There is considerable debate over whether attention deficit hyperactivity disorder (ADHD) represents a diagnostic category with distinct boundaries between the disorder and health or is better understood dimensionally, with those who have the disorder lying at the extreme end of a continuum of symptoms (
1–3). Much evidence supports a dimensional view, including several taxonometric studies suggesting that symptoms of ADHD and associated neuropsychological deficits lie on a continuum (
4,
5). Further support for a dimensional view would be gained from linking symptom dimensions to biological constructs such as brain development. In the present study, we used neuroanatomic imaging data acquired from typically developing children with no psychiatric diagnoses to explore whether high levels of hyperactive/impulsive symptoms are underpinned by neurodevelopmental patterns that resemble those seen in children with the syndrome of ADHD.
In this study, a dynamic measure of cortical change was used, since previous studies suggest that there are ADHD diagnostic differences in trajectories of cortical development (
6–9). Using a measure of cortical thickness, we previously reported that in both typically developing children and youth with ADHD, an early childhood phase of cortical increase eventually reached a peak—attained later in those with ADHD in the fronto-temporal cortex—before giving way to a phase of cortical thinning (
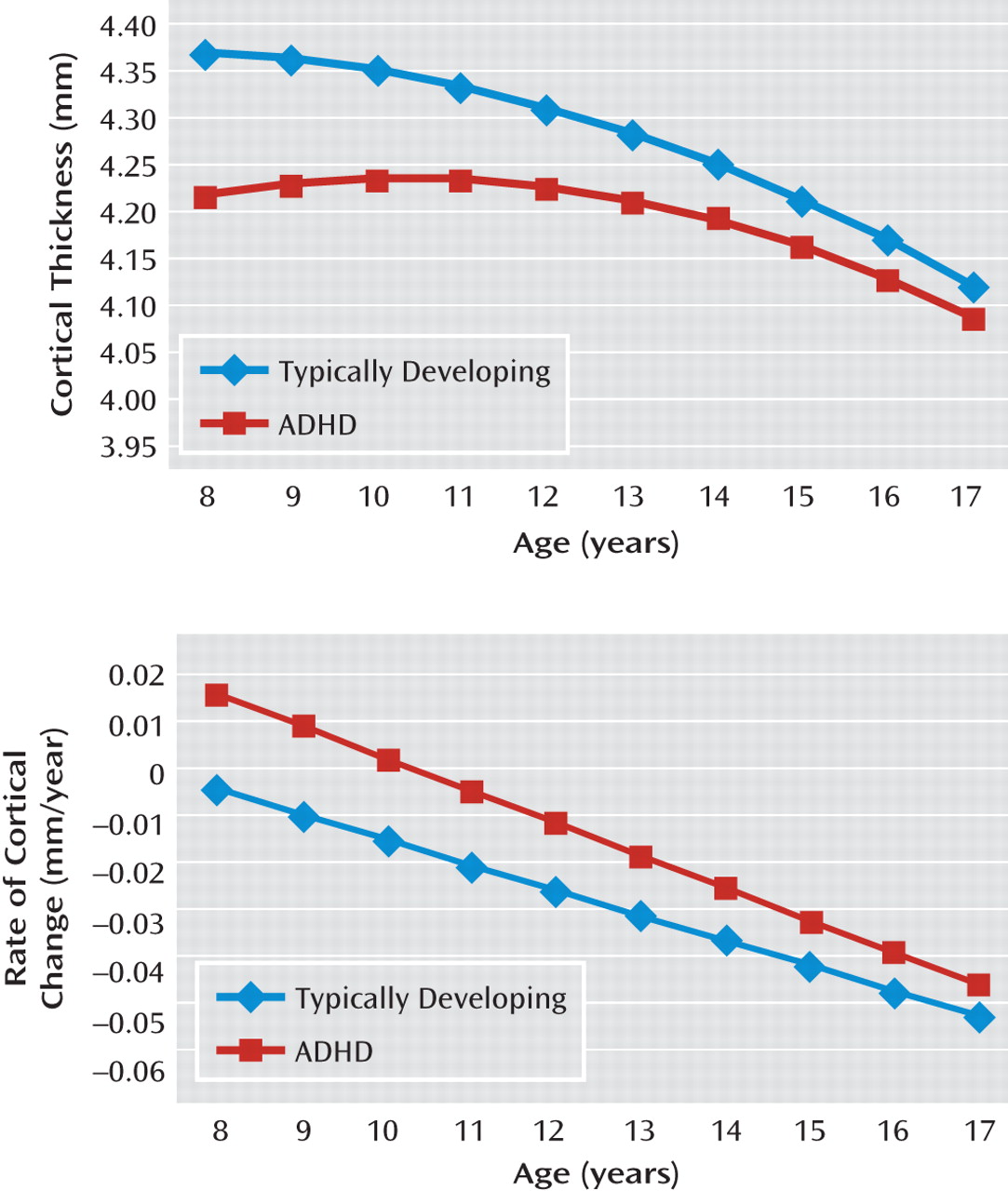
7). There were diagnostic differences in the phase of cortical thinning, with the velocity of thinning being consistently slower in youth with ADHD (see Results section). Given that cortical thinning dominates most of late childhood and early adolescence and most of our neuroimaging data lie within the same age range, cortical thinning emerges as a potential neuroanatomic marker for ADHD.
For the present study, we inquired whether similar differences in the rate of cortical thinning would occur in a typically developing group of children who have varying levels of hyperactive and impulsive symptoms. We used a version of the Conners' Rating Scales (
10), which includes a factor reflecting hyperactive and impulsive symptoms. This version does not include a factor reflecting purely inattentive symptoms, but rather a factor of learning difficulties, which while consisting of several items that reflect inattention and distractibility, includes more general learning difficulties. We did not include this learning problems factor and focused instead on the factor reflecting hyperactive and impulsive symptoms. We hypothesized that typically developing children with higher levels of hyperactive and impulsive symptoms would show cortical trajectories resembling those found in ADHD, specifically a slower rate of cortical thinning during late childhood and adolescence. To assess symptom specificity, the cortical changes associated with a measure of conduct problems in the same participants were determined, with the prediction of distinct cortical correlates for the different symptom domains.
Results
Among typically developing youth, hyperactive/impulsive symptom scores did not differ significantly by sex (boys: mean=1.43 [SD=1.59]; girls: mean=1.35 [SD=1.36]) and did not correlate with IQ (r=0.008, p=0.91). The ADHD group had greatly elevated hyperactive/impulsive symptom scores (mean=9.2 [SD=1.7]), and there was no overlap between the range of scores for the typically developing and ADHD groups (difference between groups: t=48.6, df=388, p<0.0001), in keeping with the severe phenotype of ADHD selected for this study. For the ADHD group, hyperactive/impulsive symptom scores also did not differ significantly with regard to sex (boys: mean=9.13 [SD=1.7]; girls: mean=9.3 [SD=1.7]) and did not correlate with IQ (r=0.05, p=0.53). Further details are presented in Table 1 and Table 2 of the data supplement accompanying the online version of this article, which also give the number of hyperactive/impulsive symptoms and participants' ages at each wave of scan acquisition.
For the three subgroups of typically developing youth, the mean conduct problems score was 1.6 (SD=1.9 [minimum, 0; maximum, 8]). Scores for conduct problems were higher for boys (mean=1.81 [SD=2]) than girls (mean=1.4 [SD=1.8]) but with no statistical significance. Additionally, IQ did not correlate with scores for conduct problems (r=−0.007, p=0.92).
Neuroanatomic Findings
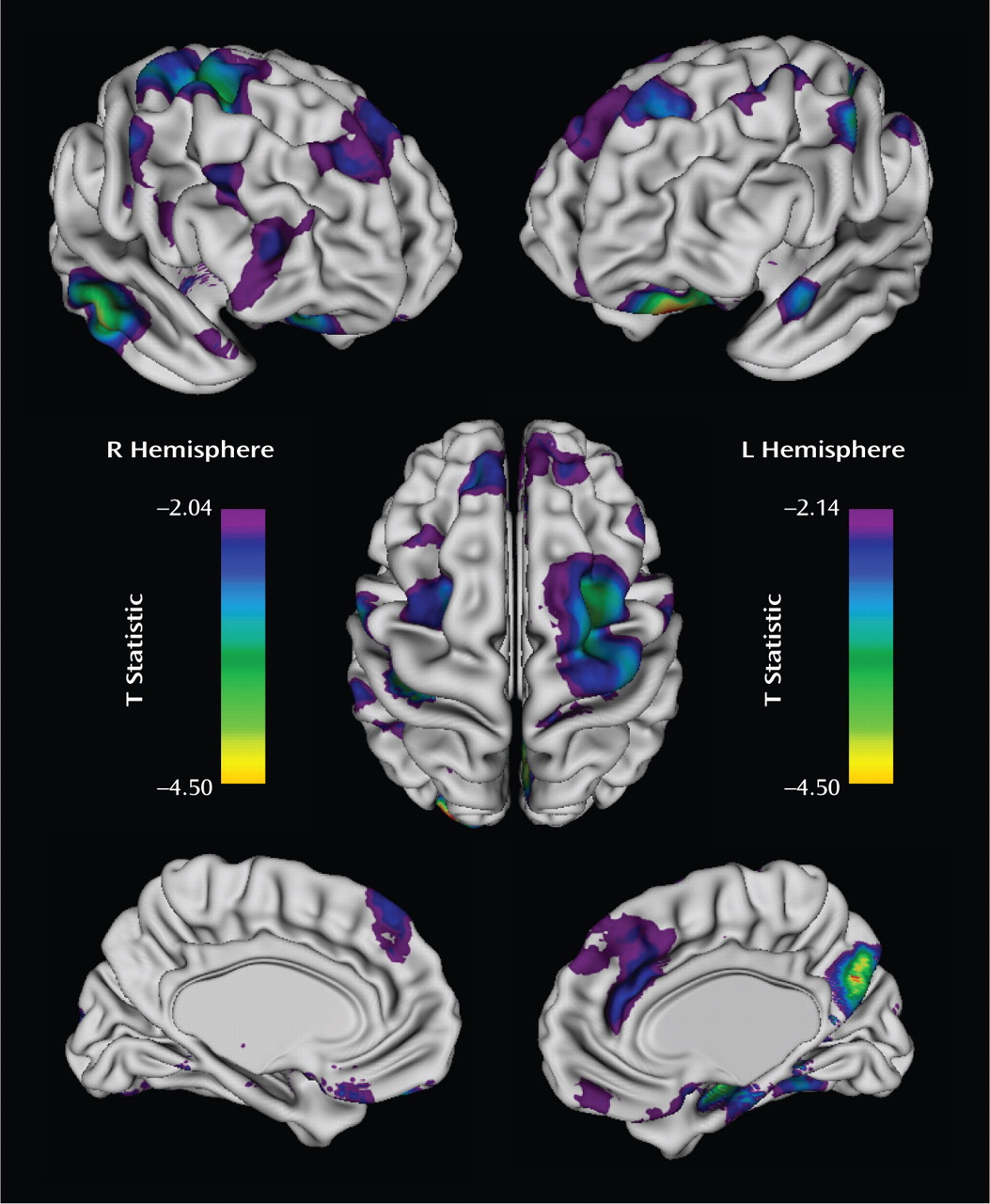
The rate of cortical thinning, predominantly in prefrontal cortical regions, varied significantly with the hyperactive/impulsive symptom score, with adjustment made for multiple comparisons using the false discovery rate procedure. There were clusters bilaterally in the middle frontal gryus, extending on the right toward the -supplementary motor area and motor strip; the medial prefrontal wall, extending toward the anterior cingulate and superior frontal gyrus; the orbitofrontal cortex; and the right inferior frontal gyrus (
Figure 2). More posteriorly, cortical thinning rates differed with hyperactive/impulsive symptom scores in the right middle/inferior temporal gyrus, parahippocampal gyrus, and precuneus as well as in the left superior gyrus. For each increase of one point in the hyperactive/impulsive symptom score, the estimated change in the rate of cortical thinning in the right prefrontal cortical regions, shown in
Figure 2, was 0.0054 mm/year (SE=0.0019 mm/year; t=2.91, df=194, p=0.004), and the estimated change in the left prefrontal cortical regions was 0.0055 mm/year (SE=0.0019 mm/year; t=2.9, df=194, p=0.004). Extent and coordinates of the local maxima of the clusters are presented in
Table 1.
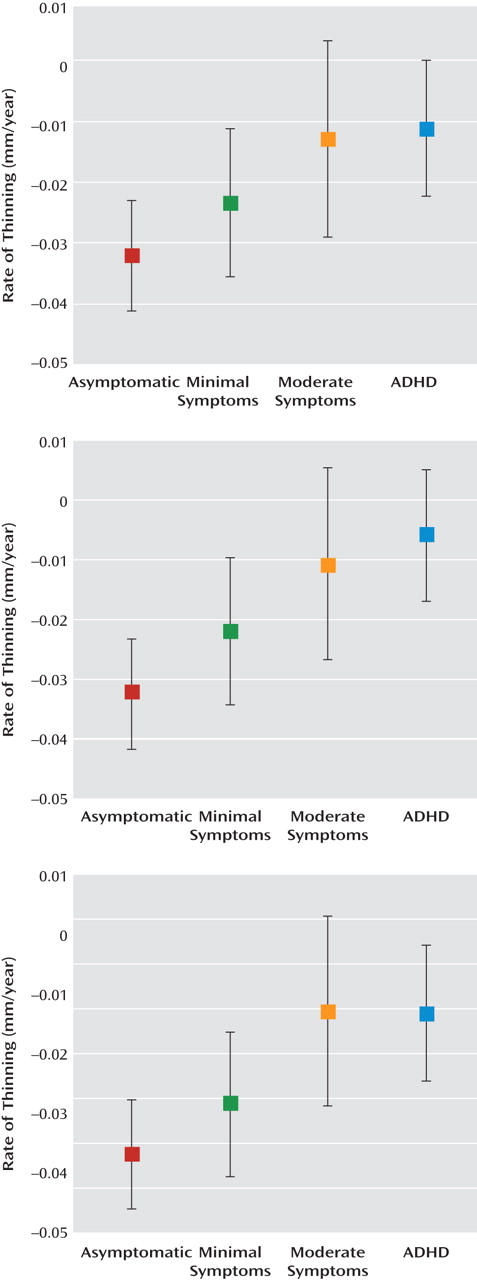
The effect of hyperactive/impulsive symptom severity can be clearly seen by comparing the rates of cortical thinning in participants who were grouped by the number of symptoms with the rates for those who had a diagnosis of ADHD (
Figure 3). For both the right and left prefrontal cortical regions (
Figure 2), the rate of cortical thinning changed in a stepwise manner with the degree of symptom severity. The rate of cortical thinning was slowest in participants with a diagnosis of ADHD and increased progressively in typically developing youth, with the moderate symptoms group having a higher rate than the ADHD group and the minimal symptoms group having a higher rate than those with moderate symptoms. Asymptomatic, typically developing youth had the highest rate of cortical thinning. In the right and left prefrontal cortical regions, pairwise contrasts showed that the asymptomatic group had a significantly higher rate of cortical thinning than both the moderately symptomatic group (right: t=2.4, df=194, p=0.02; left: t=2.3, df=194, p=0.02) and the clinical group with ADHD (right: t=3.5, df=194, p=0.0005; left: t=4.2, df=194, p<0.0001). Typically developing subjects with minimal symptoms had a significantly higher rate of cortical thinning than the ADHD group (right: t=2.2, df=194, p=0.03; left: t=3.1, df=194, p=0.002). For the posterior regions, the stepwise slowing of cortical change was less pronounced, since the ADHD group showed a similar rate of cortical thinning to typically developing subjects with moderate symptoms. However, participants in the ADHD group still had a significantly lower rate of cortical thinning than typically developing youth with no (t=3.2, df=194, p=0.001) or minimal (t=2.5, df=194, p=0.01) symptoms. This pattern of results remained when sex and IQ were entered as covariates.
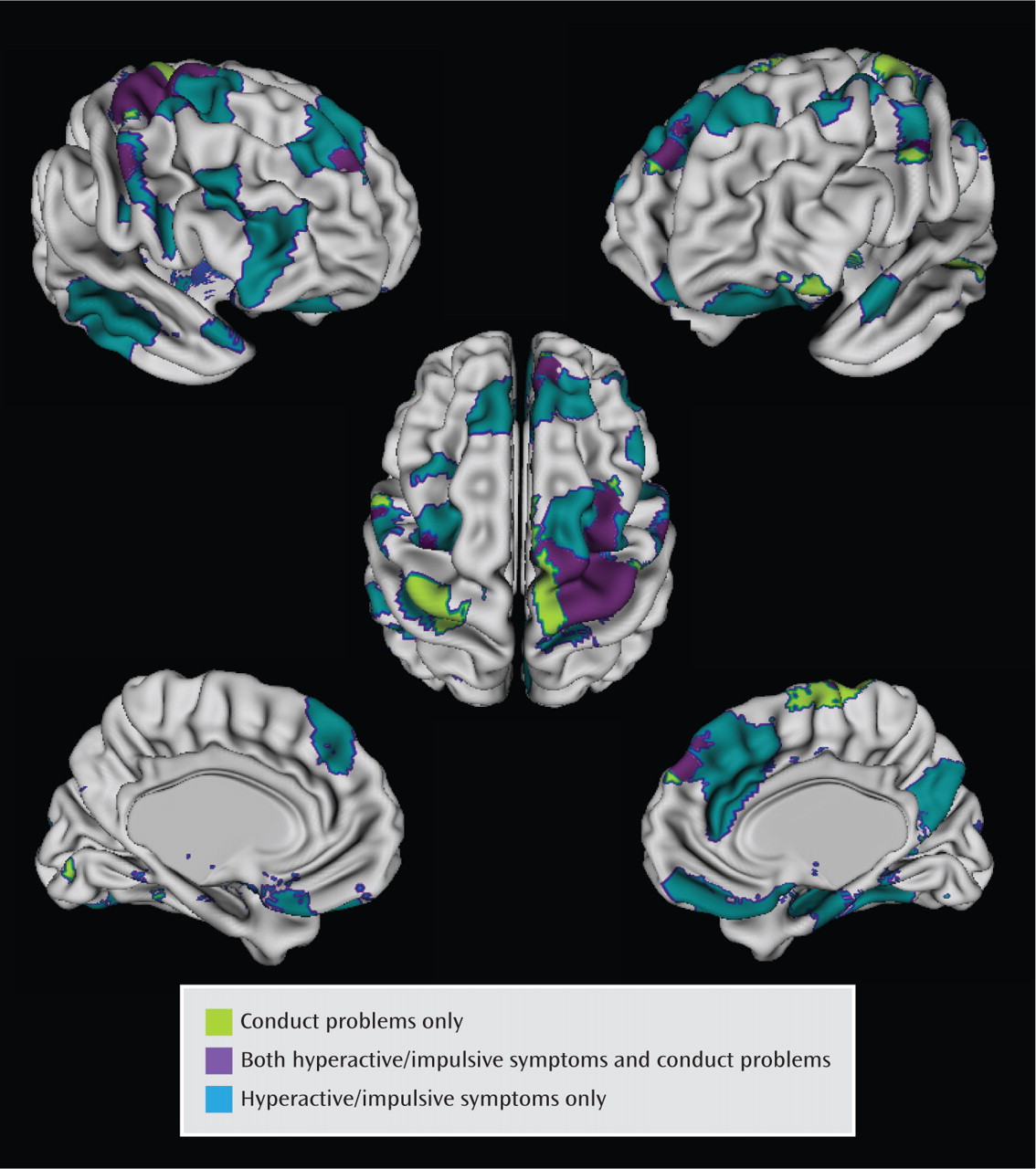
The cortical regions that varied significantly with the conduct problems score (following adjustment for multiple comparisons) were less extensive than those varying significantly with hyperactive/impulsive symptoms and centered on the superior portions of the motor and supplementary motor areas, extending into the middle prefrontal cortex on the right (
Figure 4). Here, an increasing conduct problems symptom score was associated with a slower rate of cortical thinning (for each increase of one point, the rate of thinning decreased by −0.037 mm/year [SE=0.01], t=3.2, df=194, p=0.002). In the right motor/supplementary motor and middle frontal regions, growth rates were linked to both conduct problems and hyperactive/impulsive symptom severity. However, most of the regions (89%) where cortical thinning varied significantly with the hyperactive/impulsive symptom score did not vary with the conduct problems score.
Discussion
The present study gives neurobiological support to the dimensional view of ADHD that typically developing children with no psychiatric diagnoses who have hyperactive/impulsive symptoms demonstrate neurodevelopmental changes resembling those found in youth with the syndrome of ADHD. Specifically, we found that the rate of cortical thinning during late childhood and adolescence was linked to the severity of hyperactive and impulsive symptoms. More symptoms accompanied a slower rate of cortical thinning in predominantly prefrontal cortical regions, mirroring the slower rate of adolescent cortical thinning seen in ADHD. This study also lends support to the concept of a perturbation of cortical trajectory as a fundamental deficit in the pathogenesis of ADHD, rather than reflecting features such as its treatment or comorbidities or the effects of nondisorder-specific functional impairments.
The localization of this effect may have dual significance. First, the regions overlap with cortical areas frequently reported to be structurally compromised in ADHD. Studies of gray matter density or thickness report deficits in the lateral prefrontal cortex, particularly in the right inferior and superior gyri (
25–30), as well as prefrontal medial wall deficits extending to the anterior cingulate gyrus (
26,
31,
32). Similarly, for some of the posterior regions showing a sensitivity to hyperactive/impulsive symptoms, structural alteration is reported, especially in the right middle/inferior temporal lobe (
29,
30) and posterior cingulate/precuneus (
25,
31,
33). Second, many of the cortical regions highlighted in this study are components of the neural substrates supporting certain key cognitive processes, and impairments in these domains are in turn linked to hyperactive and impulsive symptoms. These cognitive functions include the ability to inhibit responses in order to achieve later, internally represented goals (
34,
35). This cognitive control skill recruits a distributed cortico-striatal network with key involvement of the lateral prefrontal cortex, particularly the right inferior frontal gyrus (
36,
37). Aberrant processing of reward and punishment, with a tendency to prefer immediate over delayed but larger rewards, has also been reported in ADHD (
38,
39). Studies in healthy subjects implicate a neural network incorporating interactions between the ventral striatum and the limbic system, including orbitofrontal regions (
40), where we find cortical development to be linked to hyperactive/impulsive symptoms. Other investigators argue that ADHD partly results from anomalies of the spontaneous intrinsic brain activity that typifies nontask-related cognition (default-mode network), specifically its tendency to intrude into periods of active task-specific processing (
41). The default mode network in healthy subjects is thought to comprise medial (medial prefrontal cortex, posterior cingulate/precuneus) and lateral (posterior parietal) brain regions. Interestingly, anomalous resting state activity of the posterior cingulate has been reported in ADHD (
42,
43), a region where, again, we link severity of hyperactive/impulsive symptoms to cortical change. It is noteworthy that there was no effect of hyperactive/impulsive symptoms in the superior portions of the motor strip bilaterally. We reported in a previous study that this superior region of the motor strip had the distinction of attaining a peak cortical thickness earlier in ADHD (
7) and it also did not show a diagnostic difference in the rate of adolescent cortical change. Thus, we would predict and indeed found that there was no effect of the severity of hyperactivity/impulsive symptoms on cortical change in this region. The association with symptoms was confined to the middle and inferior portions of the motor strip.
In the present study, the stepwise decrease in the rate of prefrontal cortical thinning in typically developing youth, moving from those with no symptoms to those with mild symptoms and then to those with moderate symptoms, was found to extend to youth with a diagnosis of ADHD. This is in keeping with the more severe symptoms of hyperactivity and impulsivity in the clinical group, which had slower rates of thinning than the typically developing youth, affording more support for dimensionality. In the posterior cortical region, this stepwise effect was not found because the ADHD group had rates of cortical thinning comparable to typically developing youth with mild and moderate symptoms. This is not unexpected, since we found less evidence in previous work for diagnostic differences in the properties of cortical trajectories in the posterior cortex (
7).
Rates of cortical thinning also showed some links with the presence of conduct problems in typically developing youth, albeit in less extensive cortical regions. Both conduct problems and hyperactive/impulsive symptoms were linked to cortical thinning rates in the right motor/supplementary area, and some degree of overlap is unsurprising given the correlation between these symptom domains. Perhaps more striking is the degree of specificity to symptom domain: the regions where cortical thinning was linked to hyperactive/impulsive symptoms were mostly spatially distinct from those linked to conduct problems. This is congruent with reports of distinct anomalies of brain activation in children with conduct disorder and ADHD (
44) and with the lack of similarity between the structural brain anomalies reported in children with conduct disorder and those most frequently reported in ADHD (
45,
46).
The 48-item Conners' Parent Rating Scale we utilized has many strengths, including its psychometric robustness, especially regarding the factor analyses used to derive the subscales for hyperactive/impulsive and conduct problem symptoms (
10). During the course of this longitudinal study, the scale was updated (
47), but in the interest of maximizing data we continued to use the 48-item version. Both the revised and 48-item versions are similar in their composition, particularly regarding the items loading onto the factor reflecting hyperactivity/impulsivity. As mentioned earlier, the version we used did not return a factor that reflects purely inattentive symptoms. Additionally, while there is evidence from latent class analyses of the 18 DSM-IV symptoms of ADHD that the dimensions of hyperactivity and impulsivity may be in part separable (
48–50), the Conners' Rating Scales do not allow for independent assessment of these domains nor the possibility of partly distinct neural correlates.
We expected that any differences in cortical dynamics within the typically developing youth would be subtle, and thus we focused on the age period with the greatest data density, namely late childhood and adolescence. Previous investigations have established that the dominant effect of age is linear during this age period (
51,
52). Further, when our typically developing cohort was divided on the basis of symptom score, there were insufficient data to examine higher-order effects of age because of a relative lack of data for this very young age group. Future inclusion of more data from a younger age group would allow consideration of more complex growth trajectory differences. Our cohort had a relatively high socioeconomic status and IQ, reflecting the self-selected nature, the relative affluence of the geographical area surrounding the study center, and the absence of any mental illness in typically developing participants, which are factors that may limit the generalizability of the findings. We also did not systematically collect measures of potentially important environmental and lifestyle factors such as diet and tobacco, alcohol, and illicit drug use.
Alterations in cortical growth rates will be only one of the many neural changes shown to reflect or drive ADHD. Many perturbations of brain structure and function have been found in the disorder, and it is unclear whether these will also show the dimensionality we report for cortical change.