An estimated 5%–20% of patients with major depression experience psychotic symptoms, including hallucinations and delusions (
1,
2). Psychotic major depression is associated with more severe psychomotor symptoms and guilt (
3,
4), significantly greater impairment, longer illness duration, and greater likelihood of recurrence compared with nonpsychotic major depression (
1,
5,
6). However, the severity of a depressive episode does not necessarily determine whether psychotic features will be present (
7). Unfortunately, psychotic major depression is frequently misdiagnosed (
8), which can deter effective treatment.
A better understanding of the brain pathophysiology underlying psychotic major depression can lead to earlier detection and more effective treatments. Neuropsychological data from our lab have shown that patients with psychotic major depression have greater cognitive impairment than do patients with nonpsychotic major depression or healthy comparison subjects on tests of working memory, verbal memory, and psychomotor speed but not on tests of simple verbal attention (
9), which implies abnormal brain circuits associated with memory encoding and executive function (
10). However, the relationship of these cognitive impairments to neural function is not clear. For example, impairments in the function of temporal lobe memory systems, prefrontal lobe executive regions, or visual and attention networks could underlie working memory deficits in psychotic major depression.
Working memory tasks require the temporary maintenance and manipulation of information as well as sustained attention. The neurofunctional system subserving verbal working memory has been studied extensively in healthy comparison subjects using functional MRI (fMRI). Studies consistently show that the dorsolateral prefrontal cortex, typically in Brodmann's area 46, sustains activation during the delay periods in which individuals hold information in working memory (
11) and suppresses activation to nonrelevant stimuli (
12). The sustained attention and target detection component of a working memory task often activates a right hemisphere attention system, including regions of the temporoparietal cortex and inferior and middle frontal gyri. This network is consistently activated during detection of task-relevant stimuli, especially when they are infrequent (
13). In the left hemisphere, the inferior parietal region is believed to be part of a storage buffer for verbal information (
14). The hippocampus is also active during the delay period of a working memory task, but the parahippocampal gyrus, in contrast, is activated during the encoding and recognition parts of the working memory task and predicts successful long-term memory encoding (
15).
Because of the widespread brain circuitry involved, patients with various psychiatric disorders have been found to show abnormal brain activation during working memory tasks. Patients with nonpsychotic major depression show abnormal prefrontal function during a verbal working memory task, which emphasizes the importance of comparing patients with psychotic and nonpsychotic major depression (
16–22). Because patients with schizophrenia also display deficits in working memory, several neuroimaging studies have found dorsolateral prefrontal cortex deficits in schizophrenia relative to comparison subjects (
23–25) and to patients with nonpsychotic major depression (
24,
26), which suggests a common substrate for working memory deficits across diagnoses but with greater severity in schizophrenia. Given that psychotic major depression involves symptoms of both nonpsychotic major depression and schizophrenia, these patients may share common deficits in executive function. However, because high cortisol levels in psychotic major depression (
9) are likely to affect hippocampal and parahippocampal systems as well, deficits in medial temporal lobe regions may differentiate psychotic major depression from nonpsychotic major depression.
In this study, we investigated the neural correlates of a verbal working memory task in patients with psychotic major depression in order to better understand the brain circuitry underlying working memory deficits in psychotic major depression. Patients with nonpsychotic major depression and healthy comparison subjects were included to differentiate between depressive subtypes as well as between these subtypes and unaffected individuals. We predicted that the psychotic major depression group would show a unique pattern of activation that reflects abnormalities in medial temporal lobe and parietal regions and similarities to the nonpsychotic major depression group in prefrontal regions.
Results
Data for five participants with psychotic major depression, two with nonpsychotic major depression, and three healthy comparison subjects were excluded because of response box failure resulting in N-back task accuracy below 50%. Scan data from one patient with psychotic major depression, four patients with nonpsychotic major depression, and two healthy comparison subjects were excluded because of movement artifacts during more than 20% of the scan. This left 16 participants in the psychotic major depression group, 15 in the nonpsychotic major depression group, and 19 in the healthy comparison group.
Table 1 summarizes participants' clinical and demographic characteristics. There were no group differences in age, handedness, or years of education, but the groups differed in gender distribution. The groups had similar IQ, as measured by full-scale estimates of premorbid intellectual functioning.
The patients for whom medication data were available had established antidepressant medication regimens with no changes; the psychotic major depression group (N=6) had an average of 20.3 weeks of antidepressant use (range=3–56, SD=19.9) and 9.8 weeks of antipsychotic use (range=5–26, SD=9.2). The nonpsychotic major depression group (N=9) had an average of 42.6 weeks of antidepressant use (range=4–156, SD=56.4).
N-Back Task Performance Results
All participants performed the task with a high degree of accuracy (
Table 1). Response time for the 2-back task was different between groups, and further comparisons showed that this was due to significantly slower response time in the nonpsychotic major depression group compared to the healthy comparison group (p=0.002), even when controlling for group differences in gender (p=0.007). Therefore, all comparisons with the nonpsychotic major depression group during the 2-back task included response time as a covariate.
fMRI Results
Tables 2 and
3 list regions that were significantly activated during the 1-back and 2-back tasks within each group. All three groups activated regions typically associated with verbal working memory tasks, such as the left supramarginal gyrus, left inferior frontal gyrus, and left and right inferior parietal lobe.
Between-Group Analysis of Variance
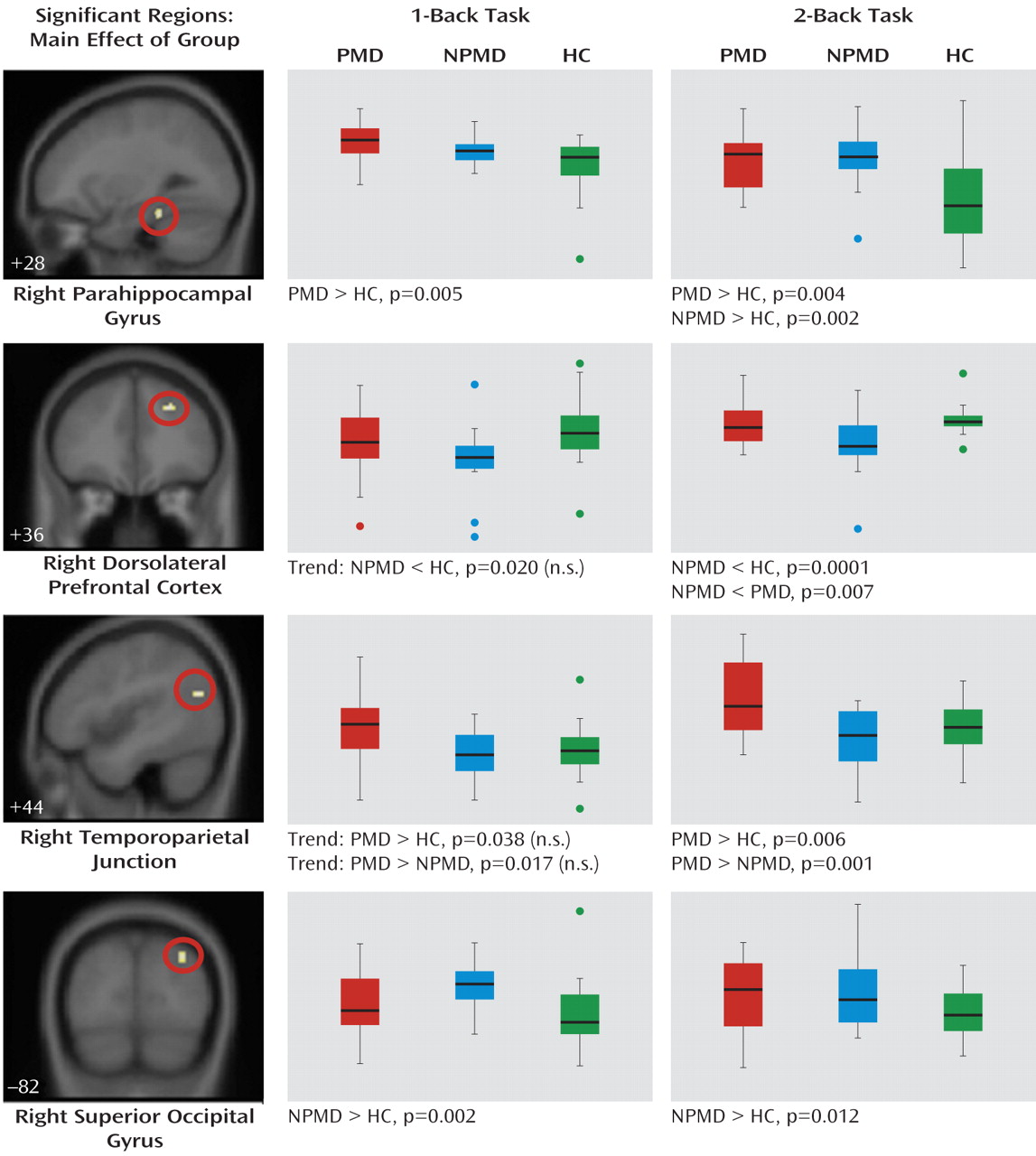
Four clusters of activation were significant (
Figure 1). Activation in the right parahippocampal gyrus was significantly greater during both the 1-back and 2-back tasks in the psychotic major depression group relative to the healthy comparison group. The nonpsychotic major depression group showed greater parahippocampal activation during the 2-back task relative to the comparison group. The nonpsychotic major depression group showed significantly less activation in the right dorsolateral prefrontal cortex during the 2-back task relative to both groups and greater occipital cortex activation relative to the healthy comparison group during both tasks. The psychotic major depression group had significantly greater activation in the right temporoparietal junction during the 2-back task relative to both the nonpsychotic major depression and healthy comparison groups and nonsignificantly greater activation during the 1-back task.
An important question is whether differences between the psychotic and nonpsychotic major depression groups can be attributed solely to differences in severity of depressive symptoms. However, group differences in brain activation did not change when covaried for HAM-D score. Also, we attempted to better understand the influence of medication on our results by examining brain activation in medicated compared with unmedicated participants. Only the nonpsychotic major depression group contained a sufficient number of unmedicated participants to perform this analysis. With the group divided into unmedicated (N=6) and medicated (N=9) subgroups, none of the regions were significantly different between subgroups.
Connectivity Results
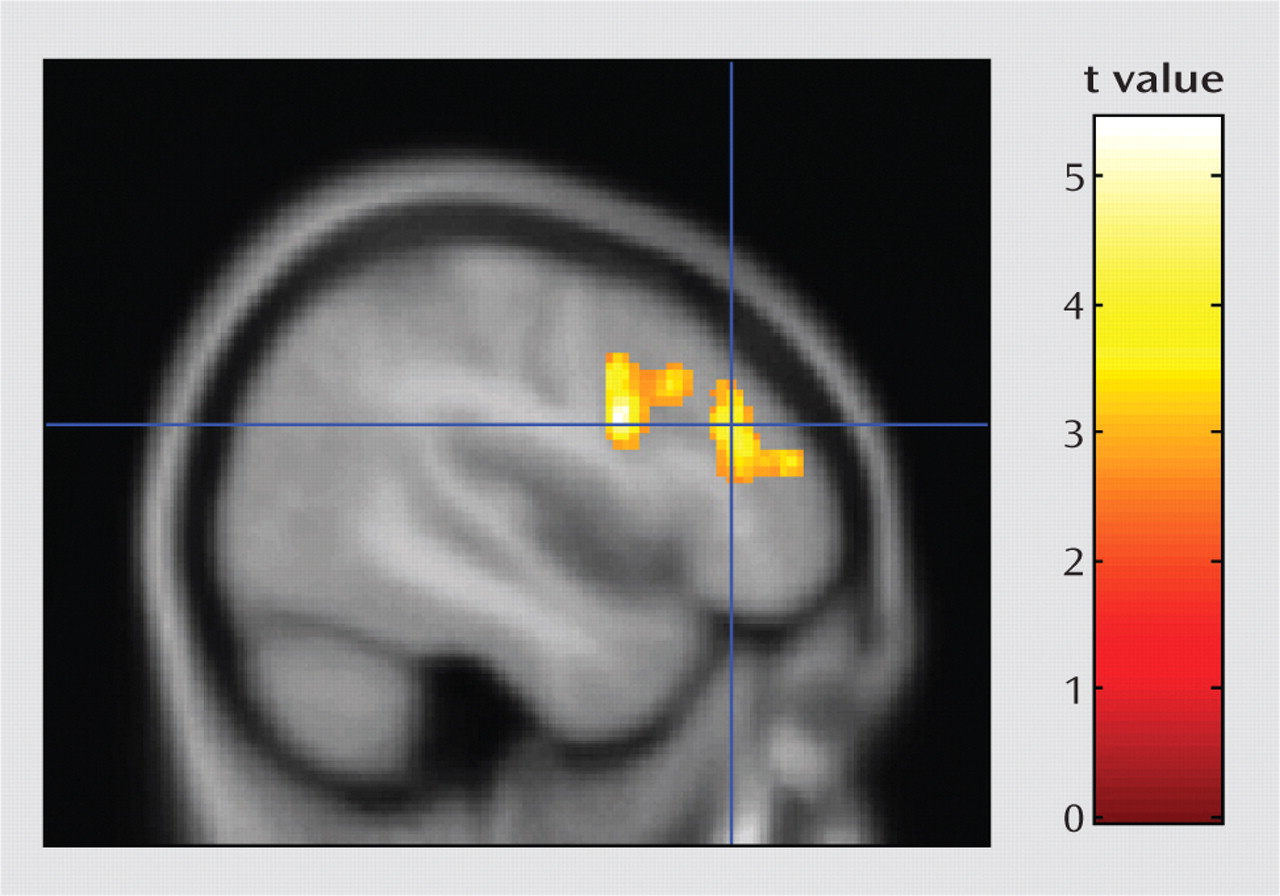
The psychophysiological interaction analysis was conducted to better understand functional circuits involving the temporoparietal junction during the 2-back task. Results showed that activation in a single large cluster in the left prefrontal cortex was significantly associated with activation in the right temporoparietal junction during the 2-back task (
Figure 2). The left prefrontal cluster included three subpeaks: the dorsolateral prefrontal cortex, the middle frontal gyrus, and the inferior frontal gyrus.
Discussion
We examined the neural correlates of working memory in patients with psychotic major depression compared to patients with nonpsychotic major depression and healthy comparison subjects. Given that several previous studies document working memory deficits in both psychotic and nonpsychotic major depression (
9–
10,
17,
20,
33), brain activation in these patient groups may reflect compensatory neural strategies as well as aberrant functional deficits. In the present study, both the psychotic and the nonpsychotic major depression groups showed increased parahippocampal activation relative to the healthy comparison group during the 2-back task, but the psychotic major depression group showed this difference during the less difficult 1-back task as well. These results suggest neural deficits common to both psychotic and nonpsychotic major depression in temporal lobe memory systems, albeit at a lower level of task demand in psychotic major depression. Only the nonpsychotic major depression group showed greater right occipital activation compared to the healthy comparison group, suggesting increased effort toward basic visual processing. The nonpsychotic major depression group also showed lower right dorsolateral prefrontal cortex activation compared to the psychotic major depression and healthy comparison groups during the 2-back task, although, contrary to predictions, no dorsolateral prefrontal cortex abnormalities were seen in the psychotic major depression group. The right temporoparietal junction was the only region where the psychotic major depression group showed significant overactivation compared to both the other groups during the 2-back task, with nonsignificantly greater activation during the 1-back task. In the psychotic major depression group, activation in the temporoparietal junction region showed psychophysiological connectivity with the left dorsolateral prefrontal cortex, which may reflect an executive function compensatory strategy. Controlling for group differences in severity of depressive symptoms did not eliminate observed differences between the psychotic and nonpsychotic major depression groups.
The temporoparietal junction region has been consistently shown in human and primate studies to be involved in reorienting attention to salient stimuli, especially when the stimuli are unexpected and important (
13). The temporoparietal junction is part of the ventral frontoparietal system, as described by Corbetta and Shulman (
13). Based on a large body of literature (
34), these authors hypothesize that the ventral frontoparietal system functions as a “circuit breaker”; the system detects unexpected stimuli that both are behaviorally relevant and require a change in the current task set, such as reorienting attention to a fire alarm while working on your computer. This system is distinct from the dorsal attention system that prepares and applies goal-directed, top-down selection of task-relevant stimuli and responses. Also, the temporoparietal junction region is posterior and ventral to the inferior parietal lobe location (Brodmann's area 40/22) that is frequently activated bilaterally in neuroimaging studies of working memory, also called the “visuospatial scratch pad.”
As part of an automatic attention system, the temporoparietal junction could be important for sensory gating processes. Sensory gating has been suggested to be impaired in psychotic major depression, as data show that patients with this disorder have difficulties distinguishing relevant from irrelevant stimuli (
35). In addition, we have observed abnormalities unique to patients with psychotic major depression during performance of the Stroop task (
10), which requires sensory gating and filtering processes to resolve response conflict. A well-known measure of sensory gating is the prepulse inhibition test of the acoustic startle reflex (
36). Although no studies of prepulse inhibition have focused on patients with psychotic major depression, it has been shown to be deficient in patients with schizophrenia but not in patients with nonpsychotic major depression (
37–39). Furthermore, a study of transgenic mice that overexpress corticotropin-releasing factor (proposed to be a model of psychotic depression) also show startle reflex gating deficits that are reversed by antipsychotic drugs (
40). Because of psychotic symptoms and problems with filtering, the psychotic major depression group studied here may be expending significantly more neural resources for sensory gating and filtering, and this may be reflected in the increased activation of the temporoparietal junction region. The temporoparietal junction region may function to continually reorient attention to the task, perhaps because of internal and external distractions, including noise from the scanner, or possibly the intrusion of psychotic symptoms. Notably, the psychotic major depression group showed insula activation during the 2-back task, and reduced insula volume has been linked to psychotic symptoms in patients with psychotic major depression (J.D. Cohen et al., unpublished 2009 data). The post hoc analysis showing functional connectivity of the temporoparietal junction with the left prefrontal cortex suggests that top-down executive control over temporoparietal junction resources may compensate for sensory gating deficits, allowing accurate task performance.
The nonpsychotic major depression group showed increased activation in the parahippocampal cortex during the 2-back task, as did the psychotic major depression group during both the 1- and 2-back tasks. Traditionally, medial temporal lobe regions and the adjacent parahippocampal cortex have not been considered to be necessary for working memory tasks but to have an essential role in memory encoding and retrieval. However, a growing body of literature suggests that parahippocampal activation during working memory tasks is associated with encoding of the stimuli and predicts subsequent recall (
15). Similar to the present study, a previous investigation (
18) showed increased parahippocampal activation in patients with nonpsychotic major depression relative to healthy comparison subjects during a verbal working memory task. The present study goes further to find a similar deficit in psychotic major depression but at a lower level of task demand than in nonpsychotic major depression.
The dorsolateral prefrontal cortex is strongly associated with working memory tasks in healthy volunteers (
11). Our study replicates previous reports of abnormal dorsolateral prefrontal cortex activation in nonpsychotic major depression. However, other studies show increased, rather than decreased, dorsolateral prefrontal cortex activation in nonpsychotic major depression during accurate task performance, usually in the left hemisphere (
20–22), but also in the right (
19). The nonpsychotic major depression group also had lower right dorsolateral prefrontal cortex activation compared to the psychotic major depression group, and this difference remained after covarying for severity of depressive symptoms. This suggests that right hemisphere dorsolateral prefrontal cortex abnormalities contribute to working memory function in nonpsychotic major depression but not in psychotic major depression. However, the connectivity analysis suggests that the left dorsolateral prefrontal cortex may compensate for aberrant brain activation related to sensory gating in psychotic major depression.
These results support the hypothesis that psychotic major depression is distinct from nonpsychotic major depression on a neurofunctional basis. The study has several limitations, however, including the small sample size and medications taken by the clinical subjects, allowing us to detect only relatively large group differences. Although our analyses covaried for gender, imbalanced gender distributions across diagnoses may have contributed to our findings. The task was not difficult enough to demonstrate the previously shown deficits in working memory that have been documented for both patients with psychotic and nonpsychotic major depression. Greater group differences might have been detected by using a task that more rigorously challenged participants' working memory abilities, and an event-related analysis could separate trials on the basis of accuracy or response time for more detailed comparisons between groups.
It is possible that group differences in medication may have influenced brain activation. However, examining the distribution of medicated and unmedicated patients in each group did not reveal patterns suggesting a strong effect of medication. Moreover, we previously reported, using this sample of patients (
9,
10), that medication status does not affect differences in cognitive test performance between symptomatic psychotic and nonpsychotic depressed groups. However, as psychiatric medications clearly affect brain function, we cannot rule out the possibility that medications influenced our results, which should thus be considered preliminary. Future studies would benefit from including only unmedicated subjects.