Working memory is engaged for the temporary storage and manipulation of information, and it underpins many higher-order cognitive processes and goal-directed behaviors (
1). Deficits in working memory are prominent in schizophrenia (
2) and have been consistently observed in functional MRI (fMRI) studies of both established (
3–
6) and first-episode patients (
7–
9).
The neurodevelopmental model of schizophrenia posits that cognitive and physiological abnormalities in persons at high risk for schizophrenia may precede the appearance of full-blown symptoms (
10–
13). Cognitive function in at-risk individuals has been assessed over multiple domains, including fluid intelligence, executive function, working memory, speed of processing, and attention. Relevant to the present study, working memory deficits appear to distinguish persons at risk for psychosis from healthy subjects (
14–
18).
Functional neuroimaging studies of individuals at risk for psychosis have found that patterns of activation similar to those observed in schizophrenia patients may precede the development of psychosis (
19). For example, alterations in prefrontal and parietal activation during working memory tasks have been reported prior to the appearance of significant behavioral change (
20,
21). However, there is little agreement regarding the direction of these functional imaging shifts. Some studies have found task-related hyperactivation in the right dorsolateral prefrontal cortex, the left inferior parietal lobule, and the anterior cingulate cortex (
22–
24). Other studies have found reduced activation in the inferior frontal cortex (including the ventrolateral prefrontal cortex and the anterior insula [
25,
26]), the dorsolateral prefrontal cortex, and the parietal cortex (
27–
29). The lack of agreement between studies may be attributed to the relatively small sample sizes (approximately 20 participants in each group), inclusion of at-risk individuals who are receiving medication, and differences in the task parameters used in working memory studies, as well as variation in behavioral performance between study and comparison samples (
30,
31).
To address the inconsistencies in earlier imaging studies, we compared a relatively large sample of individuals with at-risk mental states, identified using the Comprehensive Assessment of At-Risk Mental States (
32), with age-matched healthy comparison subjects from the community. Analyses of the imaging data emphasized interrogation of two main networks of regions that have previously shown anomalous activation related to working memory function in persons at risk for psychosis and in schizophrenia patients.
In the first of these networks—the lateral prefrontal and parietal regions strongly implicated in working memory (33, 34)—we hypothesized that the at-risk group would exhibit activation differences that reflect reduced efficiency of brain regions supporting working memory, as well as the compensation for this reduced efficiency (
35,
36). The second network that we examined is referred to as the default-mode network (
37–
39) because the areas are frequently deactivated when a person is focused on a specific task but activated when attention is not focused on the external world. We hypothesized that there would be less task-related deactivation in the default-mode network (
40,
41); this would signify reduced ability to disengage from the internal environment to perform the task at hand. Finally, to test the functional relevance of altered activation in at-risk participants, we correlated the magnitude of signal change with several established measures of symptom severity and cognition.
Method
Participants
We studied 69 persons at risk for psychosis and 40 comparison subjects between the ages of 14 and 29 years. Participants were recruited as part of the Longitudinal Youth At-Risk Study, which sought to identify and follow up ultra-high-risk persons over a 2-year period through psychiatric clinics in Singapore at the Institute of Mental Health, the Singapore Armed Forces, and community mental health services.
Participants were assigned to the at-risk group if they met criteria for any of the following three subgroups, based on the Comprehensive Assessment of At-Risk Mental States: 1) a vulnerable subgroup, comprising individuals with a family history of psychosis in a first-degree relative or with a schizotypal disorder; 2) an attenuated psychosis subgroup, comprising individuals with subthreshold psychotic symptoms; and 3) a brief limited intermittent psychotic symptoms subgroup, comprising individuals with a recent history of frank psychotic symptoms that resolved spontaneously within 1 week. At-risk participants had no history of psychiatric, neurological, or serious medical disorders or mental retardation, and they were not receiving any antipsychotic medications at the time of imaging. Persons with current substance abuse were excluded. Imaging data for three at-risk persons were excluded because of excessive head movement (>3 mm across runs). Data for an additional six at-risk participants were excluded because of poor task performance (<75% accuracy on control and maintenance-of-information conditions). Thus, data for 60 at-risk participants were analyzed. Demographic and clinical characteristics for the at-risk group are summarized in
Table 1. The small numbers of participants categorized as vulnerable or as having brief limited intermittent psychotic symptoms made subgroup analyses untenable; however, we did investigate differences in brain activity between vulnerable-only participants (subgroup 1) and those exhibiting psychotic-spectrum symptoms (subgroups 2 and 3). For the results of this analysis, see Figure S1 in the
data supplement that accompanies the online edition of this article. Six at-risk participants were later diagnosed with a psychotic disorder over a follow-up period ranging from 1 year for those recruited later in the course of the study to 2 years for those examined earlier.
In the at-risk group, 35 participants were receiving prescription antidepressants, 24 were not receiving any medications, and one had previously received chlorpromazine at a low dosage but was not receiving any medications at the time of imaging. Aside from the participant who had previously received chlorpromazine, all at-risk participants were antipsychotic naive.
Healthy comparison subjects were recruited through public advertisements in print and online media. They had no history of psychiatric disorders and were matched for age with at-risk participants. One comparison subject was excluded because of excessive head motion, and one was excluded because of poor performance, leaving 38 healthy subjects for inclusion in the analysis.
Participants underwent a battery of neurocognitive tests assessing a range of functions, including working memory, attention, and vigilance. Additionally, at-risk participants were assessed using the Global Assessment of Functioning Scale (GAF), the Positive and Negative Syndrome Scale (PANSS [
42]), the Brief Assessment of Cognition in Schizophrenia scale (
43), and the Wechsler Abbreviated Scale of Intelligence (
44). Because left-handedness is more common in schizophrenia patients, we did not attempt to balance handedness in the groups to avoid introducing bias; however, handedness was assessed using an inventory (
45) for inclusion as a covariate in imaging interactions.
The study protocol was approved by the National Healthcare Group Domain Specific Review Board (Singapore). Written informed consent was obtained from participants above age 21 or from a parent or guardian for participants under 21 (with the participant’s assent) after they received a complete description of the study. All participants were reimbursed for their participation in the study.
Experimental Design
The working memory experiment (
46) was one of four fMRI experimental paradigms administered in the functional neuroimaging battery. The other experiments (to be described in detail in separate reports) assessed associative learning and generalization (
47), implicit processing of fearful and neutral male and female faces (
48), and anticipation of or motivation to obtain a reward (
49). The working memory experiment was always administered third.
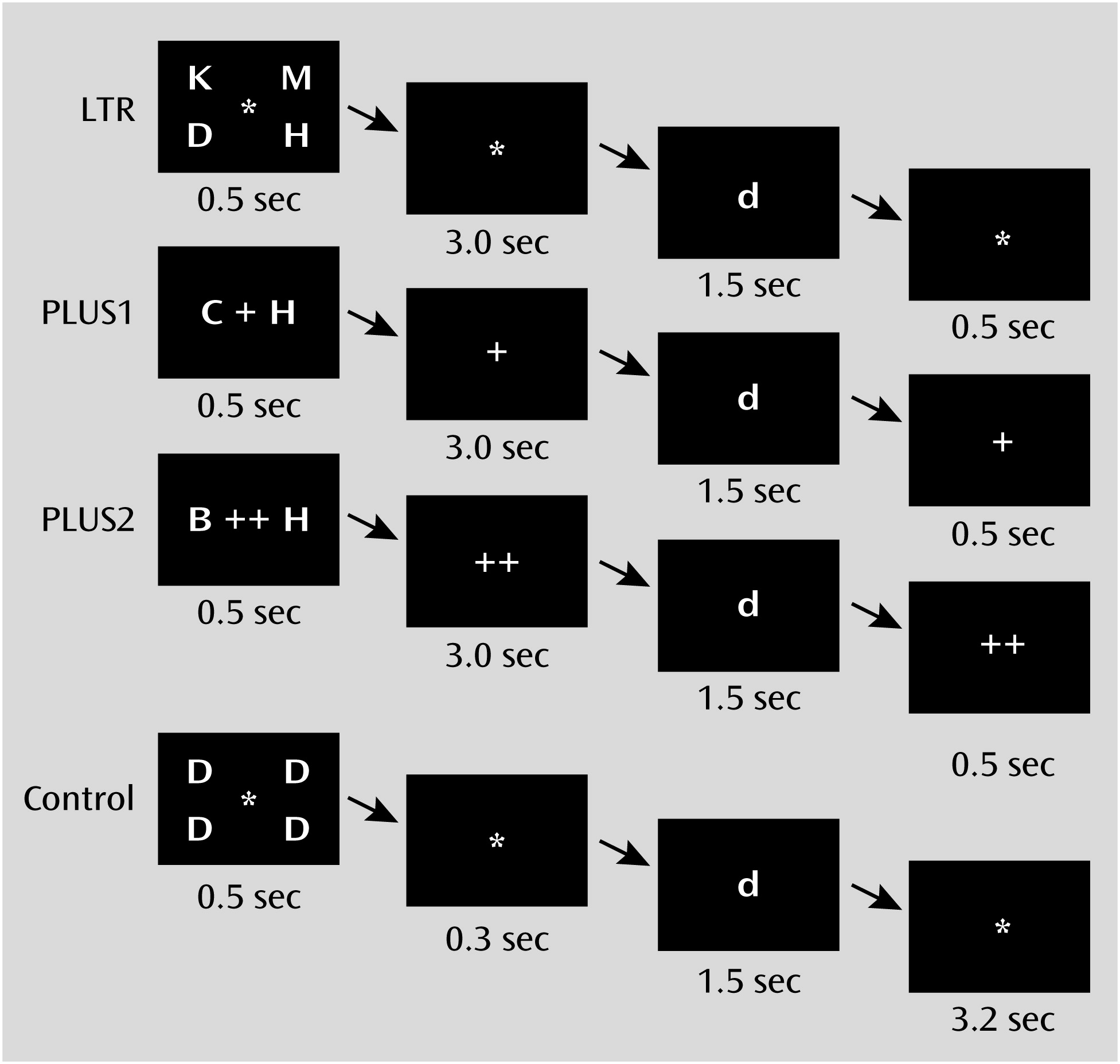
The working memory experiment, which we refer to here as the working memory task, comprised three task conditions and one control condition. Briefly, the LTR condition evaluated maintenance of information, while the PLUS and PLUS2 conditions evaluated both maintenance and manipulation of information (
Figure 1; see also the online
data supplement). Participants were required to achieve an accuracy of at least 75% on both the LTR and control conditions. In the scanner, participants were presented with three runs of alternating blocks of conditions.
Behavioral performance was assessed using accuracy and response time. These measures were entered in separate four-condition-by-two-group mixed-effects analyses of variance testing for main effects of group and condition for each variable. Significant main effects and interactions were decomposed using t tests.
Image Acquisition and Analysis
Details of the image acquisition parameters, fMRI data preprocessing steps, and statistical analyses are presented in the online
data supplement. The functional images were processed and analyzed using BrainVoyager QX, version 1.10.4 (Brain Innovation, Maastricht, the Netherlands), and custom routines were written in MATLAB (MathWorks, Natick, Mass.). The main effect of group and condition-by-group interactions was assessed using a three-condition-by-two-group mixed-effects analysis of covariance (ANCOVA). These analyses used age, sex, education, handedness, ethnicity, and accuracy as covariates. The resulting F-maps were subject to a corrected cluster significance threshold of p<0.05 (initial uncorrected voxel-level threshold, p<0.001).
The manipulation aspect of working memory was of particular interest, since it involves acting on the contents of short-term memory. We identified activation associated with the manipulation component to investigate group differences in activation within the regions examined. From the conjunction map of each maintenance plus manipulation condition against the maintenance-only condition (PLUS>LTR and PLUS2>LTR), six fronto-parietal regions known to be involved in working memory processes (
46,
50) were selected as regions of interest to be considered for between-group analyses. A three-condition-by-two-group mixed-effects ANCOVA was performed for the parameter estimates at each region of interest. A statistical threshold of p<0.008 (corrected for multiple comparisons for six regions of interest) was applied in this second-level analysis to identify significant condition-by-group interactions.
To evaluate the possible modulatory effects of antidepressant medication on brain signal (
51–
53), we repeated the above analysis with the at-risk group divided into medicated and nonmedicated subgroups.
Finally, in the at-risk group, we explored the correlation between brain activation and clinical symptom severity (using the GAF scores, the subscale and total scores of the PANSS, and the combined intensity and frequency scores of the Comprehensive Assessment of At-Risk Mental States), as well as measures of cognition (using the symbol coding and digit sequencing scores of the Brief Assessment of Cognition in Schizophrenia and the vocabulary score of the Wechsler Abbreviated Scale of Intelligence). These analyses were conducted for brain regions showing group differences in overall task-related activation and regions involved in manipulation of working memory contents. To ensure that only areas activated during the working memory task entered these analyses, brain areas were masked using the functional activation masks derived from each group for the corresponding condition or contrast.
Results
Behavioral Data
There were no significant differences between the 60 at-risk participants and 38 comparison subjects in age, handedness, sex, or ethnicity (
Table 1). At-risk participants had less education on average than comparison subjects. However, there were no significant differences in vocabulary scores between the two groups.
There was a main effect of condition on both accuracy (F=151.9, df=3, 288, p<0.001) and response time (F=459.0, df=3, 288, p<0.001) but no effect of group on either measure of behavioral performance. Increasing difficulty elicited fewer correct responses and longer response times in both groups, but there were no significant group differences. The condition-by-group interaction was not significant for either accuracy or response time.
When the analysis was repeated to take into account use of antidepressant medication, the main effect of condition remained significant (accuracy: F=153.3, df=3, 285, p<0.001; response time: F=455.6, df=3, 285, p<0.001). There was no effect of group, nor were there any condition-by-group interactions (see Table S2 in the online
data supplement).
Imaging Data
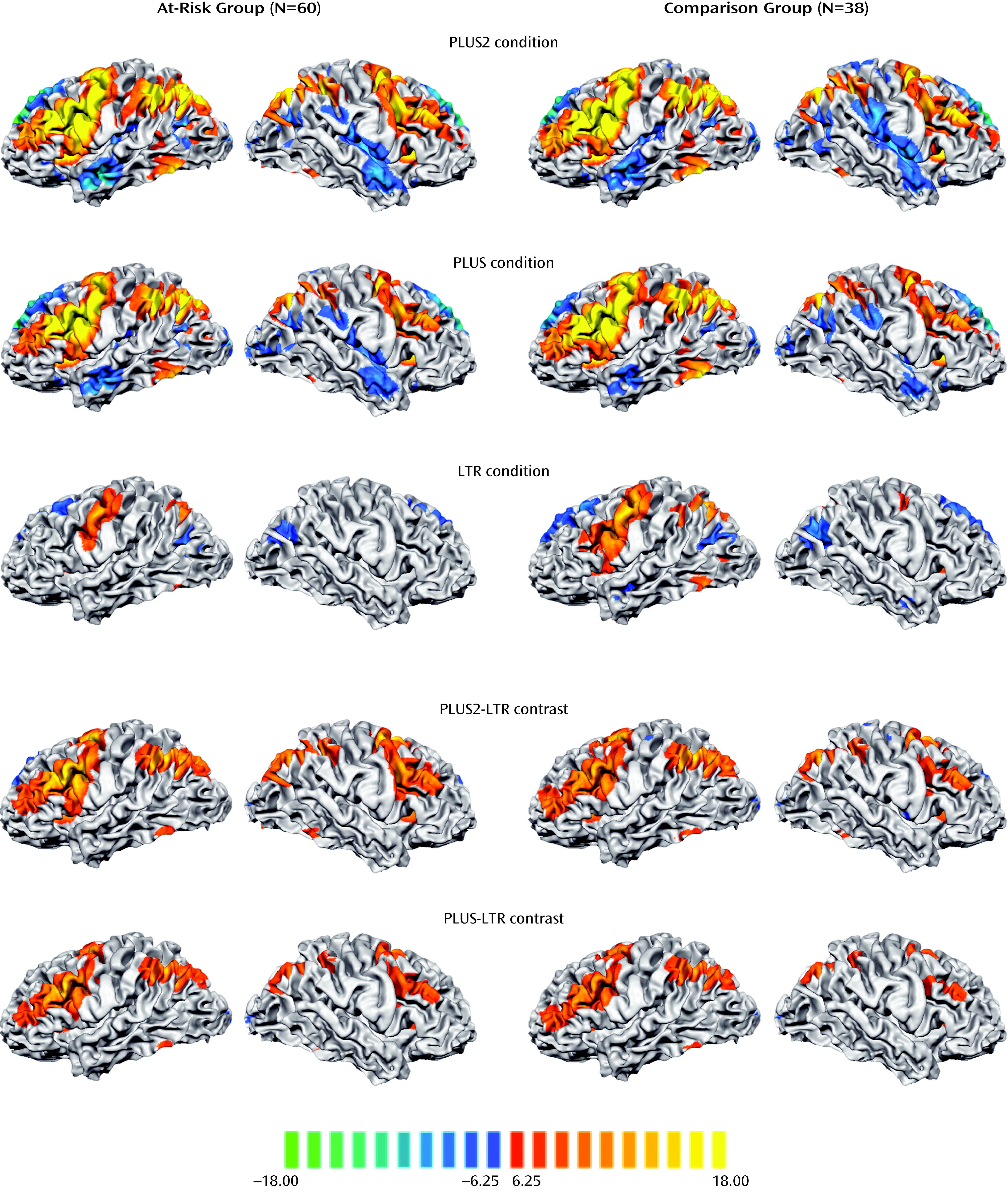
Engagement of verbal working memory across the different task conditions elicited a common network of left and right fronto-parietal regions in both the at-risk and comparison groups. As expected, activation in these regions increased in magnitude and spatial extent with increasing task difficulty (
Figure 2).
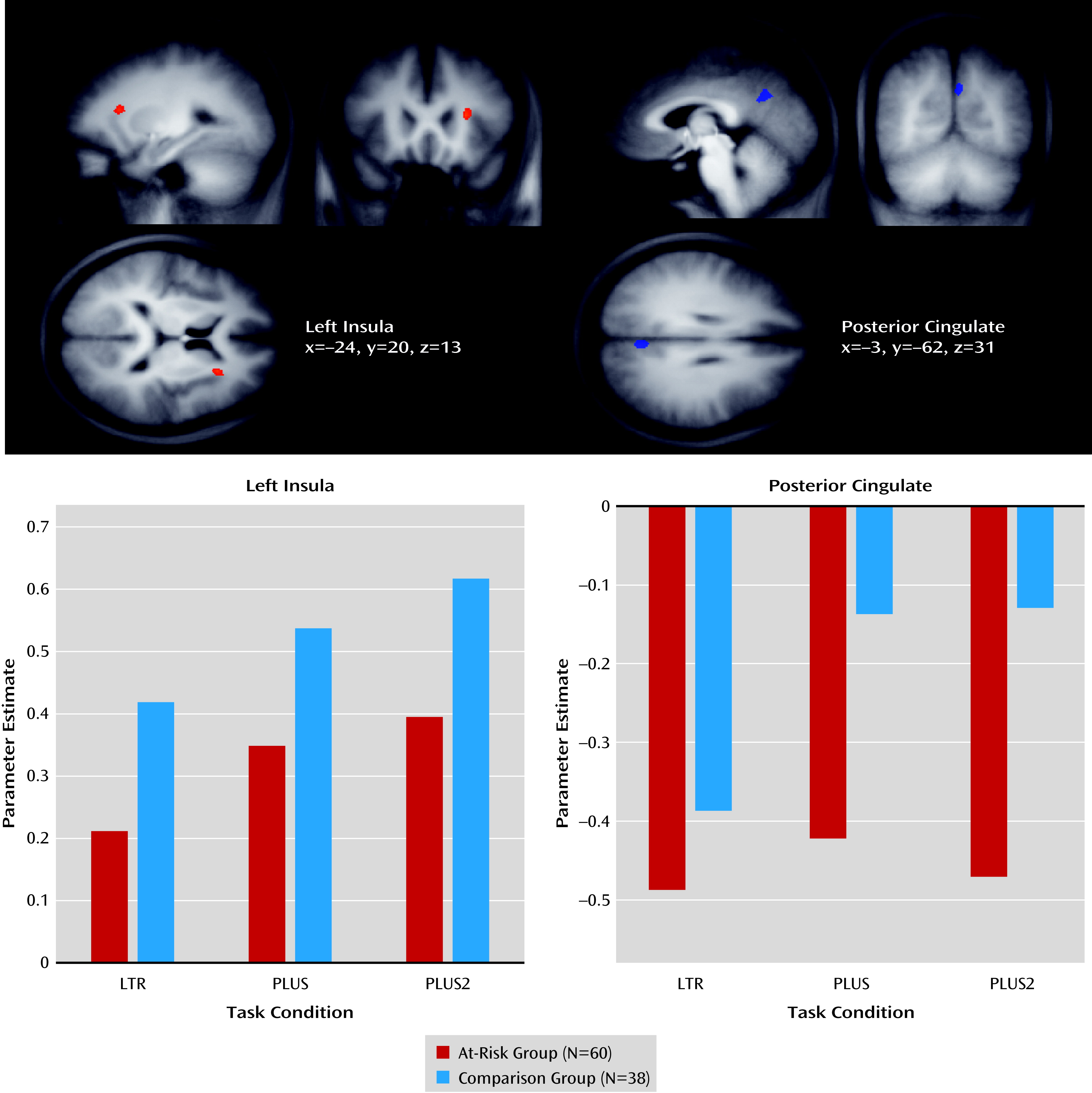
Activation in the left anterior insula and posterior cingulate cortex showed a significant main effect of group (
Figure 3; see also Table S1 in the online
data supplement). The at-risk group exhibited reduced anterior insula activation and increased posterior cingulate cortex deactivation relative to the comparison group in each of the three experimental conditions. The effects remained significant when both medicated and nonmedicated at-risk participants were accounted for (see Figure S2 and Table S2 in the
data supplement).
Condition-by-group interactions were identified from a 3×2 ANCOVA. Regions showing significant interaction effects included the right inferior frontal gyrus, right frontal eye field, right insula, precuneus, left precentral gyrus, posterior cingulate cortex, medial prefrontal cortex, left middle frontal gyrus, and left anterior middle frontal gyrus (see Table S1 in the
data supplement). The posterior cingulate cortex, medial prefrontal cortex, left middle frontal gyrus, and left anterior middle frontal gyrus revealed task-related deactivation, while all other regions revealed task-related activation. In the regions where task-related activation was observed, the at-risk group exhibited a greater increase in activation during the manipulation conditions than the comparison group. In the regions where task-related deactivation was observed, the at-risk group exhibited relatively more pronounced deactivation during the manipulation conditions than the comparison group.
Activation associated with manipulation was assessed using a conjunction of the manipulation-related contrasts (PLUS–LTR and PLUS2–LTR). Both the PLUS and PLUS2 conditions elicited greater fronto-parietal activation than the LTR condition in both the at-risk and comparison groups (
Figure 2). From the maps showing manipulation-related activity, we analyzed six regions of interest that were centered on the locus of peak signals in the frontal and parietal lobes (see Table S1 in the
data supplement).
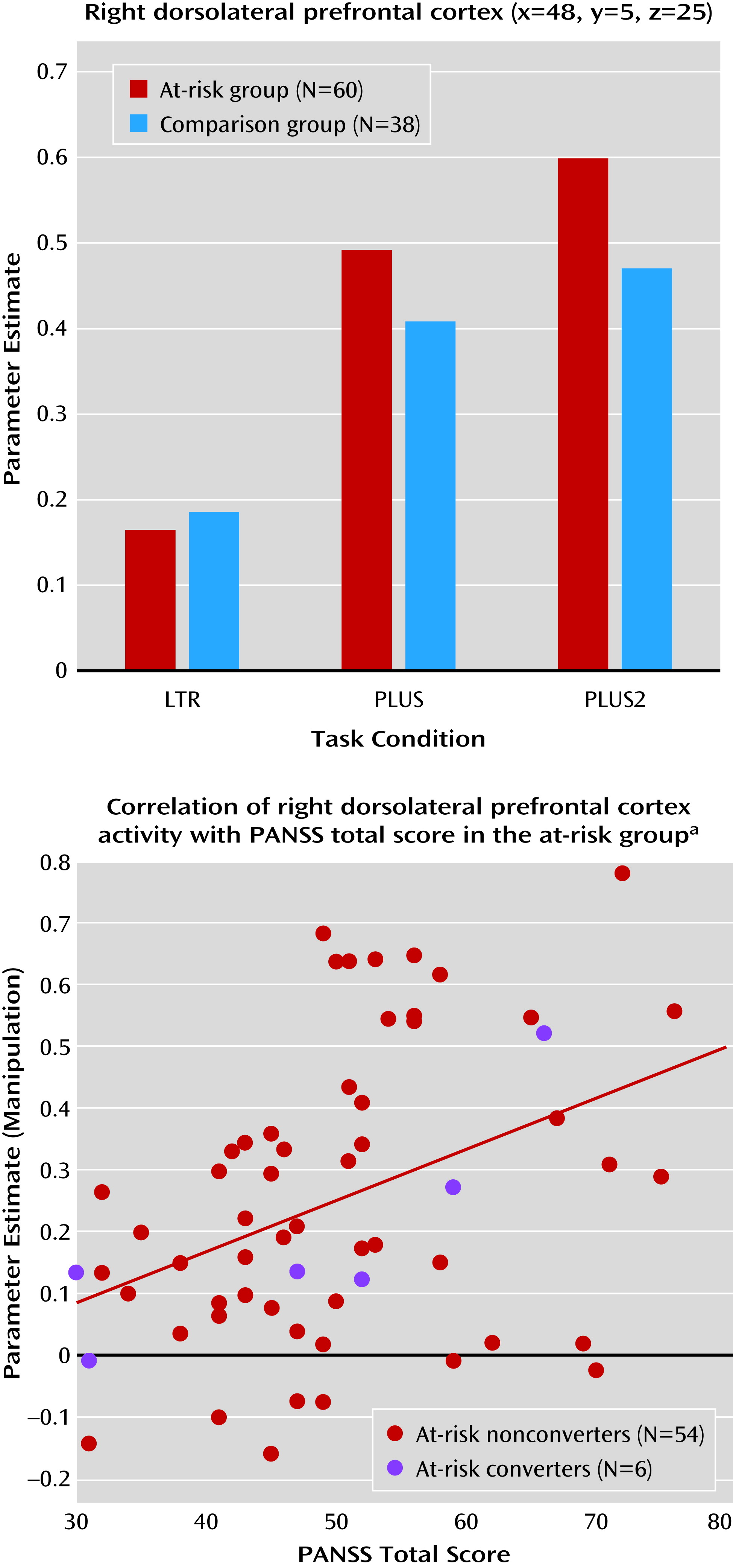
The only region of interest that showed significant condition-by-group interaction was the right posterior dorsolateral prefrontal cortex (F=5.94, df=2, 192, p=0.004) (
Figure 4). The at-risk group exhibited greater increase in manipulation-related activity than the comparison group. Both medicated and nonmedicated at-risk participants exhibited greater MR manipulation-related activity than comparison subjects, although the between-group differences were less pronounced (F=3.20, df=4, 190, p=0.02) (see Figure S2 and Table S3 in the
data supplement).
Association Between Brain Activation and Symptom Severity
There was a significant correlation between manipulation-related activation and PANSS total scores (r=0.40, df=58, p=0.001) in the right dorsolateral prefrontal cortex (
Figure 4). Correlations with PANSS positive, negative, and general psychopathology scores in the right dorsolateral prefrontal cortex were positive but nonsignificant. None of the other symptom scales or measures of cognition were correlated with MR signal in contrasts of interest.
Discussion
This study represents one of the largest functional imaging studies comparing persons at high risk for developing psychosis with healthy comparison subjects. We observed altered patterns of activation in the at-risk group as they maintained and manipulated letters in working memory. These group differences remained significant after accounting for differences in education and antidepressant use (see the online
data supplement).
At-risk participants exhibited reduced activation in the left anterior insula compared with comparison subjects in all task conditions. When manipulating letters, the at-risk group displayed more pronounced task-related deactivation of the default-mode network than the comparison group. A region-of-interest analysis of MR signal in regions known to be involved in working memory found greater manipulation-related activation in the right dorsolateral prefrontal cortex. In the at-risk group, the magnitude of right dorsolateral prefrontal cortex signal during working memory manipulation correlated with PANSS symptom scores.
Absence of Significant Behavioral Differences Between Groups
The equivalence of behavior between the at-risk and comparison groups, despite differences in education, across all three conditions allowed us to attribute the altered patterns of activation to the at-risk mental state (
23,
29,
31,
54), as opposed to poorer performance in at-risk participants. Additionally, the comparability of performance across medicated and nonmedicated participants reassures us that the imaging findings were unlikely to be a result of antidepressant therapy. The large size of our sample allowed us to perform subgroup analysis with reasonable power.
Reduced Insula Activation in At-Risk Participants
Reduced anterior insula activity was observed across all task conditions in the at-risk group and was our most robust finding. The anterior insula and anterior cingulate cortex are part of a salience network (
55) that supports high-level executive processes.
The insula has been proposed as the key region involved in detecting salient stimuli and facilitating switching between the task-positive central executive network and task-negative default-mode network (
56). Decreased anterior insula activity in schizophrenia is thought to result in a reduced ability to detect and process salient stimuli and thus an inability to switch between relevant brain networks in order to access attention and working memory resources required for performing the task (
57,
58), even under conditions of matched performance (
59). This could explain why group differences in insula activation were observed even in the LTR condition, which imposes minimal cognitive load. This finding contrasts with those in the dorsolateral prefrontal cortex and posterior cingulate cortex, where group differences emerged only at higher cognitive loads.
Greater Task-Related Deactivation in At-Risk Participants
Task-related deactivation in the default-mode network, in particular the midline nodes, is associated with reallocation of processing resources that occurs when attention is shifted from introspectively oriented mental activity, such as when a participant is at rest, to externally focused goal-directed behavior (
60–
62).
There have been conflicting reports regarding the direction of altered task-related deactivation within the default-mode network in patients with schizophrenia or their first-degree relatives (
63–
65). For example, decreased task-related deactivation accompanied by increased resting-state connectivity within the default-mode network has been reported in patients and first-degree relatives (
38). Reduced task-related deactivation corresponding with lowered cognitive performance occurs in other settings, such as in Alzheimer’s disease (
66) and following sleep deprivation (
46), and could be expected in the at-risk group. On the other hand, some patients with schizophrenia exhibit increased task-related deactivation within the default-mode network in association with changes in resting-state connectivity (
39). In our at-risk group, increased task-related deactivation during task performance was observed in the default-mode network nodes, including the medial prefrontal cortex, posterior cingulate cortex, and left middle frontal gyrus. While this could reflect the need for greater effort in suppressing self-directed thought in at-risk persons but not in schizophrenia patients, further studies are necessary to clarify this issue.
Elevated Prefrontal Cortex Activation During Manipulation in At-Risk Participants
It has been suggested that persons with a schizophrenia spectrum disorder exhibit increases in prefrontal activation as a compensatory change because of deficits in other cortical regions (
67). Greater activation in the right dorsolateral prefrontal cortex, a region implicated in executive processes within working memory, could be compensatory for altered recruitment of the anterior insula. Within the prefrontal cortex, the dorsolateral prefrontal cortex contributes relatively more to manipulation of short-term memory, while the ventrolateral prefrontal cortex contributes primarily to information maintenance (
68–
70). Previous neuroimaging studies suggest that executive functions in working memory, associated with parietal and dorsolateral prefrontal cortex function, may contribute more to working memory impairment in schizophrenia (
6,
7,
71) than the impaired maintenance of information.
Most fMRI studies of working memory involving patients with an established diagnosis of schizophrenia and some studies of first-episode schizophrenia have reported reduced performance accuracy. However, the associated functional imaging findings have been mixed. Some studies have reported reduced activation in prefrontal and parietal regions in patients compared with comparison subjects (
5,
8,
29), while other studies have reported increased activation in these regions (
4,
35,
72).
In a study of patients with schizophrenia, there was a greater increase in activity with elevated cognitive load in patients with good task performance compared with those with poor performance (
73). This suggests that in high-performing patients, reallocation of processing resources to prefrontal areas may allow behavioral performance to be maintained at a level comparable to that of comparison subjects. By contrast, in lower-performing patients, this capacity to adaptively recruit additional brain regions has been found to be exceeded and reflected in decreased prefrontal activation (
30,
74,
75). This pattern of neuroimaging findings resembles adaptation to cognitive aging (
76,
77), in which participants who perform slower exhibit greater activation in the dorsolateral prefrontal cortex. In the present study, our finding of hyperactivation in the right dorsolateral prefrontal cortex in the at-risk group, together with preserved behavioral performance, suggests an adaptive response to the at-risk mental state. While the between-group similarities in performance may reflect a reduced psychometric sensitivity of the task in this population, it is noteworthy that neuroimaging contrasts were sensitive to the at-risk condition when performance measures were not.
Alternatively, the positive correlation between PANSS symptom severity and manipulation-related activation in the right dorsolateral prefrontal cortex may simply denote greater difficulty in engaging task-driven cognition in at-risk participants. Symptomatic individuals may have to cope with intrusive thoughts or auditory hallucinations. This explanation is supported by studies of patients as well as first-degree relatives that found increased prefrontal cortex activity to be correlated with higher symptom severity (
24,
78).
Limitations
While this study suggests altered fMRI activation patterns in at-risk persons without their manifesting overt working memory impairment, these remain baseline results. Six of our 60 at-risk participants presented with a first episode of psychosis after at least 1 year (see Figure S3 in the online
data supplement). This low conversion rate is one of the inherent difficulties in prodromal psychosis research. At-risk individuals are heterogeneous, presenting with a range of symptoms, including anxiety and depression (58% of at-risk participants in our sample were receiving antidepressants), and often have functional deficits. Therefore, the group differences could be related to general psychopathology rather than specifically psychosis risk; continued follow-up to identify individuals who convert will be required to relate neuroimaging findings to specific risk of psychosis.
The healthy comparison subjects are likely higher functioning than the at-risk participants based on education level. However, neither task performance nor brain activity was correlated with education, and when education was included as a covariate in the analyses, the results remained significant. Furthermore, the vocabulary scores of the Wechsler Abbreviated Scale of Intelligence, often used as a proxy for IQ, were not significantly different between the two groups.
Conclusions
We found that while not apparent at the behavioral level, individuals classified as being at risk for psychosis manifested functional neuroimaging changes consistent with altered processing of salient stimuli. These may affect the manipulation of working memory contents in a manner resulting in overactivation of the dorsolateral prefrontal cortex, as well as abnormal default-mode network function. Of functional significance, dorsolateral prefrontal cortex signal alteration was associated with symptom severity. Together, these findings highlight the potential utility of detecting early brain changes to facilitate early treatment for at-risk persons.
Acknowledgments
The authors thank the research staff involved in the recruitment and assessment of the participants of this study.