Bipolar disorder is associated with a high risk for suicide completion compared with other major psychiatric disorders (
1,
2). The rate of suicide attempt, the strongest predictor of suicide, is also high (
3). Lithium, a well studied and widely used mood stabilizer, has been suggested to have antisuicidal properties based on secondary analyses of randomized controlled trials (
4,
5), naturalistic studies (
6,
7), meta-analysis (
8,
9), and open-label treatment trials (
8,
9).
Despite the weight of evidence drawn from naturalistic studies favoring an antisuicidal effect for lithium, such studies cannot control for several key issues. For example, outcomes of such studies may be influenced by “confounding by indication,” since it is unknown whether physicians are less likely to prescribe lithium to patients deemed at risk for suicide because of its potential for lethal outcomes in overdose. Also, because of concerns regarding toxicity, physicians may monitor lithium levels regularly, thereby permitting intervention for nonadherent patients. Finally, lithium clinic data (
7) may stem from patients who have come to acknowledge their illness and need for ongoing treatment, perhaps leading to better medication compliance. Such clinics may also have built-in close clinical monitoring, which may decrease suicidal behavior.
Few double-blind randomized controlled trials have examined mood stabilizers and suicidal behavior in exclusively bipolar samples (see reference
9 for a review), and existing studies have excluded actively suicidal individuals (
10,
11). This dearth of studies reflects the ethical and logistical barriers to conducting randomized controlled trials to test whether a drug prevents suicide or suicide attempt. Examples of such barriers include the ethical impossibility of assigning such high-risk participants to placebo (
12) and the practical barriers to studying low-frequency events, which generally require large samples and years of follow-up. However, a randomized controlled trial based on comparator medication groups addresses the ethical concerns, and studying a sample of suicide attempters over a long study period addresses the second issue, as this group is likely to have a higher base rate of the outcome of interest.
Given the importance of preventing suicidal behavior and the absence of prospective, controlled, randomized, double-blind medication treatment trials in bipolar patients at high risk for suicidal behavior, we conducted a 2.5-year, double-blind, randomized, parallel-group study to determine whether lithium was superior to valproate in preventing the occurrence of suicidal events in bipolar patients in a major depressive or mixed episode who had a past suicide attempt. The sample size provided the statistical power to detect a relative risk of 5 or greater.
Method
This clinical trial was conducted between 2000 and 2007. Participants were recruited through a combination of emergency department referrals, referrals from other outpatient services, and self-referral in response to advertisements. All participants provided written informed consent as approved by the New York State Psychiatric Institute Institutional Review Board.
We sought to recruit an inclusive, generalizable group and therefore minimized inclusion and exclusion criteria. To be included, patients had to have a DSM-IV diagnosis of a bipolar disorder (bipolar I or II disorder or bipolar disorder not otherwise specified) based on the Structured Clinical Interview for DSM-IV; be in a depressive or mixed episode; have at least one past suicide attempt; and be 18 to 75 years of age. Exclusion criteria were lack of capacity to provide informed consent; pregnancy or lactation; active medical problems, including substance abuse problems requiring detoxification; contraindication to use of lithium or valproate; a history of nonresponse to adequate dosages of either lithium and valproate in the past 2 years; contraindication to the use of adjunctive antidepressants (paroxetine, bupropion, or venlafaxine) if in a depressed state or adjunctive antipsychotics (olanzapine, perphenazine, or haloperidol) if in a mixed or psychotic depressed state.
Intervention
Participants were randomly assigned to treatment with lithium or valproate. Target blood level ranges for the comparator medications were 0.6–1.0 mEq/dl for lithium and 45–125 5g/ml for valproate. Blood levels were checked regularly, and dosages were adjusted by an unblinded, nontreating physician who advised the pharmacy of any prescription changes, so that the double blind was preserved. We aimed to maintain patients on therapeutic dosages of lithium or valproate for the duration of the study.
To protect this high-risk population, an algorithm provided for adjunctive treatment at the time of randomization (double-blinded comparator plus open-label adjunctive treatment) and for adjustment and switching of adjunctive medication in cases where initial interventions failed to result in remission. Depressed bipolar patients were randomly assigned to receive either lithium plus paroxetine (up to 60 mg/day if needed) or valproate plus paroxetine, because addition of an antidepressant to a mood stabilizer was recommended by treatment guidelines for bipolar depression when the study was initiated. Patients who continued to meet criteria for major depression on paroxetine had up to two consecutive trials with bupropion (up to 400 mg/day) and/or venlafaxine (up to 375 mg/day) (
13). Depressed, psychotic bipolar patients were randomly assigned to receive either lithium plus paroxetine (or bupropion or venlafaxine) and olanzapine (up to 25 mg/day if needed) or valproate plus paroxetine (or bupropion or venlafaxine) and olanzapine. Mixed bipolar patients were randomly assigned to receive lithium plus olanzapine or valproate plus olanzapine. Patients who could not tolerate or who did not respond to olanzapine were treated with a second- or third-line antipsychotic, perphenazine (up to 64 mg/day) and haloperidol (up to 20 mg/day), sequentially. Patients who entered randomization with a history of nonresponse to first-line adjunctive medications went immediately to second- or third-line medications.
The study was divided into three treatment stages: acute stabilization, continuation, and maintenance. During acute stabilization, patients received treatment drug plus adjunctive drug as described above until they achieved 2 weeks with a score below 7 on the Hamilton Depression Rating Scale (calculated from the Schedule for Affective Disorders and Schizophrenia, Change Version [SADS-C;
14]) and a mania score <10 on the SADS-C. At the point of stabilization, they entered the continuation phase and continued on their assigned study drug and their adjunctive treatment for 6 months. After the continuation phase, adjunctive antidepressants were tapered if the patient did not meet criteria for full or partial remission of a mood episode. Adjunctive antipsychotics, if required for acute stabilization, were tapered 2 months after remission of mixed or psychotic states. After 6 months (or 2 months in the case of mixed episode) of continuation treatment, patients entered the maintenance phase, in which the goal was to maintain them on mood stabilizers alone if they were euthymic.
Patients who developed a new episode after remission and required rescue medication received adjunctive antidepressants if they had a depressive episode and adjunctive antipsychotics if they had a manic, mixed, or psychotic episode. Patients who required hospitalization for any reason could remain in the study on double-blind medication as long as the hospitalization occurred at our institution.
Clinical Characterization
Baseline symptoms were assessed using the SADS-C. Axis I and II pathology was assessed at baseline with the Structured Clinical Interview for DSM-IV Axis I and II Disorders. Follow-up interviews assessed levels of psychopathology (using the SADS-C and the Global Assessment Scale [
15]), suicidal behavior (using the Columbia Suicide History Form [available from J.J.M.]), and related constructs including ideation and planning (using the Beck Scale for Suicide Ideation [
16]). Over a 2.5-year period encompassing the acute, continuation, and maintenance phases, patients participated in regularly scheduled interviews at a minimum of 10 time points, including at study entry. During the acute phase, patients were assessed at week 1, week 2, week 4, month 2, month 3, and month 6 after randomization. In the continuation phase, assessment occurred at months 2, 3, and 6. In the maintenance phase, patients were assessed every 3 months (six visits over 18 months). Thus, under optimal conditions, a patient would be assessed at four visits during the acute phase (2 months total in the acute phase), one visit during the continuation phase (discontinue antipsychotic after 2 months), and six visits during the maintenance phase. Interviews were also conducted on the occasion of a suicide event, hospitalization, or entry into open treatment.
Randomization
Participants (N=98) were randomly assigned to treatment with lithium or valproate. Randomization was stratified by recentness of attempt (<1 year ago or not) and bipolar subcategory (bipolar I versus bipolar II or not otherwise specified).
Blinding
Patients, study psychiatrists, and assessors were blind to treatment assignment. Because the lithium and valproate pills differed in appearance (as did their respective matched placebo pills), we used the “double dummy” approach to maintain the double blind. Patients received two containers of identical pills, one with active medication and the other with placebo pills identical in appearance to the medication to which they had not been assigned. Patients were instructed to take the same number of pills from each of the containers, as indicated by the pharmacy. All blood level determinations were reported to an unblinded, nontreating administrative research psychiatrist who then instructed the pharmacy on prescription. If the medication was being titrated up in response to a low blood level, the patient would be instructed to take an increased number of pills from each of the two containers.
Outcome Measures
The primary outcome measures were time to suicide completion, time to suicide attempt, and time to suicide event. Suicide completion was defined as self-inflicted death for which there was evidence of at least some intent to end one's life. Suicide attempt was defined as a potentially self-injurious behavior carried out with at least some intent to end one's life. Given the tension between the ethical requirement to intervene when imminent risk of suicidal behavior is suspected (
12) and the likelihood that such action would suppress the outcome of interest, we defined an additional outcome measure: suicide event. Suicide event was operationalized as a suicide attempt or an episode of suicidal ideation with a plan (the patient acknowledges one or more of planning items 12 and 15–18 in the Scale for Suicide Ideation) requiring a change in treatment, such as addition of a rescue medication or hospitalization.
Statistical Analysis
The primary outcome measure was assessed by survival time to any of the three events described above. Baseline variables for the two treatment groups were compared using t tests and chi-square statistics. The primary analysis was an intent-to-treat analysis conducted using the log-rank test, a nonparametric test for right-censored data. Two separate models were fitted, one for suicide attempts only and one for any suicide event (suicide completion, suicide attempt, or suicidal ideation with a plan requiring intervention).
Results
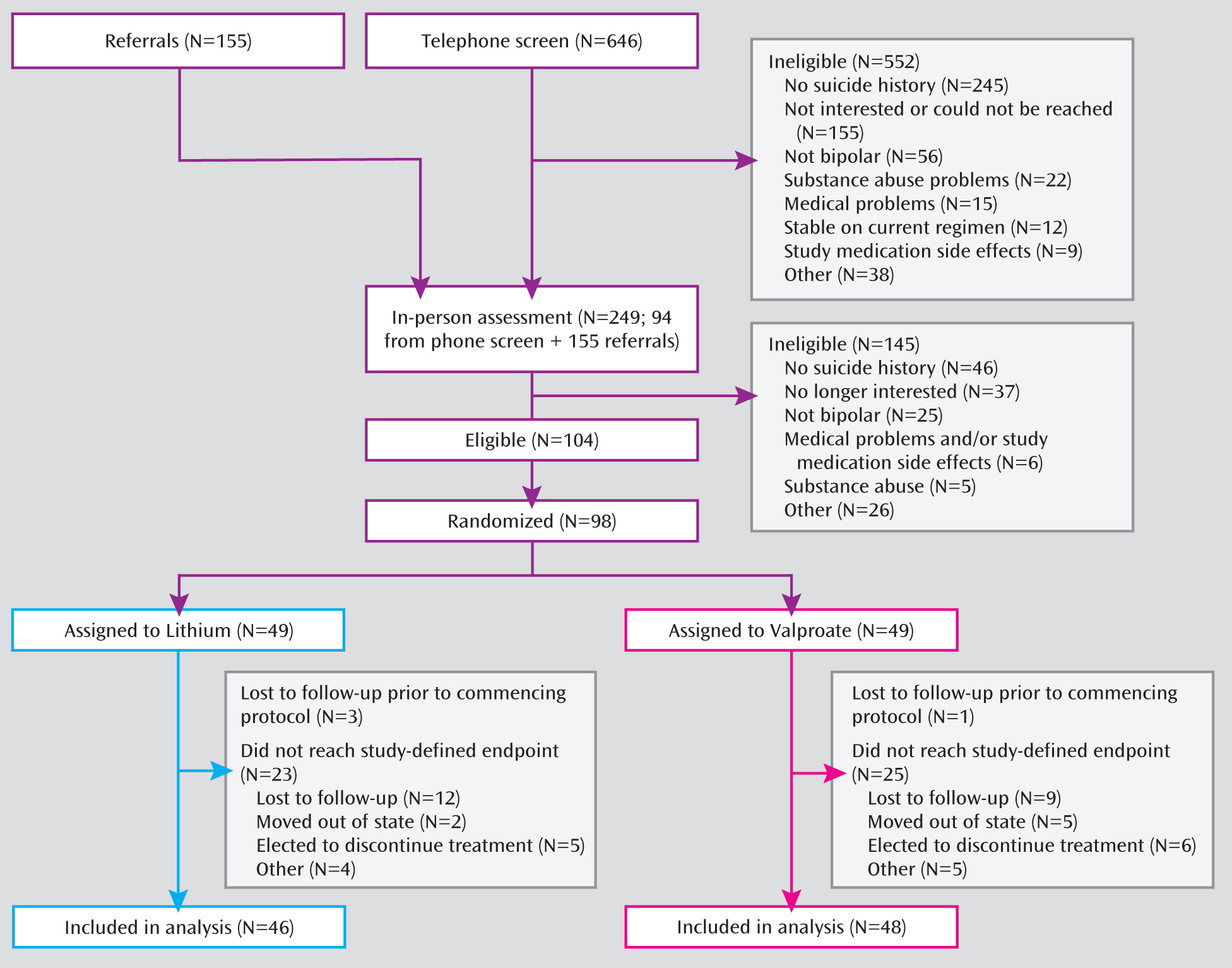
A total of 646 individuals were screened for inclusion, of whom 98 were eligible for the study, agreed to participate, and underwent randomized treatment assignment (
Figure 1). Participants who were lost to follow-up did not differ in age, sex, or bipolar subtype from those who remained in the study. However, those lost to follow-up had more previous psychiatric hospitalizations and were more likely to report a history of childhood abuse.
The demographic and clinical characteristics of the two treatment groups did not differ, indicating successful randomization (
Table 1). Eighty-three patients were in a major depressive episode at study entry, and 15 were in a mixed state. The mean score on the Hamilton Depression Rating Scale (derived from the SADS-C) was 34.
Exposure to Intervention
Average time in the study was not significantly different between the lithium and valproate groups (495 days and 550 days, respectively).
Primary Outcome Measure
There were no suicide deaths during the study. A total of 18 suicide attempts were made by 14 patients, six from the lithium group and eight from the valproate group. All 14 suicide attempters were female. There were 45 suicide events (attempt, hospitalization, or rescue) by 35 patients, 16 from the lithium group and 19 from the valproate group. The event rate did not vary by sex.
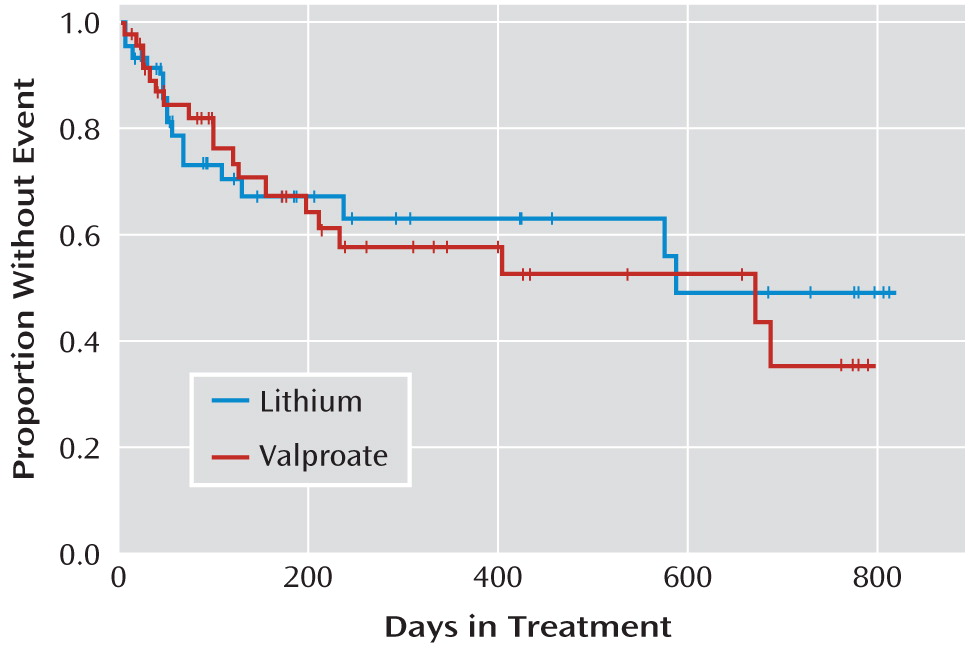
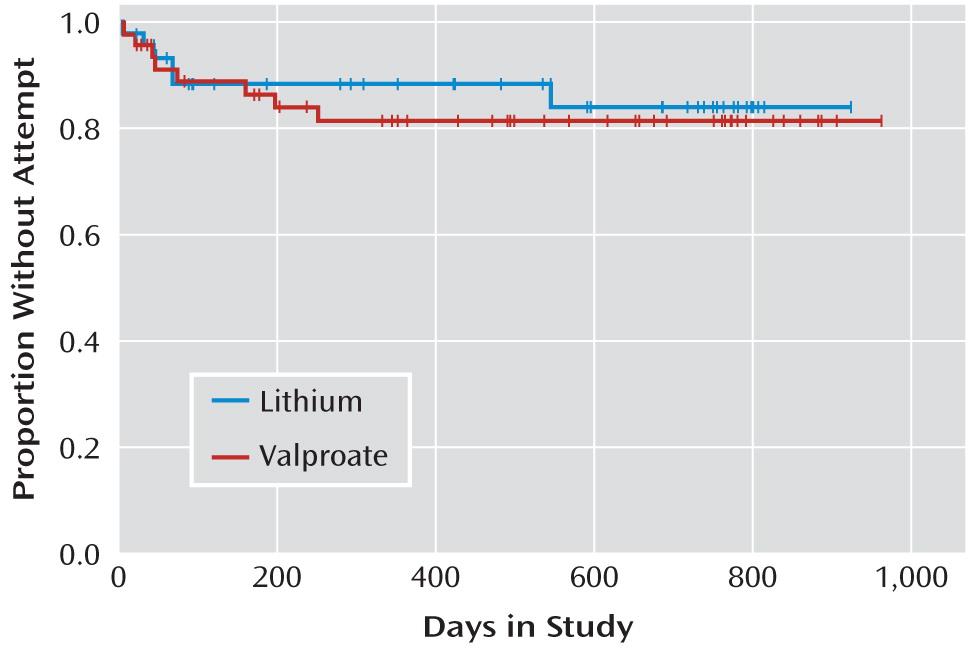
In an intent-to-treat analysis, the log-rank test showed no differences between treatment groups in time to suicide event (
Figure 2) or in time to suicide attempt (
Figure 3). The median time to suicide event, dropout, or end of treatment was 138 days for the valproate group and 114 days for the lithium group. The median time to suicide attempt, dropout, or end of study was 480 days for the valproate group and 507 days for the lithium group. There were no differences between treatment groups in proportion achieving remission from acute (index) episode, time to hospitalization for any reason, or time on study medication (data not shown).
Serious Adverse Events
In addition to 45 study-defined suicidal events (attempt, ideation, hospitalization), other serious adverse events reported were pregnancy (N=2), skin rash (N=1), hand tremor (N=1), and gunshot wound, not self-inflicted (N=1).
Discussion
Contrary to our hypothesis, this randomized controlled trial revealed no difference in time to suicide attempt or suicide event between patients treated with lithium and those treated with valproate. While evidence from naturalistic studies and meta-analyses of largely open-label treatment trials suggests an antisuicidal effect of lithium, other mood stabilizers have been less studied with respect to suicidal behavior. Valproate is a widely used first-line mood stabilizer in bipolar disorder, and several randomized controlled trials have shown comparable efficacy to lithium in acute and maintenance treatment (
17). Most studies examining valproate and lithium effects on suicidal behavior in bipolar disorder have found no difference between lithium and valproate/divalproex groups. This was the case in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial (
18), as well as in two retrospective record reviews (
19,
20). Notably, consistent with lithium discontinuation studies, the latter two studies found that the risk for suicidal behavior increased significantly when medication was discontinued, whether it was valproate or lithium. Data from pharmacoepidemiologic studies, while limited because of their retrospective nature and lack of randomization, are instructive nonetheless. One large pharmacoepidemiologic study reporting an advantage for lithium over valproate/divalproex in terms of rates of suicide and suicide attempt during treatment (
6) also noted an increase in risk for suicide event when either drug was discontinued. Data from Oregon Medicaid patients revealed a greater odds ratio for suicide attempts among divalproex users compared with lithium users, but no significant difference for suicide death (
21). In another health plan data set study, no differences emerged between lithium and anticonvulsant monotherapy, including divalproex, in posttreatment suicide attempts, and both lithium and anticonvulsants resulted in a significant reduction in posttreatment attempt rates compared with pretreatment rates (
22).
To our knowledge, this is the only randomized, double-blind trial comparing these two drugs in a sample of high-risk suicide attempters with bipolar disorder, and the result is consistent with the findings of three of four studies that have compared these two treatments using other methodologies. The lack of difference between treatment groups on suicidal outcomes observed in this study may be because 1) lithium's antisuicidal effect is more modest than that we could test in this study; 2) the putative antisuicidal effect of lithium is due to factors not tested here, such as regular contact with clinical care, careful monitoring of blood levels and thereby enhancement of adherence, or confounding by indication; 3) the low rates of clinical remission (the mean decrease in depression scores was 10 points, which, although substantial, nonetheless left patients with significant symptoms) observed in both groups over the course of the study contributed to an equally elevated risk for events in both groups; or 4) valproate and lithium have comparable efficacy with respect to suicidal behavior. This latter explanation is supported by the observation that discontinuation of either lithium or valproate is observed to increase risk for suicidal behavior in all published studies that have examined this issue (
6,
19,
20). This suggests that it is perhaps continuity of treatment—any treatment—that attenuates risk for suicidal behavior in bipolar disorder.
A much higher suicide attempt and event rate was observed in this study than in most other studies. For example, Baldessarini and colleagues' 2006 meta-analysis of 45 mostly open-label trials (
9) found a suicide or suicide attempt event prevalence of 0.435% per year on lithium, compared with 2.63% per year off lithium. In the present study, the prevalence for suicide attempt was 13.4% per year, with no difference between groups. This marked rate of suicide events and attempts most likely reflects the high-risk nature of the sample, which comprised depressed or mixed-episode bipolar patients with past suicide attempts, high baseline depression scores, and low rates of remission, rather than a lack of effect on suicidal behavior for either drug. However, we cannot rule out the possibility that there was no suicidal behavior risk reduction effect in either treatment arm. Nonetheless, the findings do suggest either that both agents work equally with respect to risk for a suicide event in this high-risk population or that any difference between the two treatments is not large. Given the evidence drawn from observational studies supporting an antisuicidal effect of lithium treatment, one interpretation is that valproate and lithium have similar beneficial effects on suicidal behavior in high-risk patients presenting with a depressed or mixed episode. If that is the case, the trend in prescribing practices away from the use of lithium as a first-line treatment for bipolar disorder (
23) and the shift to greater use of anticonvulsants or atypical antipsychotics as mood stabilizers is less alarming than originally posited.
Limitations
The lack of difference between comparator drugs may be due to the modest sample size. Power analysis based on the attempt rate reported in a study of suicide attempters with affective disorders yielded an estimation of 232 subjects, and this enrollment target was not reached. However, the power analysis was based on an attempt rate much lower than that observed in this study. Moreover, this study also had as an outcome measure “any suicide event,” which included hospitalization for suicidal ideation with a plan. This is a more common occurrence, so differences should be detectible in a smaller sample. Post hoc power analysis (conducted using SSP1 [
http://cct.jhsph.edu/javamarc/SSP1/SSP1v4.htm]) to compute the effect size that would be detectable with 80% power given the observed event rate in the valproate group (40%) and equal dropout rates of 50% revealed 80% power to detect a lithium:valproate relative risk of 1:5. Notably, the relative risk for suicidal behavior described in a meta-analysis of studies of bipolar disorder has been reported to be 4.91 in favor of lithium compared to no lithium, which is very close to the 1:5 ratio we calculated (
9). A related limitation is that the study was not powered to detect suicide deaths or suicide attempts, and thus we can most reliably comment on suicide events, which were more plentiful. Also, the loss to follow-up was higher than expected—50% compared with the 40% we originally calculated—although it fell within the range reported for studies in comparable populations of ≥1 year's duration, and those lost to follow-up did not differ in most demographic and clinical features from those who remained in the study (data not shown). Furthermore, research psychiatrists in this study were not asked to state which study medication they believed patients were receiving. An additional concern is that the study was not multicenter, although single-center studies have the advantage of less variability in important study parameters.
Generalizability
Suicidal behavior is prevalent in bipolar disorder, and most clinical studies of the disorder exclude suicidal individuals. To maximize generalizability, we included patients with comorbid disorders, including substance use disorders, as comorbidity is the rule in bipolar disorder (
24). The use of an adjunctive medication algorithm also increased generalizability, as we were able to enroll patients who were not likely to respond to single-drug treatments, and polypharmacy is common in bipolar disorder. Still, it is notable that this study population represents a severely ill group of bipolar patients, with high baseline depression scores and very few participants who achieved sustained remission over the 2.5-year course of the study.
Future Directions
Although our results suggest that the risk ratios estimated on the basis of naturalistic data are too large, it may well be worth seeking evidence for more modest effects. Indeed, given the gravity of the outcome, suicide attempt, and the fact that each attempt increases the risk for future attempts and suicides, one could argue that demonstrating a relative risk of less than 5 would be of value. Of course, the magnitude of the protective effect must be considered in the context of risk of death with lithium overdose, which casts a long shadow on its potential benefits. Indeed, three of the 18 attempts in this sample were overdoses with study drugs, which participants knew might be lithium. The rate of overdoses with study medication notwithstanding, based on this data, it appears that a relative risk as small as 1:2 would be worth demonstrating. Major funding sources in the United States have generally not been inclined to fund large-scale studies to detect modest effect sizes in psychiatric disorders, as has been done for cardiovascular disease and cancers. Nonetheless, studies of antisuicidal interventions in bipolar disorder, with the high suicide attempt rate and attendant morbidity and with the impressive suicide mortality, may warrant such an investment. A multisite study to demonstrate even modest differences that could change current prescribing practices in the United States may be life saving.