The mechanisms underlying the chronic, relapsing nature of alcoholism are not well understood. Several medications are efficacious in the treatment of alcoholism and in initiating abstinence (
1), but more than two-thirds of alcohol-dependent individuals return to drinking within weeks or months of initiating recovery (
2,
3). While psychosocial factors contribute to relapse susceptibility (
2), there is increasing interest in the neurobiological markers that account for the chronic, relapsing nature of the illness.
Previous evidence clearly indicates chronic alcohol-related neurotoxicity and brain atrophy. MRI studies examining lobular volumes or regions of interest find smaller volumes most consistently in the cortical gray matter of the frontal lobes or fronto-temporal region (
4–6) but also in posterior cortical regions (
7,
8), subcortical regions (
7), and the cerebellum (
9). Whole-brain voxel-wise analyses support the existence of gray matter deficits in each of the cortical lobes (
10–13), the thalamus, and the cerebellum (
11,
12). More severe gray matter deficits have been reported in alcoholics who relapse than in those who abstain (
14,
15). Assessing volumes in specific regions of the amygdala, hippocampus, and ventral striatum after only 1 week of alcohol abstinence, Wrase and colleagues (
16) recently reported smaller amygdala volumes in relapsed compared with abstinent alcoholics. While this previous research suggests a role for alcohol-related brain atrophy in the chronic, relapsing nature of the illness, no previous study has assessed whether brain volumes during sustained alcohol abstinence are predictive of time to alcohol relapse.
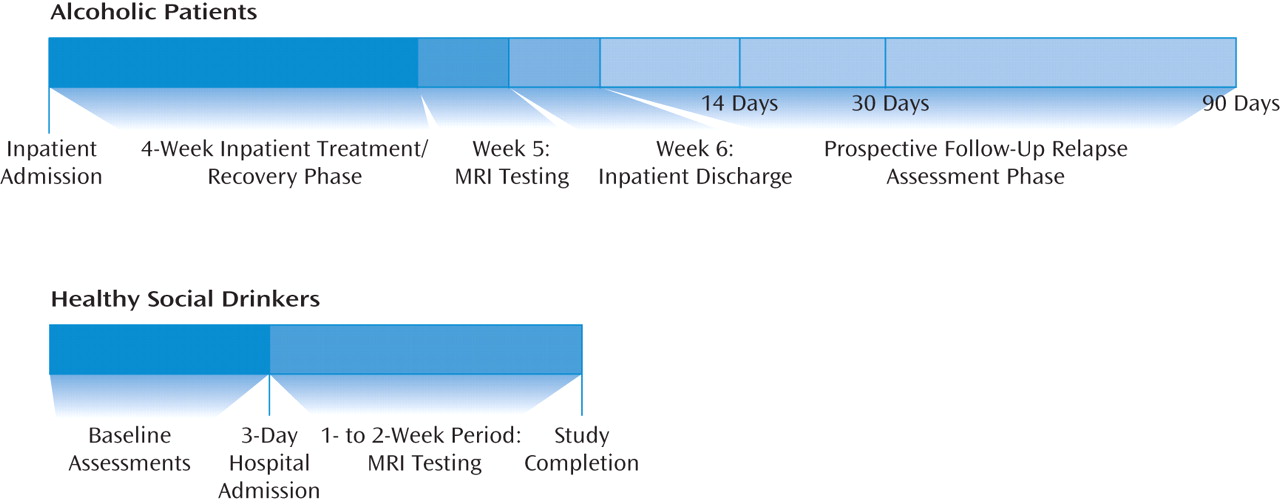
In this study, building on previous research, we assessed gray matter volumes using automated segmentation and registration of high-resolution MR structural brain images; a whole-brain analysis implemented voxel-based morphometry to assess gray matter volume differences of alcohol-dependent men and women receiving inpatient treatment after 1 month of abstinence, as compared with social-drinking healthy comparison subjects. All alcohol-dependent patients were then followed prospectively with face-to-face interviews at 14, 30, and 90 days after discharge from inpatient treatment to assess alcohol relapse outcomes. We expected to find gray matter volume deficits in the alcohol-dependent relative to the healthy -comparison group and an association between gray matter volume deficits and prior history of chronic alcohol abuse. Furthermore, because medial frontal regions play an important role in behavioral control and decision-making functions that are likely to contribute to relapse risk (
17,
18), we specifically hypothesized that gray matter volume deficits in these regions would be independently predictive of shorter time to alcohol relapse.
Results
There were no differences between the alcohol-dependent and healthy comparison groups in sex, race, and lifetime prevalence of mood and anxiety disorders. The patient group was older on average and had a lower mean IQ than the comparison group (
Table 1), and these measures were included as covariates in the group difference analyses and in the relapse prediction analyses. As expected, the patient group also had a significantly higher mean number of days of alcohol use, a greater mean number of drinks per day, and a greater mean number of years of alcohol use.
Group Differences in Voxel-Based Morphometric Analyses
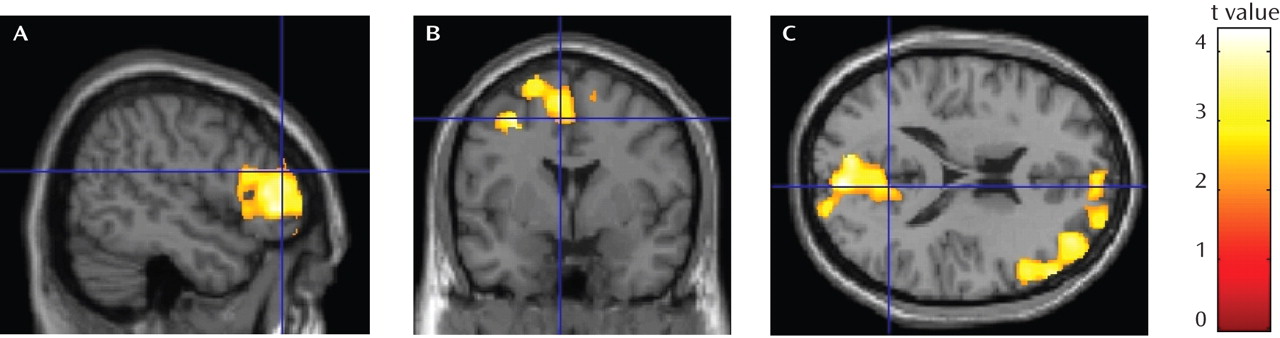
As shown in
Table 2 and
Figure 2, the alcohol-dependent group showed significantly lower gray matter volume than the comparison group in three regional clusters: the lateral prefrontal cortex, including the right dorsolateral and inferolateral prefrontal cortex (family-wise error p<0.004, cluster size=8,060 voxels); a medial frontal cluster including the dorsal anterior cingulate gyrus, the medial and lateral superior frontal gyrus (including the supplementary and presupplementary areas), the middle frontal gyrus, and the posterior cingulate gyrus (family-wise error p<0.013, cluster size=6,513 voxels); and a parietal and occipital cortex cluster including the precuneus and cuneus regions and the posterior cingulate (family-wise error p<0.009, cluster size=6,886 voxels). Montreal Neurological Institute space coordinates from SPM output were converted to Talairach coordinates (
40) using the Nonlinear Yale Montreal Neurological Institute to Talairach Conversion Algorithm (
41). Anatomical regions for coordinates within significant clusters were identified using the Automated Anatomy Library (
42) for Montreal Neurological Institute space and confirmed using the Talairach Daemon (
43) for Talairach space within the WFU (Wake Forest University) PickAtlas (
44). No clusters were found for which volumes in the alcohol-dependent group were significantly larger than in the comparison group.
Correlation of Regional Volume Differences With Baseline Alcohol Use Measures
After controlling for sex, a significant negative correlation was observed between volume in the medial frontal cluster and both the number of years of alcohol use (r=−0.38, df=42, p<0.01) and the number of days of alcohol use during the 90 days preceding treatment (r=−0.32, df=42, p<0.03). Volume in the lateral prefrontal cluster also correlated significantly with both the number of years of alcohol use (r=−0.32, df=42, p<0.03) and the number of days of alcohol use during the 90 days preceding treatment (r=−0.32, df=42, p<0.03). No significant correlations were observed between gray matter volume in the parietal-occipital cluster and baseline alcohol use measures.
Relapse Rates
Alcohol use information pooled from the Substance Use Calendar, urine and breath test results, and collateral information indicated that relapse rates were 25% (11/44) at day 14, 43% (19/44) at day 30, and 68% (30/44) at day 90.
Regional Volume Difference and Prediction of Relapse Outcomes
Age, IQ, gender, and years of education as well as clinical variables such as lifetime mood disorders, lifetime anxiety disorders, depressive symptom scores on the Beck Depression Inventory, lifetime years of alcohol use, and baseline alcohol use measures (total use, number of days of use, and amounts per occasion of use) were assessed independently for prediction of relapse using proportional hazard regression models. Greater number of years of alcohol use (χ2=6.37, df=1, 44, p<0.01; hazard ratio=1.06, 95% CI=1.01-1.11) and greater amount of total alcohol use in the baseline 90-day period (χ2=4.24, df=1, 44, p<0.04; hazard ratio=1.001, 95% CI=1.00-1.001) were each significantly predictive of shorter time to alcohol relapse. Variables that predicted shorter time to relapse but fell short of statistical significance included higher age (χ2=2.66, df=1, 44, p<0.10; hazard ratio=1.04, 95% CI=0.99-1.09) and lower IQ (χ2=2.64, df=1, 44, p<0.10; hazard ratio=0.97, 95% CI=0.93-1.01).
After controlling for age, IQ, years of alcohol use, and total alcohol use in the 90 days preceding inpatient treatment, smaller gray matter volume remained significantly predictive of shorter time to relapse for the medial frontal cluster (χ
2=6.7, df=5, 44, p<0.009; hazard ratio=0.52, 95% CI=0.31-0.85) and the parietal-occipital cluster (χ
2=9.28, df=5, 44, p<0.002; hazard ratio=0.52, 95% CI=0.34-0.79). Smaller gray matter volume fell short of significance for the lateral frontal cluster (χ
2=4.74, df=5, 44, p<0.06; hazard ratio=0.68, 95% CI=0.48-0.96). The hazard ratios indicated that for each 1-ml reduction in gray matter volume in the medial frontal cluster (hazard ratio=0.52) and in the parietal-occipital cluster (hazard ratio=0.52), there was a 48% increase in risk of earlier relapse. Similarly, for relapse to heavy drinking, Cox proportional hazards regression analyses indicated that gray matter volume was significantly associated with time to relapse to heavy drinking in the medial frontal cluster (χ
2=4.66, df=5, 44, p<0.04; hazard ratio=0.56, 95% CI=0.33-0.95) and the parietal-occipital cluster (χ
2=7.56, df=5, 44, p<0.006; hazard ratio=0.55, 95% CI=0.36-0.84) and fell short of significance in the lateral frontal cluster (χ
2=2.91, df=5, 44, p<0.09; hazard ratio=0.74, 95% CI=0.52-1.05). Smaller gray matter volume in the medial frontal and the parietal-occipital cluster predicted shorter time to relapse to heavy drinking (by 44% and 45%, respectively).
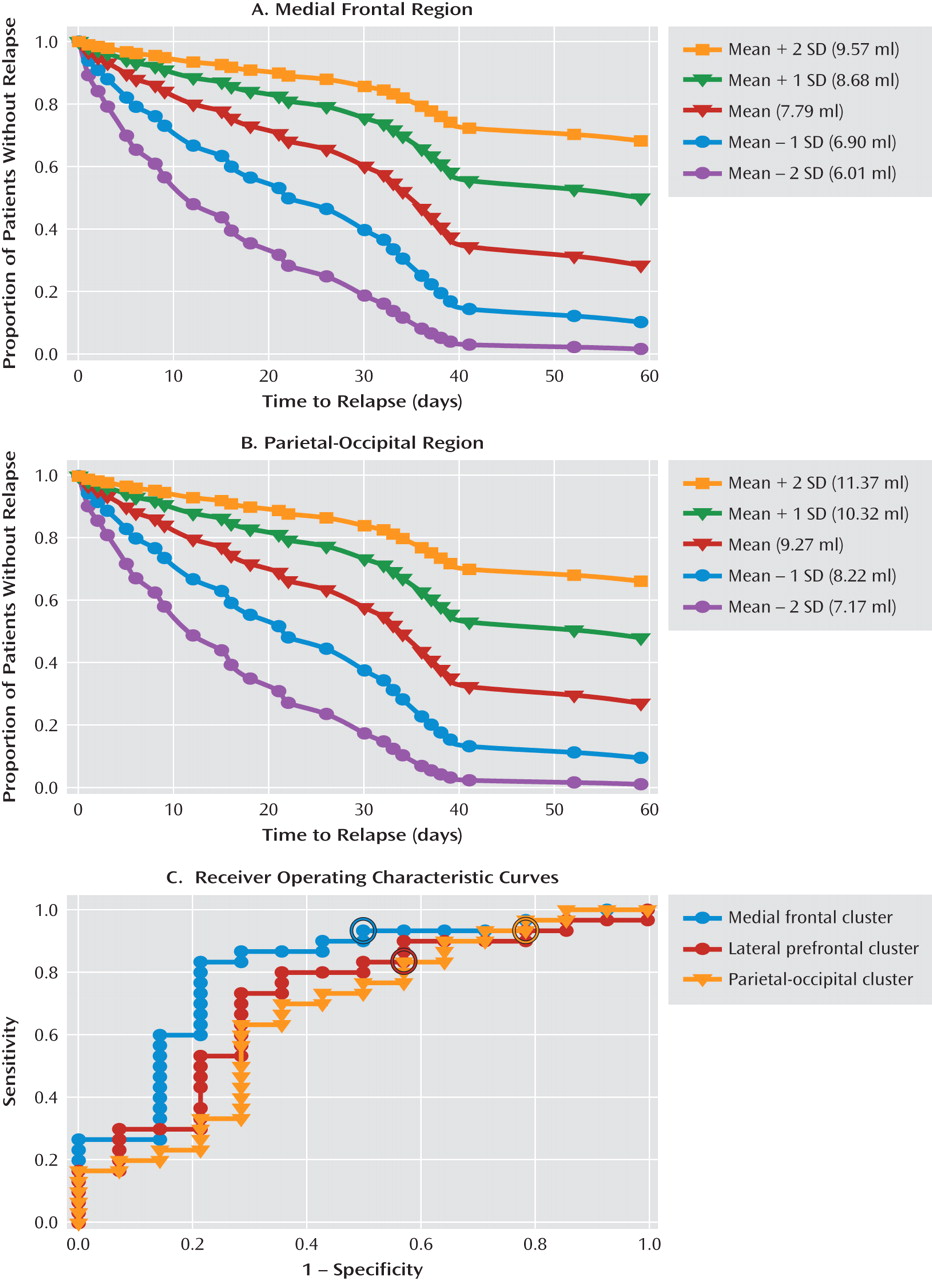
Figure 3 illustrates the estimated survival functions for the gray matter volume mean and the mean plus one and two standard deviations in either direction for the medial frontal cluster (
Figure 3A) and the parietal occipital cluster (
Figure 3B) with covariates kept constant.
In addition, nonparametric receiving operator characteristic (ROC) analysis was applied to assess the sensitivity and specificity with which gray matter volume for each of the three region clusters identified those who relapsed compared with those who did not. The ROC curves for the medial frontal, lateral frontal, and parietal-occipital clusters are shown in
Figure 3C. Logistic regression analyses indicated significant probabilities for classifying those who relapsed and those who did not for each of the three regions, along with the optimal cutoff values for gray matter volume for each cluster.
Discussion
Our findings in this study indicate significantly smaller gray matter volume in medial frontal, lateral prefrontal, and posterior parietal-occipital regions of the brain in a sample of alcohol-dependent patients engaged in inpatient treatment after 1 month of abstinence relative to healthy comparison subjects. The observed gray matter volume deficits were located in the medial frontal cortex, including the medial frontal gyrus and the anterior cingulate gyrus, but extending laterally in the superior and middle frontal gyri, the lateral prefrontal regions, primarily including the dorsolateral and inferolateral prefrontal cortex, and lastly a posterior region centered on the parietal-occipital sulcus, overlapping the precuneus, cuneus, and posterior cingulate regions. No areas were found in which the alcohol-dependent sample had larger gray matter volume than the comparison group. These results are consistent with previous studies and provide further evidence of gray matter volume reductions in 1-month abstinent recovering alcoholics relative to comparison subjects. Modest significant correlations were also observed between volume deficit in alcoholic patients and number of years of alcohol use and between volume deficit and baseline number of days of alcohol use prior to treatment. More importantly, using a prospective follow-up study design and after controlling for the influence of demographic variables (age and IQ) and alcohol use history variables (years of alcohol use and total amount of alcohol used for the 90-day period preceding treatment), smaller gray matter volume in the medial frontal cluster and the posterior-occipital cluster were each predictive of shorter time to any alcohol relapse and to heavy drinking relapse. In addition, the medial frontal cluster most accurately classified relapsers versus nonrelapsers at 80%, with a sensitivity of 93.3% and a specificity of 50%.
In support of our hypothesis, our findings indicated that smaller gray matter volume within two regions of medial frontal cortex cluster-the dorsal anterior cingulate (Brodmann's area 24/32) and the presupplementary (Brodmann's area 6) and supplementary motor regions-was predictive of shorter time to relapse and greater relapse risk in the alcohol-dependent sample. The medial frontal cortical regions are involved in cognitive control with top-down processing of sensory inputs, internal states, thoughts, and actions that guide behavior in pursuit of internal goals (
45). Cognitive control makes possible flexible, adaptive task-relevant behavior when more automatic responses are inadequate or inappropriate in the interest of long-term goals. Effective cognitive control therefore transcends habitual stimulus-response associations (
46). Although this study did not establish a link with functional impairment, the volume deficits in the medial frontal cortical cluster would suggest disruption of cognitive control functions associated with atrophy in these regions. Greater atrophy in these brain regions could therefore weaken a recovering alcohol-dependent patient's ability to override strong, habitual responses to environmental cues, stress, or otherwise cognitively challenging situations and increase his or her susceptibility to relapse, as observed with the association between brain atrophy in this region and relapse risk.
More specifically, the dorsal-caudal region of the anterior cingulate (Brodmann's area 24), which was part of the medial frontal cortex cluster that predicted alcohol relapse, makes up the anterior cingulate cognitive division (
47). Evidence suggests a role for the anterior cingulate cognitive division in maintaining attention to goal-relevant stimuli when distracting stimuli conflict with these goals (
48), and the inhibition of incorrect actions or facilitation of correct responses (
49). Deficits in these executive functions are characteristic of poor impulse control (
50) and are known to occur with addiction (
51). Altered impulse control has been observed in recently abstinent alcohol-dependent individuals (
52). In a recent study using low-resolution brain electromagnetic tomography (
53), abnormalities in Brodmann's areas 24 and 32, as measured by reduced visual P3 amplitudes, were observed in alcohol-dependent patients relative to comparison subjects, and in the high-impulsivity participants from the entire sample when compared to the low-impulsivity participants. It is possible that overall gray matter volume loss in Brodmann's area 24/32, as well as the degree of gray matter recovery during abstinence, may have an effect on impulse control and risk of relapse. The presupplementary (Brodmann's area 6) and supplementary motor areas are involved in switching response sets when task rules change (thus altering a stimulus-response association) but also in error and conflict processing. Humans with lesions in these regions demonstrate impaired switching between response sets (
54), which could result in difficulties in adaptively responding to alcohol cues in the environment and thus an elevated risk of relapse.
Although not hypothesized, we also found significantly smaller gray matter volume in the alcohol-dependent group in a region surrounding the parietal-occipital sulcus and extending anteriorly into the posterior cingulate gyrus. Gray matter volume deficits relative to comparison subjects have been previously observed in the parietal and occipital lobes of long-term abstinent alcoholics (6 months to 21 years [55]). Our alcohol-dependent sample had been abstinent for 1 month at the time of their imaging sessions, so our finding is consistent with evidence of persistent deficits in posterior gray matter volume. The impact of dysfunction in parietal-occipital areas on alcohol use and relapse is unclear. However, in a functional MRI study (
56), abstinent alcohol-dependent patients showed significantly lower activation to visual stimuli in the occipital lobes bilaterally. In a study using multiple regression analyses to examine the relative contributions of tasks assessing visuoperceptual processes, explicit declarative memory, and frontal executive function in the performance of a visuoperceptual learning task (
57), the results suggested that male alcoholics invoked executive processes to perform at normal levels, while male control subjects used basic visuoperceptual processes. Alcohol-dependent individuals therefore may be using frontal executive processes to compensate for deficits in visual processing. Interestingly, the authors suggested that the inefficient use of higher executive processes to perform a low-level cognitive task could leave insufficient reserves of higher-level attentional capacity available for additional cognitive demands. To the extent that these executive processes are unavailable to alcohol-dependent individuals because they are being used to compensate for deficits in visual processing, the resources necessary to exert cognitive control may be unavailable, thus increasing vulnerability to alcohol use. Our finding that reduced gray matter volume in visual processing areas of alcohol-dependent subjects predicted shorter time to relapse is consistent with this explanation. The fact that smaller gray matter volume in this region predicted alcohol relapse outcomes suggests a need to examine structural and functional atrophy in this region more closely in both those at risk for alcohol dependence and those at greatest risk of relapse.
There are important clinical implications to our findings. First, MRI volume assessment of medial frontal and posterior parietal-occipital brain regions could be further developed as neural markers for identifying alcoholic patients entering treatment who are at highest risk of relapse and treatment failure. For example, estimated survival functions for gray matter volume values that are one and two standard deviations above the mean (
Figure 3A,B) show surviving alcohol relapse and maintaining abstinence at 60 days since discharge, indicating good outcome. By contrast, estimated survival functions for gray matter volume values that are one and two standard deviations below the mean show precipitous decreases in surviving risk of relapse, to levels below 20%. In particular, data from the ROC curves showed that the medial frontal cluster most accurately identified relapsers versus nonrelapsers at an 80% accuracy rate. Second, on further validation in future studies, use of such neural markers in clinical assessment could inform treatment planning by prescribing tailored interventions to improve brain atrophy and associated function early in abstinence in alcoholics who are at greatest risk of relapse. Recent preclinical data with evidence of microglial cell proliferation and neurogenesis during recovery from chronic alcohol exposure in laboratory animals (
58) suggest that in alcoholics with significantly smaller gray matter volume, longer-term rehabilitation would be of benefit both for recovery from brain atrophy and in decreasing relapse risk. Finally, our data in this study support the development of pharmacological and other neural therapies that promote neurogenesis and cell proliferation to increase gray matter volume in critical regions in order to decrease relapse vulnerability and promote recovery from alcoholism.
Several limitations of this study are noteworthy. Because of the small number of women included in the sample, we were unable to examine sex-specific effects on gray matter volume deficits. Although the effects of sex were accounted for in the analyses, larger studies that assess sex differences in the association between gray matter volume deficits and relapse risk are recommended, as there is some evidence of greater sensitivity to neurotoxicity in women than in men (
59). Furthermore, some recent evidence (
60) indicates that MRI findings in treatment-seeking alcoholics may not be generalizable to non-treatment-seeking alcoholics in the community; and while the present study is especially relevant to treatment-seeking clinical alcoholic samples, it would be of benefit to assess gray matter volume deficits in non-treatment-seeking community alcoholic samples. Finally, our relapse assessments used self-reports and urine toxicology assessments that assess alcohol use through levels of alcohol metabolites for a limited time window of up to 80 hours, and hence our study lacked objective long-term assessments of alcohol use. On the other hand, these methods are widely used clinically, and the relapse rates we observed are consistent with those reported in the literature. Despite these limitations, this study is the first to link gray matter volume deficits in a sample of treatment-receiving alcoholics early in abstinence to subsequent time to relapse, and to provide predictive estimates of relapse risk. The findings provide clear evidence that smaller gray matter volumes in specific medial frontal and posterior brain regions play an important role in alcoholism relapse risk and clinical outcome.