Stroke is the second leading cause of death and the sixth leading cause of disease burden globally (
1). Depression is an independent risk factor for stroke and the leading cause of years lost to disability (
1–
3). The prevalence of antidepressant use has increased in many countries, including the United States (
4) and Taiwan (
5). Whether treating depression with antidepressants reverses depression-related cardiovascular complications remains inconclusive (
6–
9), and concerns about the cerebrovascular effects of antidepressants have increased since a growing body of evidence has shown that antidepressants (especially selective serotonin reuptake inhibitors) may induce bleeding complications (
10–
12) and vasoconstriction of the large cerebral arteries (
13–
15). In this regard, the benefit-risk profile of the cerebrovascular effect of antidepressant use remains unclear (
14).
Despite negative findings in randomized trials (
16) and case-control studies (
17–
20), recent cohort studies revealed that current users of antidepressants had a higher risk of ischemic stroke (
21) and hemorrhagic stroke (
22). The discrepant findings may be partially explained by small sample sizes, lack of controls for potential confounders (such as confounding by indication [
14]), and comparison between current users and nonusers in most of the case-control studies. The current users category contained both new users and long-term regular users. Antidepressants may have acute adverse effects but chronic beneficial effects on stroke risk by treating depression or psychological distress. Thus, the sum of different cerebrovascular effects in new users and long-term users may confound the results (
23).
To overcome the limitations of previous studies, we conducted a case-crossover study to analyze the association between stroke risk and antidepressant exposure in a nationwide population-based claims data set. The case-crossover design is an efficient method for examining the association of transient drug exposure with acute effects (
23,
24). In this design, study subjects serve as their own controls, removing between-subject time-invariant confounders, which remain unmeasured or unknown (
23). We hypothesized that acute exposure to antidepressants, particularly those with high inhibition of the serotonin transporter, would increase the risk of stroke.
Method
Data Source
Taiwan introduced a single-payer National Health Insurance program, covering 98% of the Taiwanese population, on March 1, 1995. Since 1996, the National Health Insurance reimbursement claims data have been transferred to and managed by the National Health Research Institute in Taiwan to establish the National Health Insurance Research Database, a medical claims database. The database includes patients' demographic characteristics, diagnoses, medical expenditures, and prescription claims data (
25). Each prescription record contains type of medication, dosage, time of prescription, and duration of drug supply. Information that could be used to identify beneficiaries and medical care providers is scrambled by the Bureau of National Health Insurance (
26). All investigators must sign an agreement that guarantees patient confidentiality before using the database.
Data for this study were obtained from the ambulatory and inpatient claims database. The database has been used for the study of several diseases (
26–
28), including stroke (
28). We included data from all individuals who were enrolled between January 1, 1997, and December 31, 2007.
Study Subjects
We defined patients with an incident cerebrovascular event as those with a hospitalization for a primary diagnosis of a cerebrovascular event under ICD-9-CM codes 430, 431, and 432.x for hemorrhagic stroke; 433.x, 434.x, and 435.x for ischemic stroke; and 436.x for other stroke. The index date was defined as the date when the case subjects were diagnosed as having a first hospitalization for a stroke.
In this study, the patients in whom an incident stroke was suspected had to be at least 18 years of age at the time of first hospitalization for stroke (index day) in the years 1998 to 2007 (N=489,852). Three groups of patients were excluded: those who had any inpatient or outpatient diagnosis of cerebrovascular disease with an ICD-9-CM code (430–438.x) in 1997; those who had diagnoses of head injury (ICD-9-CM code: 800.x–804.x, 850.x–854.x, or 959.x) at the time of first hospitalization for stroke; and those who had been hospitalized within 1 year before the index date (so that we would have a full 1-year observation period). After these exclusions, 334,249 patients were eligible for the study. Among them, 26,171 (7.8%) had at least one antidepressant prescription within 1 year before the onset of stroke. We further excluded patients who had prescriptions of melitracen-flupentixol (N=1,957), which combines a tricyclic agent and a conventional antipsychotic. After these exclusions, 24,214 patients remained in the study.
Case-Crossover Design
The case-crossover design is one method for examining the effect of transient exposures on acute outcomes (
24). In this study design, each patient serves as his or her own control. The odds ratio was estimated by the ratio of patients exposed only during the 14-day case period (1–14 days before the index date) to patients exposed only during the 14-day control period (15–28 days before the index day). We also computed odds ratios using two other time windows, set at 7 days (i.e., 1–7 days and 8–14 days before the index day, for the case and control periods, respectively) and 28 days (i.e., 1–28 days and 29–56 days before the index day, for the case and control periods, respectively) for sensitivity analysis.
Characteristics of Study Subjects
In addition to age and sex, we assessed general health status by the Charlson comorbidity index, which is the sum of the weighted score of 19 comorbid conditions (
29) and is widely used to control confounding in epidemiological studies (
30). The presence of mood disorders was defined as any of ICD-9-CM codes 296.x, 300.4, and 311 at outpatient diagnosis within the year before onset of stroke. New-onset mood disorders were defined as the first date of diagnosis of a mood disorder during the study period after observation for 2 years without any diagnosis of mood disorders before the index date. Durations of antidepressant treatment were assessed by the number of antidepressant prescriptions during the year before stroke; this measure reflected the number of times an antidepressant medication was prescribed, including refills of the same prescription.
Exposure to Antidepressants
We identified antidepressants according to the Anatomical Therapeutic Chemical (ATC) classification system (
31). Antidepressants were classified into four groups according to their proposed mechanisms of action: tricyclic antidepressants, selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, and other antidepressants.
Based on the dissociation constant (K
d) for the serotonin transporter, antidepressants with serotonin transporter reuptake inhibition were categorized into three groups: high K
d (<1 nmol/liter), intermediate K
d (1–10 nmol/liter), and low K
d (>10 nmol/liter). For the degree of inhibition of norepinephrine reuptake, we used the K
d for the norepinephrine transporter and categorized antidepressants into three groups: high (<100 nmol/liter), intermediate (100–1,000 nmol/liter), and low K
d (>1,000 nmol/liter) (
32,
33).
The exposure variable in this study was antidepressant medication, which was retrieved from the medical expenditure and prescription claims data (including refills) in the national database. The presence of exposure to an antidepressant was defined as the prescription of a particular antidepressant at least 1 day during the case or control time periods. Defined daily dose, which was defined as “the assumed average maintenance dose per day for a drug used for its main indication in adults,” was used to quantify an individual's average daily dose. We assessed the average daily dose by cumulative doses divided by cumulative exposure days during the case or control periods. For the analyses of the dose-response effect of antidepressants on stroke risk, the average daily dose was categorized into four ranges in relation to defined daily dose (
31): 0, >0 to <0.5, ≥0.5 to <1, and ≥1 defined daily dose.
Time-Variant Confounding Factors
The confounding variables analyzed included health care utilization, measured as the number of outpatient visits during the case or control periods, and medication related to strokes, defined as the prescription presence of any of the following medications (grouped by ATC classification) prescribed for at least for 1 day during the case or control periods (
31): antipsychotics (N05A), antithrombotic agents (B01), antidiabetes agents (A10), diuretics (C03), antihypertensives (C07, C08, and C09), and lipid-modifying agents (C10).
Data Analysis
For the case-crossover analyses, we used conditional logistic regression models following the PHREG procedure in SAS, version 9.13 (SAS Institute, Cary, N.C.), to estimate the odds ratios and their 95% confidence intervals (CIs). The statistical significance of relationships was assessed by using 95% CIs or a p threshold of 0.05. The adjusted odds ratios of stroke for antidepressant use were estimated after controlling for the number of outpatient visits, mood disorders, and proposed confounding medication exposures.
We performed subgroup analyses by stratifying the various characteristics of the patients, including age, sex, Charlson comorbidity index (
29), duration of treatment (number of prescriptions), and mood disorders. We tested the interactions between antidepressant exposure and these patient characteristics in the whole sample. We further conducted conditional logistic regression analyses to explore whether new-onset mood disorders were predictive of risk of stroke and to test the potential confounding effect of new-onset mood disorders on the relationship between antidepressant use and risk of stroke.
We further compared odds of the exposures of antidepressants between the case and control periods after the onset of stroke for the ischemic and hemorrhagic strokes stratified by the characteristics of antidepressant use, based on average daily dose, type of antidepressant, and degree of inhibition of serotonin or norepinephrine transporters. We also explored a potential dose-response relationship between stroke and antidepressant exposure by using conditional logistic regression analyses and considering average daily dose as a continuous variable. The trend toward an increasing risk of stroke with a higher average daily dose was determined by the Wald chi-square test. Finally, sensitivity analyses using 7-day and 28-day time windows were performed to test for the robustness of the results.
Results
Data for a total of 24,214 stroke patients with at least one antidepressant prescription in the year before a first hospitalization for stroke were analyzed (
Figure 1). The mean age at stroke onset was 68.6 years (SD=12.0); 48.3% of the study subjects were women, 36.3% had mood disorders, and 75.9% had ischemic strokes. The mean number of antidepressant prescriptions in the previous year was 5.3 (SD=6.5, range=1–138). The demographic characteristics, stroke types, and number of prescriptions are summarized in
Table 1, and rates of antidepressant types are listed in
Table 2.
The data in
Table 3 show that antidepressant use in the 2 weeks before the stroke was associated with a higher risk of stroke by 48%, adjusting for health system utilization and proposed confounding medications. In patients with mood disorders, univariate analysis revealed that new-onset mood disorders were associated with a higher risk of stroke (odds ratio=1.33; 95% CI=1.10–1.60). Including new-onset mood disorders as one of the covariates in multivariate analysis, we found that the stroke risk with antidepressant use was slightly lower, with the adjusted odds ratio dropping from 1.48 to 1.44 (95% CI=1.27–1.64), and the effect of new-onset mood disorders was not statistically significant (adjusted odds ratio=1.16; 95% CI=0.96–1.41, p=0.13).
There were no interactions between antidepressant use and age, the Charlson comorbidity index, or mood disorders on the risk of cerebrovascular events. However, the stroke risk with antidepressant use in the 2 weeks before the stroke was negatively associated with the number of antidepressant prescriptions in the previous year (p<0.001). There was no statistical association between antidepressant use in the 2 weeks before the stroke and the risk of stroke for patients with prescription numbers ranging from three to five in the previous year. Antidepressant use was associated with a lower stroke risk among patients with more than six antidepressant prescriptions. The greater stroke risk with antidepressant use was mainly attributable to patients who had one or two prescriptions in the previous year.
Table 4 summarizes the relationship between stroke type and antidepressant type among patients with fewer than three antidepressant prescriptions in the year before the stroke. We found that an excess risk of stroke with antidepressant use was more prominent for the ischemic type (adjusted odds ratio=2.52; 95% CI=2.23–2.84) than for the hemorrhagic type (adjusted odds ratio=1.92; 95% CI=1.49–2.47) of stroke, despite overlapping of the 95% confidence intervals of the two stroke types. We also observed a trend toward an increasing risk of stroke with a higher average daily dose (p trend <0.001, Wald χ
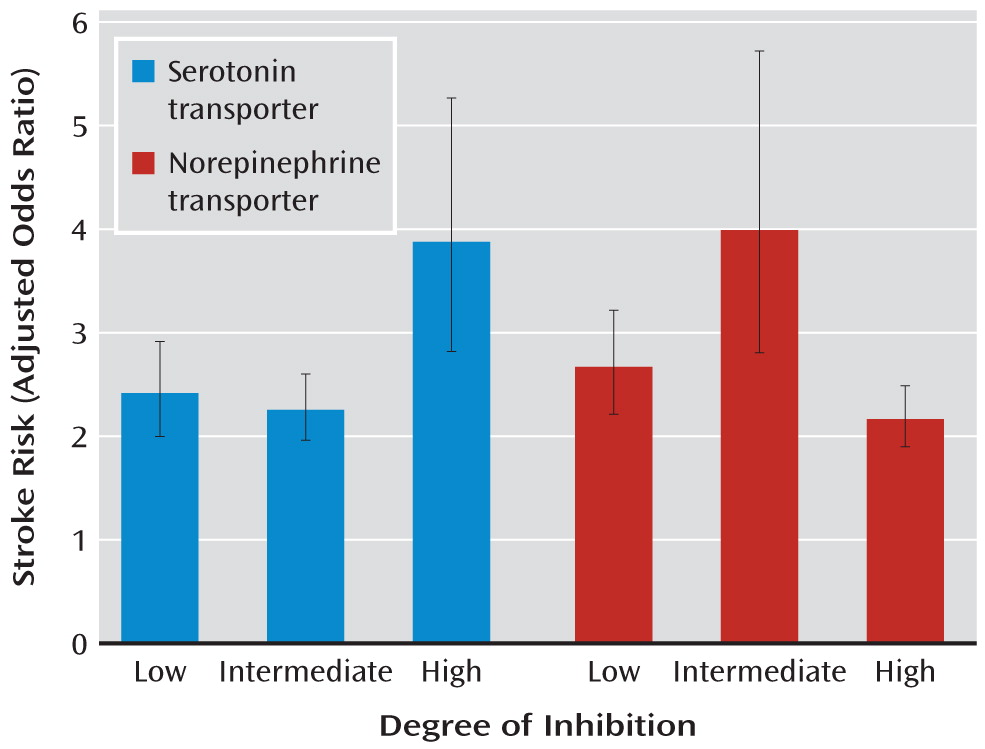
2=194.5, df=1) after adjusting for confounding factors. In addition, the risk of stroke associated with antidepressants with high inhibition of the serotonin transporter was greater than with antidepressants with low or intermediate inhibition (
Figure 2). Antidepressants with high inhibition of the norepinephrine transporter were associated with a lower risk of stroke than those with low or intermediate inhibition. There was no significant difference between low and intermediate inhibitors of norepinephrine transporter.
We performed sensitivity analysis with 7-, 14-, and 28-day time windows as the case and control periods for our results (
Table 5). The results were grossly consistent for the three time periods. However, compared with the use of low serotonin transporter inhibitors, the stroke risk with high inhibitors remained but was statistically nonsignificant in the 7-day and 28-day windows.
Discussion
This is the first study to investigate the association between use of antidepressants and risk of cerebrovascular events in a nationwide population-based cohort using a case-crossover design with a population-wide sample. We found that antidepressant use was associated with a 48% greater risk of stroke, after taking confounding factors into account, and that the magnitude of associations was greater in high-potency inhibitors of the serotonin transporter than in low- and intermediate-potency inhibitors.
Our findings are in agreement with those of previous studies showing that antidepressant use was associated with an increased risk of stroke, both ischemic (
21) and hemorrhagic (
22) types. Contrary to the findings from Chen and colleagues (
34), in which a much smaller sample was studied, our findings here provide strong evidence supporting the thesis that degree of inhibition of the serotonin transporter is associated with risk of hemorrhagic stroke. Our findings are compatible with those of studies showing that a high inhibition of the serotonin transporter has a more potent antiplatelet effect and is associated with a higher risk of abnormal bleeding in other organ systems (
12,
35). Surprisingly, we also found that antidepressants with high inhibition of the serotonin transporter were associated with a greater risk of ischemic stroke. A possible mechanism is antidepressant-induced vasoconstriction, which is mediated by the serotonin receptors on smooth muscle cells and can prompt thromboembolic formation in atherosclerotic cerebral arteries (
14,
36). However, even the use of low-potency inhibitors of the serotonin transporter is associated with an excess risk of stroke, regardless of stroke type. Thus, other underlying mechanisms for stroke risk in relation to antidepressant use warrant further investigation.
This is the first study to explore the role of norepinephrine transporter inhibition in the relation between antidepressant use and stroke risk. We found that low or intermediate inhibitors of the norepinephrine transporter were associated with higher stroke risks than were high inhibitors. A possible explanation is that high norepinephrine transporter inhibition helps prevent vasovagal reaction (
37), which reduces the possibility of hypoperfusion of cerebral circulation and lowers the stroke risk. However, highly selective norepinephrine reuptake inhibitors, such as reboxetine, are not available for patients who participate in the National Health Insurance program. Further studies are needed to explore the cerebrovascular effect of norepinephrine reuptake inhibition and other underlying mechanisms of stroke risk.
One new finding of this study is that the risk of stroke increased with average daily antidepressant dose. The increasing trend was significant (p<0.001) across the three different time windows we used. Another new finding is that the magnitude of stroke risk with antidepressant use varied according to the duration of antidepressant treatment. The increasing stroke risk associated with antidepressant use is largely attributable to patients who had few antidepressant prescriptions in previous years. Patients who used antidepressants over longer periods (three or more prescriptions) had no excess risk of stroke. This may partially explain the inconsistent findings of previous studies (
17–
20), in which the category of current users included both new users and long-term users. The different cerebrovascular effects of antidepressants among new users and long-term users would move the estimated effect toward the null. The failure to show the effect of duration of treatment on the relationship between antidepressant use and stroke in previous studies may be due to limited case numbers (
17–
19).
In contrast, we found that there may be a protective effect of antidepressant use on stroke risk among long-term users. The antiplatelet effect of antidepressants may play a role in preventing thrombus formation. Depression and psychological distress themselves are risk factors for stroke, and antidepressants may eliminate the harmful effects of these factors after an adequate duration of treatment. However, the interpretation of such findings is not clear. In a case-crossover study, a protective effect would be indicated by the rates of nonuse being higher during the case period than during the control period. Thus, another possible explanation for such findings is that withdrawal from long-term antidepressant use may increase the risk of stroke. Indeed, antidepressant discontinuation syndrome is associated with autonomic nervous system instability (
38), which may have an adverse effect on the cardiovascular and cerebrovascular systems. Furthermore, the depletion of susceptible cases might explain the decreased stroke risk among patients with long-term antidepressant use (
39). Those who are likely to have had strokes due to antidepressants may have already had them before the observation period and therefore were not included in this study.
In addition to use of antidepressants, we found increased prescription rates of other medications and more number of outpatient visits in the case period. The clinical circumstance whether patients received an antidepressant prescription or not might be different. The conditions that patients used antidepressants would also increase the likelihood that they received other drugs and had increased number of outpatient visits. For example, patients with mood disorders would be more likely to have somatic complaints; therefore, they may seek professional help more frequently and used more drugs. However, after adjusting these confounding factors, the associations between antidepressant use and stroke risk were still significant.
Our study has several potential limitations. We identified incident cases as first-time hospitalization for stroke; although patients with any record of stroke in 1997 were excluded, remote recurrent cases may have been misclassified as incident cases. We excluded cases with hospitalization within 1 year before the stroke. Thus, the patients included as cases may have been healthier than those excluded from the study. Moreover, patients who died of stroke before hospitalization were not included in the study.
One of the major limitations of the case-crossover design is that the exposure frequency changes over time. For example, the prevalence of antidepressant use increased from 2.2% in 1997 to 4.4% in 2004 in the general population in Taiwan (
5). However, the effect of this time trend bias may be negligible since the study time windows were short. Second, the case-crossover design controls all between-subject time-invariant confounding factors but not time-variant factors. Our results may have been influenced by the confounding factors of acute indications, such as abrupt emotional distress. However, we found that stroke risk with antidepressant use was not changed after adjustment for new-onset mood disorders. Moreover, mood disorders may have occurred insidiously, in which case the onset date would not have been accurately measured in this study. Thus, the association between antidepressant use and stroke might, to a varying degree, be related to the change in the severity of underlying psychiatric disorders between the case and control periods.
Several limitations inherent in using claims databases also need to be taken into consideration. First, because patients' identification was scrambled for protection of privacy, we have no way to assess for medication adherence. However, nonadherence would most likely result in nondifferentiated misclassification of the exposure, which would lead to underestimation of the actual risk. Second, there are no published studies of the accuracy of stroke-related coding in the National Health Insurance Research Database, although some studies examining the validity of stroke diagnoses in claims data showed high positive predictive values (
40). Identifying patients hospitalized for a primary diagnosis of stroke, as we did, would minimize potential misclassification of cases. Third, a number of other potential confounding factors that might affect stroke risk, such as body mass index, alcohol use, and smoking status, were not available in the database. However, we used a case-crossover design for controlling such unmeasured variables. Finally, our findings may or may not extend to the population outside Taiwan. Further investigations are needed to explore such associations in different populations and ethnic groups.
The strengths of this study are the use of a nationwide sample (National Health Insurance covering 98% of the population of Taiwan), a very large sample, a well-defined method for identifying cases of stroke, detailed data on exposure to antidepressants and other medications, controls for all between-subject time-invariant confounding factors by using the case-crossover study design, and a sensitivity analysis to confirm the robustness of the results.