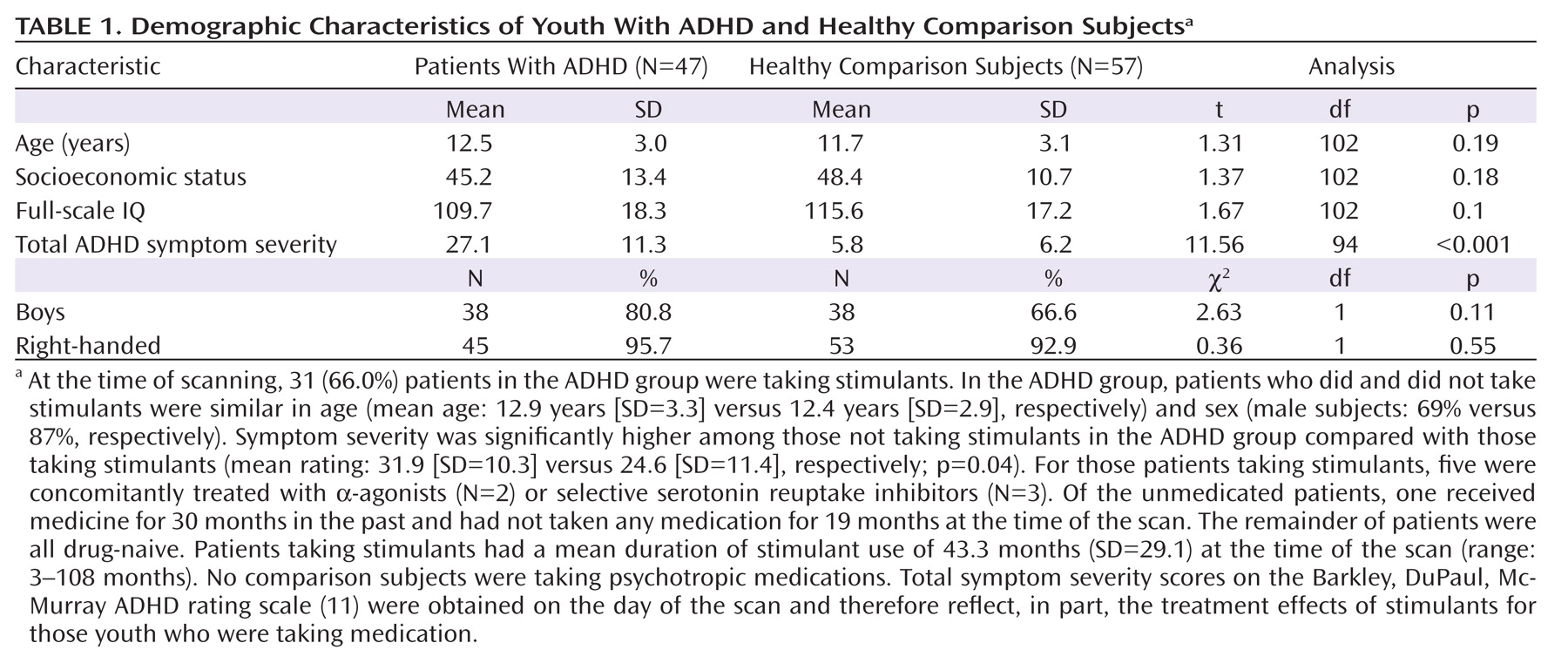
We acquired MRIs in 47 children with ADHD and 57 healthy comparison children, aged 7–18 years (
Table 1). All patients met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM–IV) (
10), criteria for combined-type ADHD. Exclusion criteria for patients with ADHD were a history of obsessive-compulsive, bipolar, psychotic, anxiety, tic, conduct, or pervasive developmental disorders. In the ADHD group, a total of 23 (48.9%) patients had a co-occurring lifetime diagnosis of depression (N=12), oppositional defiant disorder (N=12), and/or specific developmental disorder (e.g., reading, mathematics, written expression, or motor coordination problems [N=7]).
We estimated symptom severity using the Barkley, DuPaul, and McMurray ADHD rating scale (
11). We excluded comparison participants who had a history of medical, psychiatric, or neurologic disorders. Additional exclusion criteria for both diagnostic groups were any previous seizure, head trauma with loss of consciousness, current or previous substance abuse, or IQ <70. The Institutional Review Boards of Yale and Columbia Universities approved the study. Parents provided written informed consent, and participants provided verbal assent.
Surface Morphology
We evaluated the surface morphology of the basal ganglia by computing the signed Euclidean distance from each point on the surface of the basal ganglia nuclei of an individual participant to the corresponding point on the basal ganglia nuclei of a healthy comparison reference template. We encoded outward deformations using positive distances and inward deformations using negative distances.
We previously validated these procedures to identify known regional increases and decreases in volumes at the surface of brain structures (
12). Procedures are detailed elsewhere (
13–
15) and summarized in the present study. We applied a two-step procedure for surface morphometry of the basal ganglia. First, we coregistered each participant's brain with the template brain using a similarity transformation. The parameters estimated for this transformation are global scaling, three translations, and three rotations of the brain. The translation and rotation parameters were applied to place the caudate, putamen, and globus pallidus into the template space. The global scaling parameters controlled for any scaling differences in these structures caused by differing whole-brain volumes. Second, we independently and rigidly coregistered each basal ganglia nucleus and then nonlinearly warped it to the exact size and shape of the corresponding nucleus in the template, allowing precise identification of corresponding points along the surface of these regions. Next, we unwarped each region while preserving the labels assigned to corresponding points on the surface of each region. We then computed the distance from each point on the surfaces of the basal ganglia nuclei for each participant to the corresponding point on the template. Therefore, at each point on the surface of a basal ganglia nucleus in the template brain, we had a set of 57 distances for the healthy comparison group and a set of 47 distances for the ADHD group. Finally, these two sets of distances were compared statistically to determine local regions of significant differences between the groups of participants (see discussion on template selection).
Template Selection
Conceivably, localization and interpretation of point correspondences may depend on the selected template (
14). Therefore, we selected the template brain by a two-step process. First, we selected as a preliminary template the brain of the healthy participant that best represented, demographically, the healthy participants in the study. The remaining healthy subjects were coregistered to this preliminary template. We then computed the distances between the corresponding points on the surface of the basal ganglia nuclei (as detailed in the discussion on surface morphology). Second, we determined the final template by selecting the brain for which all points across the surface of the basal ganglia nuclei were closest to the average of the computed distances. The surface morphometry was then repeated for our entire cohort of healthy and affected participants using the final template brain.
We used a single representative brain as a template rather than an averaged brain because a single brain has well-defined tissue interfaces, such as CSF gray matter or gray-white matter interfaces. Averaging images for a template blurs these boundaries and increases registration errors that are subtle but important when distinguishing subtle effects across populations. In addition, precise surface morphometry requires a brain with smooth gray and white surfaces that are devoid of topological defects, which cannot be reconstructed by averaging brains from several participants.
Statistical Analysis
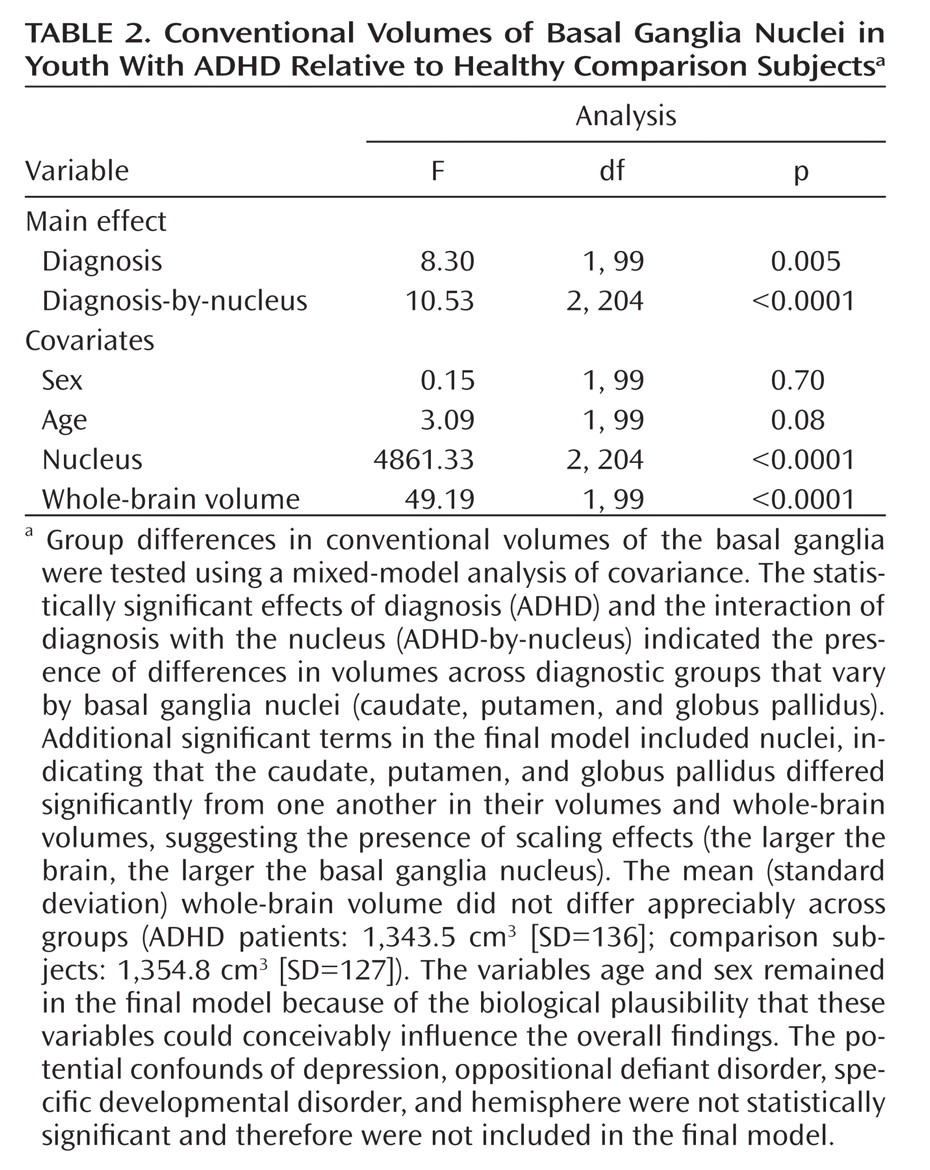
For conventional volumes, to test the a priori hypothesis that patients with ADHD have different volumes of basal ganglia nuclei relative to healthy comparison subjects, we calculated the statistical significance of the main effects of diagnosis (between-factor) and the interaction of diagnosis with the nucleus (within-factor) in a mixed-model analysis of covariance (PROC MIXED, SAS Institute, Inc., Cary, N.C.) with repeated measures over a spatial domain (caudate, putamen, and globus pallidus). Covariates included in the mixed model for assessment of conventional volumes were whole-brain volume (to control for scaling effects), age, sex, and hemisphere (left or right). To control for possible confounds, lifetime diagnoses of comorbid depression, oppositional defiant disorder, or specific developmental disorders (e.g., reading, mathematics, written expression, or motor coordination problems) were used in confirmatory models to assess the effects of these comorbid conditions. Statistically nonsignificant terms (p≥0.10) were eliminated from the final mixed model. The variables age and sex remained in all final statistical models because of the biological plausibility that these variables could influence the overall findings. All dependent measures were normally distributed, as assessed using the Kolmogorov-Smirnov test.
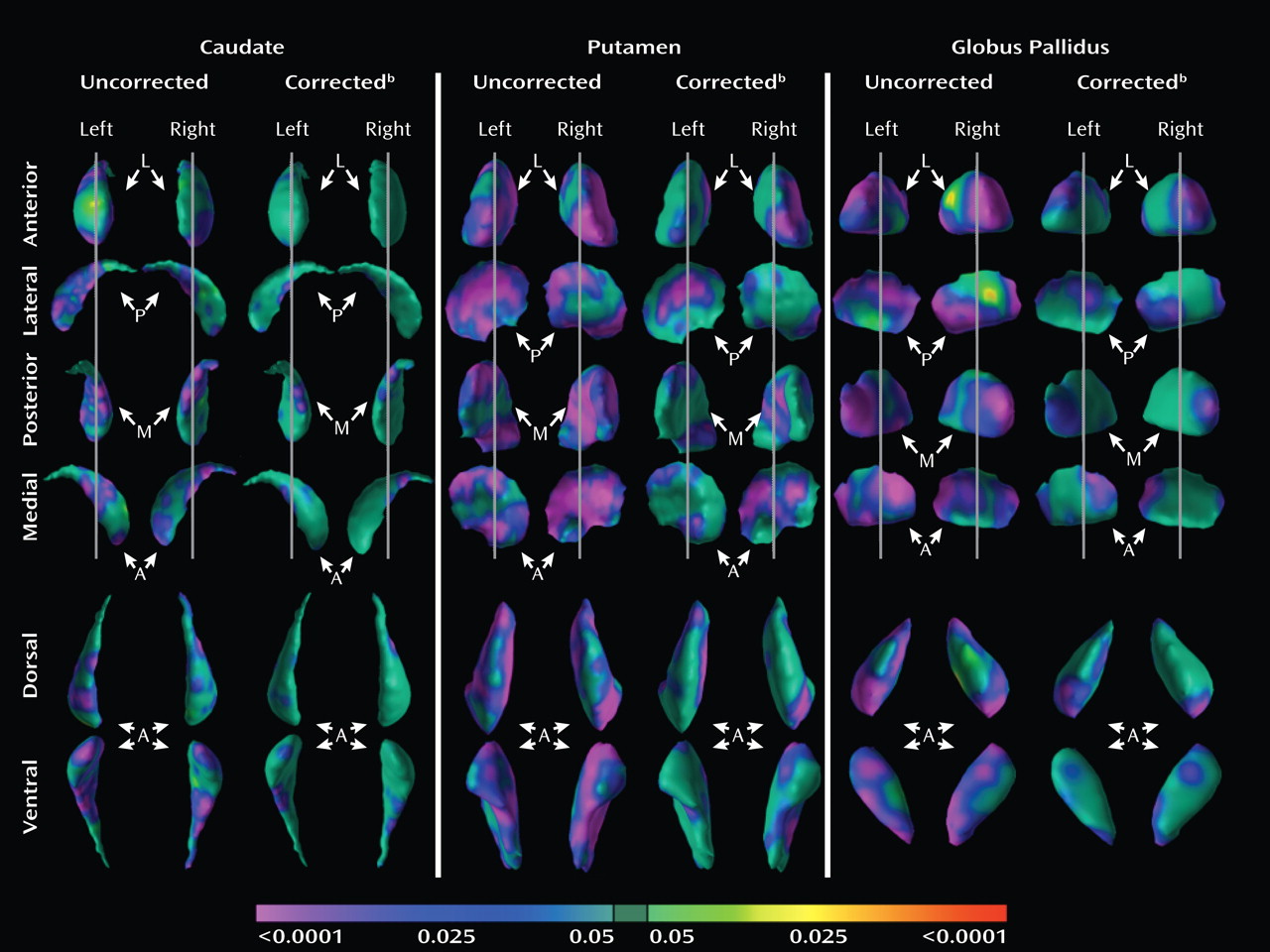
For analysis of surface features, the distances between each point on the surface of the basal ganglia for each participant and the corresponding point on the template basal ganglia were compared statistically between the two diagnostic groups using multiple linear regression while covarying for age and sex. Because global scaling parameters controlled for any scaling differences across brains, covarying for whole-brain volume was not necessary. We used the theory of Gaussian random fields to correct p values appropriately for the multiple comparisons performed across the basal ganglia surface (
16).
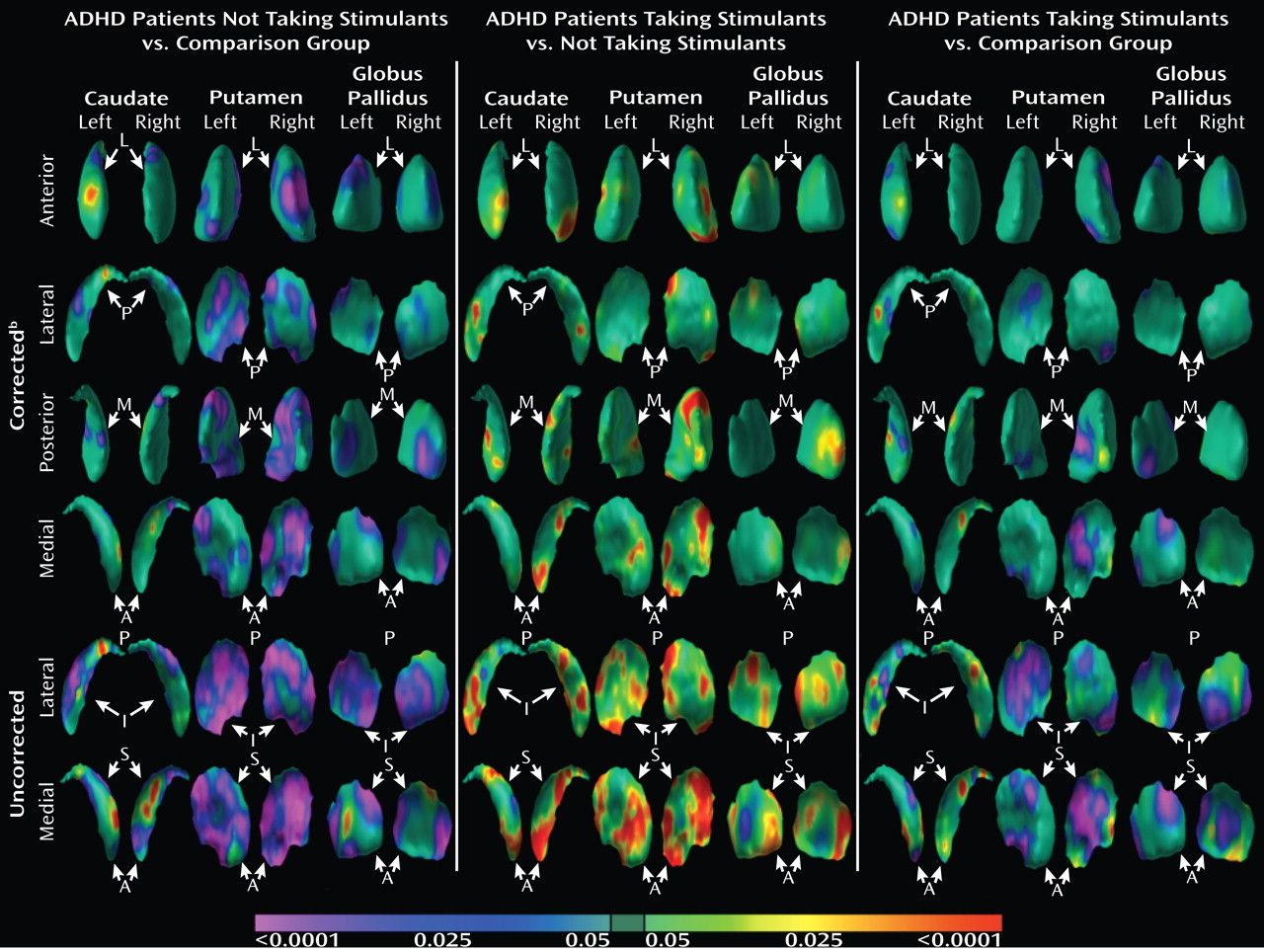
We used two complementary approaches to test the hypothesis that the morphological features of basal ganglia nuclei in youth with ADHD who are currently treated with stimulant medication differ from the basal ganglia of patients not taking stimulant medications. First, we analyzed the effects of stimulant use in the ADHD group alone. For conventional volumes, we tested the main effect of stimulant use (between-subject factor) and the interaction of stimulant use with nuclei (within-subject factor) in a mixed-effects model (while covarying for age, sex, and whole-brain volume). For surface morphology, we tested the significance of stimulant use in a multiple linear regression model while covarying for age and sex. Second, we assessed the main effects of diagnosis (as detailed above) on surface features in the subgroup of ADHD youth who were taking stimulants (N=31) relative to healthy comparison subjects and in the subgroup of ADHD youth not taking stimulants (N=16) relative to healthy comparison subjects.
Symptom Severity
In the ADHD group, we explored correlations of basal ganglia nuclei volumes or surface features with symptom severity at the time of scanning, as measured with the Barkley, DuPaul, and McMurray ADHD rating scale (
11).
Discussion
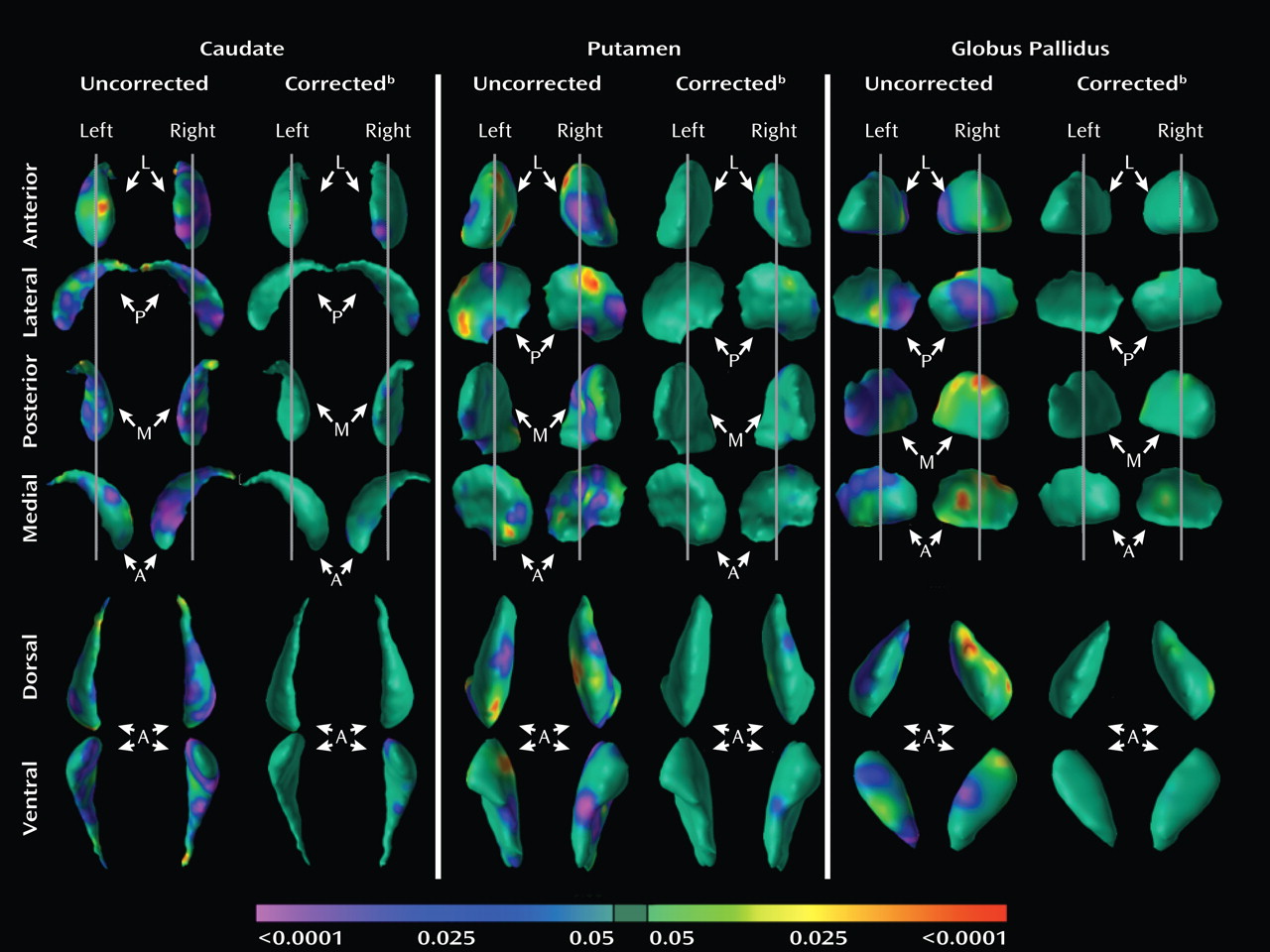
Analyses of overall conventional volumes detected differences between ADHD youth and healthy comparison subjects only in the putamen. Surface analyses demonstrated that this reduced volume derived from local inward deformations in most portions of the surface of the putamen, and they further identified significant inward deformations in the caudate and globus pallidus. Many of the locations of these inward deformations in the ADHD group corresponded with locations where overall symptom severity correlated significantly with local inward deformations, with more pronounced inward deformations accompanying more severe symptoms. Surface analyses also demonstrated that outward deformations of these nuclei were associated with ADHD youth treated with stimulants relative to those who were untreated at the time of the scan. These outward deformations were in locations similar to those where inward deformations were detected in ADHD youth relative to comparison subjects.
The decreased conventional volume of the putamen in the ADHD group is consistent with findings from previous anatomical imaging studies of ADHD (
6–
8). In addition, the similarity in conventional volumes of the caudate across groups corroborates the findings from several other studies that also controlled for scaling effects by covarying for whole-brain volume (
3,
17–
19). Our finding that globus pallidus volumes were similar between diagnostic groups conflicts with findings of reduced globus pallidus volumes reported in a study of a demographically similar cohort (
18), although our improved signal-to-noise characteristics (accomplished with two signal averages during image acquisition compared with one in prior studies) and our better spatial resolution (1.2 mm slice thickness in the present study compared with 2 mm) likely improved the precision and accuracy of our anatomical measurements. Finally, the findings of our analyses comparing surface morphology across groups are consistent with those from a study of ADHD youth that employed similar analytic procedures (
9). That study reported reductions in overall conventional volumes across all three basal ganglia nuclei, as well as inward deformations across the surfaces of all three nuclei, particularly in the anterior portions and midbodies of each structure. The similarity of results across studies corroborates the existence of structural disturbances within the basal ganglia of individuals with ADHD.
Our surface analyses provide a degree of spatial detail of the basal ganglia nuclei that overall conventional volume measures cannot provide, permitting us to identify highly localized anatomical disturbances in these nuclei and allowing us to infer the presence of corresponding disturbances in the anatomical pathways that contain those regional abnormalities in youth with ADHD. Neuroanatomical tracing studies in animals (
20–
25), as well as diffusion tensor imaging (
26) and functional connectivity studies (
27) in humans, suggest that ventral, anterior, and posterior portions of the putamen, caudate, and globus pallidus can be partitioned topographically into limbic, associative, and sensorimotor systems, respectively, based on the topographically organized input to these nuclei from cortical and subcortical areas and on the topographically organized projections that these nuclei send back to cortical areas via the thalamus.
We detected inward deformations located in the limbic (primarily ventral) portions of the basal ganglia nuclei in ADHD youth, consistent with prior studies of persons with ADHD that report abnormalities in limbic structures such as the orbital frontal cortex and amygdala (
14,
28). The limbic portions of the putamen, caudate, and globus pallidus connect anatomically and interact functionally with the orbital frontal cortex, amygdala, and nucleus accumbens to form the distributed limbic neural circuit that guides reinforcement-based learning (the acquisition and selection of appropriate behavior) (
29–
32). Therefore, morphological aberrations in the limbic circuits that support reinforcement learning (
33–
35) may account for the difficulties that ADHD youth have with delayed gratification and with selecting inappropriate behaviors in a given environmental context. The inward deformations that were located generally in the associative (anterior) portions of the basal ganglia nuclei, combined with a prior report of thinning in the lateral prefrontal cortex in ADHD youth (
5), may represent an altered associative neural circuit in persons with ADHD. Given that the associative portions of the basal ganglia nuclei and the lateral prefrontal cortex together guide executive functioning (
36–
38), the behavioral consequence of an altered neural circuit for associative learning likely includes impaired executive functioning, which is arguably the hallmark of ADHD (
39–
41). The inward deformations located generally in the sensorimotor (primarily posterior) portions of the basal ganglia nuclei accord well with other reports of anatomical abnormalities in the sensorimotor cortices of ADHD youth (
42,
43). The sensorimotor portions of the basal ganglia, together with the sensorimotor cortex, drive motor learning and control (
44–
48). Deficits in the sensorimotor neural circuit may underlie the dysfunction in fine and gross motor control and the impairments in learning and execution of complex motor behaviors that are characteristic of ADHD (
39,
49–
53).
Although the neurobiological mechanisms that cause inward deformations of the basal ganglia in ADHD youth are unknown, one possibility is that dopamine dysfunction in ADHD may alter local cytoarchitecture within basal ganglia nuclei. Several candidate genes related to dopamine neurotransmission have been associated with ADHD (
54). One of these, the dopamine-transporter gene, removes dopamine from the synaptic cleft through a reuptake mechanism (
55). Abnormally high levels of the dopamine-transporter gene reported in persons with ADHD (
56–
59) may diminish concentrations of synaptic dopamine. The basal ganglia may be particularly sensitive to diminished levels of dopamine, since basal ganglia nuclei receive substantial projections from midbrain-dopamine afferents (
60) and contain the largest relative concentration of the dopamine-transporter gene in the brain (
61,
62). Indeed, prior studies of the histological features of the basal ganglia in animals (
63–
66) and humans (
64,
67,
68) suggest that deficits in dopamine concentrations produce anatomical alterations in basal ganglia neurons, including reductions in the number of synapses, decreases in the density of dendrite spines, and decreases in dendritic arborization and length. These cellular changes, when affecting large-scale populations of neurons in the basal ganglia, could produce the local volume reductions of the basal ganglia that we detected in the ADHD group. Moreover, this putative mechanism could also account for the location of the most prominent volume reductions in the putamen, which is the largest recipient of midbrain-dopamine afferents of all the basal ganglia nuclei (
62,
69,
70). An exquisite modulation of the basal ganglia by dopamine supports the acquisition and execution of a broad range of behavioral actions that are orchestrated by limbic, associative, and sensorimotor basal ganglia circuits (
71–
73). Thus, the local volume reductions that span the limbic, associative, and sensorimotor portions of basal ganglia nuclei may be associated with the heterogeneous symptoms of ADHD, which include context inappropriate behaviors, deficits in working memory, and impaired motor control (
35,
74–
76).
If local volume reductions of the basal ganglia in persons with ADHD reflect the morphological modifications that occur in response to a deficit in dopamine, then the local volume increases of basal ganglia in youth with ADHD who are treated with stimulants may represent the morphological changes that occur in response to the relative improvement in dopamine levels that the stimulants produce. Indeed, previous imaging studies in youth with ADHD demonstrate an association between stimulant treatment and normalized gray matter volume in various brain regions (
77–
79). Stimulants bind to and block the dopamine transporter gene, effectively increasing synaptic and extracellular dopamine levels (
80,
81). Therefore, stimulants may alleviate the deleterious cellular effects that the deficit of dopamine has on target basal ganglia neurons in untreated persons with ADHD. Although the exact anatomical modifications produced by a stimulant-induced increase in dopamine in humans are unknown, animal studies suggest that stimulants induce changes in gene expression and dendritic architecture within the basal ganglia in a direction opposite of that seen with deficient dopamine (
82–
86). Thus, the significant local volume reductions of the basal ganglia in ADHD youth not taking stimulants and the attenuation of these reductions in those youth receiving stimulants may reflect the architectural modifications that occur in response to deficient and relatively corrected dopamine concentrations, respectively.
Limitations
Although we cannot exclude entirely the possibility that co-occurring illnesses, sex, or age effects influenced our findings, including these effects as covariates in our statistical models and conducting separate analyses of ADHD subgroups indicated that these effects did not appreciably affect our findings. In addition, the cross-sectional design of this study precludes strong inferences that the morphological abnormalities of the basal ganglia represented either the causes or consequences of ADHD. The cross-sectional design similarly precludes strong inferences that the seemingly morphological normalizing effects of stimulant medications represented the direct effects of these medications on the basal ganglia or indirect influences from their effects on regions that are connected with the basal ganglia or even some sort of ascertainment bias related to unknown clinical features that determined which participants were or were not taking stimulant medications. Longitudinal imaging studies of ADHD youth that are combined with randomized studies of stimulant medications are needed to identify more conclusively the causal effects that ADHD and stimulant medications have on the morphology of basal ganglia nuclei.