Two of the most widely used medications in contemporary clinical practice are antidepressants and nonsteroidal anti-inflammatory drugs (NSAIDs) (
1), and the co-occurrence of pain and depressive symptoms is common (
2). A recent report suggested that NSAIDs interfere with some behavioral and biochemical effects of selective serotonin reuptake inhibitors (SSRIs) in mice (
3). The same study also presented an analysis of the multicenter Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study of antidepressant effectiveness, suggesting that NSAID-treated patients with major depression were less likely to achieve remission with citalopram than patients who were not treated with NSAIDs.
To accomplish the first, we used a tool for automated determination of treatment outcome in large electronic medical record systems, the i2b2 (Informatics for Integrating Biology and the Bedside) treatment-resistant depression framework (
7), using methods that have previously demonstrated sensitivity to other adverse treatment effects outside of the field of psychiatry (
8). We hypothesized that we would observe an association between NSAID use and treatment resistance in more than 1,500 antidepressant-treated patients with major depressive disorder. We then reanalyzed data from this cohort and from the original STAR*D study to better characterize the potential confounding effects of medical comorbidity on the observed association.
Results
The first cohort was drawn from 1,528 patients with at least one billing code diagnosis of major depressive disorder (ICD-9 codes 296.2–296.3) and who were identified as having treatment-resistant or treatment-responsive depression using the natural language processing single-visit and longitudinal classification algorithms. Of this cohort, 1,245 (81%) patients were exposed to NSAIDs or NSAID-like medications, and 283 (19%) were unexposed.
Table 1 summarizes this cohort’s sociodemographic and clinical characteristics.
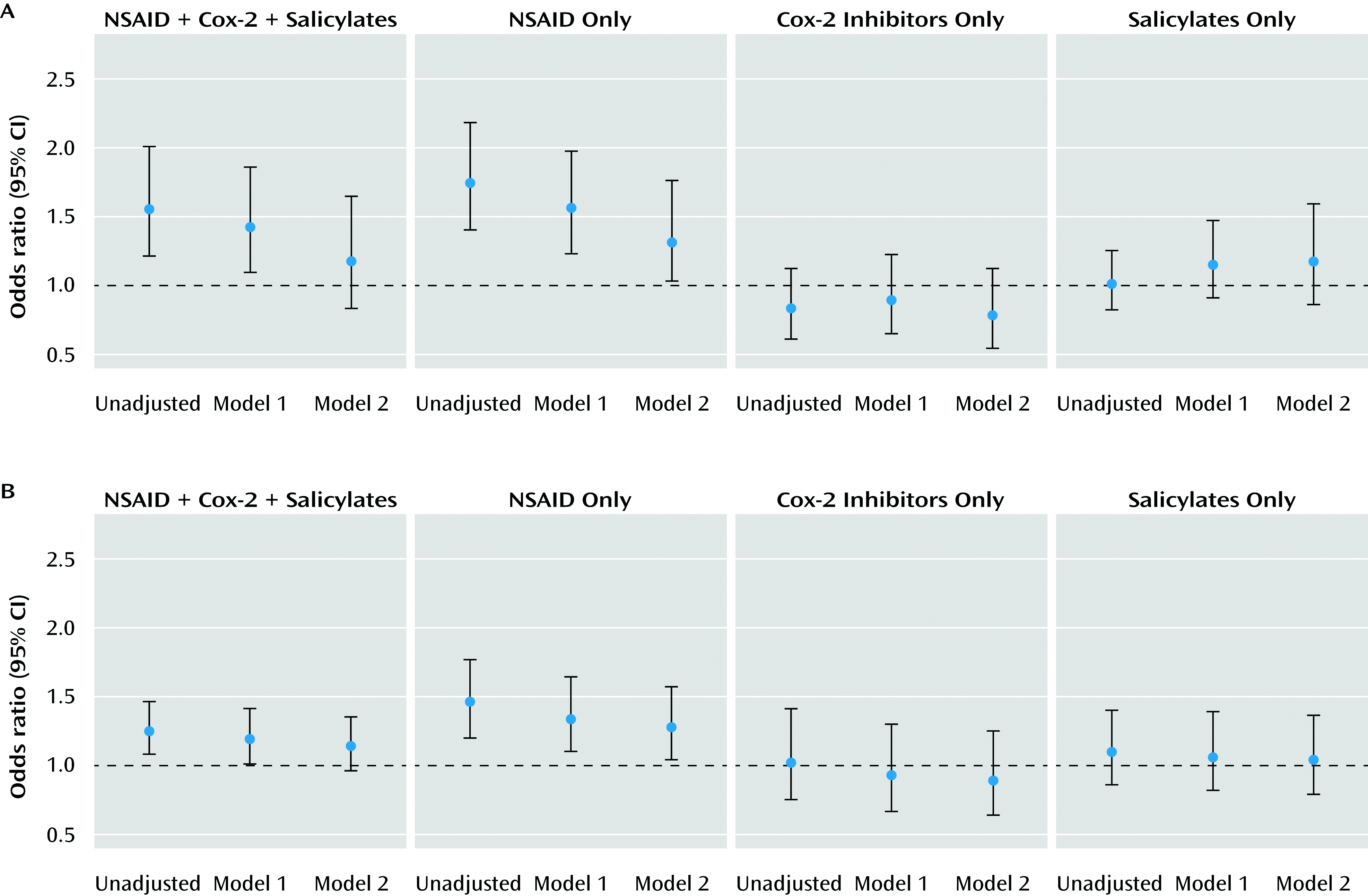
Patients with NSAID exposure were significantly more likely to be older, female, and non-White, as well as to have public rather than private insurance. As anticipated, NSAID-exposed patients also demonstrated significantly greater medical comorbidity and use of health care services. Crude and partially adjusted odds ratios for association between NSAID exposure and outcome category are listed in
Table 2 and illustrated in
Figure 1. NSAID exposure in the broad analysis was significantly associated with risk for treatment-resistant depression. This effect appeared to be confined to the NSAIDs-only group, since no significant association was observed in the COX-2 inhibitor and salicylate treatment groups (
Table 2). In the fully adjusted model (model 2 in
Table 2), which incorporated medical comorbidity and health care utilization measures, the beta coefficient for NSAID use changed by more than 10%, a conventional albeit imperfect indicator of confounding (
19), and was no longer statistically significant (odds ratio=1.17, 95% confidence interval [CI]=0.83\x{2013}1.64, p=0.38). When the analysis was limited to NSAIDs only, the effect in the fully adjusted model was likewise reduced but remained significant (odds ratio=1.31, 95% CI=1.03–1.76, p=0.04).
We conducted a secondary analysis in the i2b2 treatment-resistant depression cohort to distinguish between chronic NSAID use and intermittent use. Of the 1,245 patients who were prescribed at least one NSAID, 1,012 (81%) had chronic treatment (>2 prescriptions, excluding as-needed basis only) and 233 (19%) had intermittent treatment (≤2 prescriptions or as-needed basis only prescriptions). Demographic and clinical characteristics were similar between patients with chronic NSAID use and those with intermittent use (see Table S1 in the
data supplement accompanying the online edition of this article). In the fully adjusted model (model 2), patients with chronic NSAID use continued to have increased risk for treatment-resistant depression relative to patients without NSAID use (odds ratio=1.47, 95% CI=1.03–1.76, p=0.02), but no increased risk was observed in patients with intermittent use. (For details of the results in the secondary analysis, see Table S2, Table S3, and Figure S1 in the online
data supplement.)
We next reanalyzed the STAR*D cohort to better characterize potential confounding variables, particularly those that might mediate confounding by indication (i.e., medical comorbidity). Patients who were exposed or unexposed to NSAIDs or NSAID-like medications differed on most sociodemographic and clinical characteristics (
Table 3). As previously reported by Warner-Schmidt et al. (
3), without adjusting for confounding variables, NSAID exposure was associated with nonremission at level 1 in STAR*D (crude odds ratio=1.23, 95% CI=1.06–1.44, p<0.01) (
Table 4). However, in fully adjusted logistic regression models including terms for Cumulative Illness Rating Scale severity in each category, the effect size was markedly diminished and no longer statistically significant. We observed a pattern similar to that in the i2b2 treatment-resistant depression cohort: the significant effects appeared to be limited to the NSAIDs-only treatment group rather than the NSAID-like treatment groups.
To better understand the potential for confounding by pain, we conducted two follow-up analyses. First, we examined narcotic exposure among non-NSAID-treated patients in STAR*D. Although this analysis might carry the risk of the same potential confounding effects observed in the Warner-Schmidt et al. (
3) study, it does not consider the same molecular mechanisms. As we expected, narcotic exposure was associated with nonremission (
Table 4). We observed similar evidence of confounding; in a fully-adjusted model, the effect was no longer statistically significant.
Second, we examined the effect of NSAID treatment chronicity in STAR*D. We defined a priori a chronic/subacute treatment group (with initiation of NSAID treatment 14 or more days before entering STAR*D, N=337) and an acute treatment group (with initiation of NSAID treatment within 14 days of entering level 1 of STAR*D or during level-1 treatment, N=262). In our comparison of the chronic/subacute treatment group and the no-NSAIDs group, a significant effect on odds of nonremission was observed (fully adjusted model odds ratio=1.44, 95% CI=1.10–1.90, p<0.01). In our comparison of the acute treatment group and the no-NSAIDs group, a substantially more modest and nonsignificant effect was observed (fully adjusted odds ratio=1.07, 95% CI=0.80\x{2013}1.42, p=0.66).
Finally, we examined NSAID association with response to CBT in level 2 of STAR*D, again to consider an intervention with a presumed different mechanism of action. Among 147 patients receiving CBT (alone or as augmentation of citalopram), 25 (17.0%) also received concomitant NSAID treatment during this level. As observed in the level-1 analyses, NSAID administration was associated with greater likelihood of nonremission in an unadjusted model, with more modest effects in a fully adjusted model, although none of these effects were statistically significant, most likely because of the modest sample size.
Discussion
In this pharmacovigilance study using data from a large health care system, we confirmed a significant association between NSAID exposure and poorer antidepressant treatment outcome in major depressive disorder. In both of the cohorts we analyzed, this effect appeared specific to treatment with NSAIDs rather than with agents that have similar mechanisms of action. Importantly, we also identified evidence of potential confounding effects using measures of medical comorbidity. In a reanalysis of data from the STAR*D study used to support the original report of NSAID risk (
3), we likewise identified evidence of confounding using multiple complementary approaches. Notably, the outcomes with CBT at level 2 in STAR*D revealed the same pattern observed for outcomes with citalopram at level 1. This was surprising, since the mechanism of action of CBT is presumably not through direct effects on cytokines. Likewise, citalopram-treated patients who were receiving narcotics at level 1 were also less likely to remit, an effect partially explained by medical comorbidity.
A body of literature suggests that general medical comorbidity is associated with poorer treatment outcomes in major depression (
5,
20).
In particular, the presence of painful symptoms seems to be associated with greater depression severity and poorer outcomes (4). A portion of the observed effects in both of the cohorts we analyzed appeared to be confounded by this phenomenon. However, a more modest effect remained even after adjustment for these confounding factors.
Two key questions follow from our observations drawn from the two different data sets. The first is whether the observed association, after adjusting for confounding variables, is considered clinically significant and actionable. That is, should clinicians aim to avoid NSAID treatment in depressed patients receiving antidepressant treatment? The finding by Warner-Schmidt et al. (
3) is scientifically valuable, even if it does not inform practice, in that it elucidates a putative mechanism of antidepressant effect in vitro. One prediction arising from our data set could be that salicylates and COX-2 inhibitors might be preferable to NSAIDs when indicated for antidepressant-treated patients, absent broader considerations of cost and safety. However, in light of the modest effect size we observed in fully adjusted models, additional investigation in large cohorts or randomized studies is warranted before practice can be altered in this way.
The second question is that of what the appropriate next step in investigation should be. When pilot studies or pharmacovigilance studies identify potential beneficial effects, the gold standard for confirming efficacy is considered to be a randomized controlled trial. On the other hand, when there is preliminary evidence of harm, the next step is less clear. For example, we would probably not proceed with a trial in which some individuals are randomly assigned to smoke cigarettes. At minimum, based on our analysis, there should be sufficient evidence of association to motivate further investigations using large independent data sets, ideally ones with prospectively collected outcomes and detailed exposure data.
A key limitation of our analysis is that, as with any data set that does not include randomization, we cannot fully exclude unmeasured confounding variables. In particular, while we are able to characterize proxy measures of medical comorbidity, electronic medical record data are insufficient to identify specific comorbidities such as pain, which might be more closely associated with NSAID use. In addition, while electronic medical record-based data sets have the advantage of including rich data on medical comorbidity, they often include only limited data on psychiatric comorbidity or on crucial details of pharmacotherapy, such as adherence. Likewise, adequacy of antidepressant treatment can be established by duration (
7) but not necessarily by dosage. The precision with which depression treatment outcomes may be assigned in these types of data sets is still less than that of a prospective outcome study that employs systematic use of clinician ratings. The systematic incorporation of scale-based measures in routine clinical practice would greatly improve the utility of future pharmacovigilance studies.
One notable aspect of our analysis could have led to an underestimate of strength of association. In both data sets, there was insufficient detail available to reliably estimate the daily dose, which precludes characterization of dose-response. On the other hand, in both cohorts, we were able to estimate the chronicity of NSAID use. Confidence in our findings is increased by the observation of a stronger effect in the chronic/subacute NSAID-treatment group and little or no effect in the acute NSAID-treatment group.
Despite these limitations, we emphasize the potential utility and efficiency of electronic medical record-based pharmacovigilance systems for rapid identification or confirmation of risk, as well as the consistency of observations between our electronic medical record-based cohort and the more traditionally ascertained STAR*D cohort. Our analysis was initiated within 7 days of the initial report by Warner-Schmidt et al. (
3), building on previous investigations using the same methodologies to elucidate association between rosiglitazone and myocardial infarction (
8,
21) and between high-serotonin-affinity antidepressants and gastrointestinal bleeding or stroke. By integrating across multiple large data sets, it should be possible to detect early evidence of harm, which might then motivate closer follow-up analysis in more targeted or focused data sets.