One important question that remains largely unanswered is how anatomical and functional brain deficits are related early in the course of schizophrenia. Previous anatomical studies have revealed reductions of gray matter volume mainly in the thalamus and the temporal and frontal cortex (
3–
5), perhaps most notably in the temporal cortex in drug-naive first-episode schizophrenia (
12–
15). In contrast, the major functional deficits revealed by functional MRI (fMRI) in drug-naive first-episode schizophrenia patients have been localized more widely in prefrontal, temporal, and parietal regions (
16–
18), especially in the ventromedial prefrontal cortex (
19) and in the default mode network, where deficits have been related to specific clinical features (
20,
21). Thus, while there are overlaps and differences in brain regions where structural and functional abnormalities have been identified, few studies have examined these deficits together in the same sample. Another important question is whether anatomical and functional deficits are different in patients with prominent negative symptoms, which is important because these patients have different clinical manifestations and a diminished treatment response (
22,
23).
Our aim in this study was to characterize alterations of gray matter volume and amplitude of low-frequency fluctuation in 100 drug-naive first-episode schizophrenia patients, the interrelationship of these abnormalities, and their relationship with illness duration and negative symptom severity.
Results
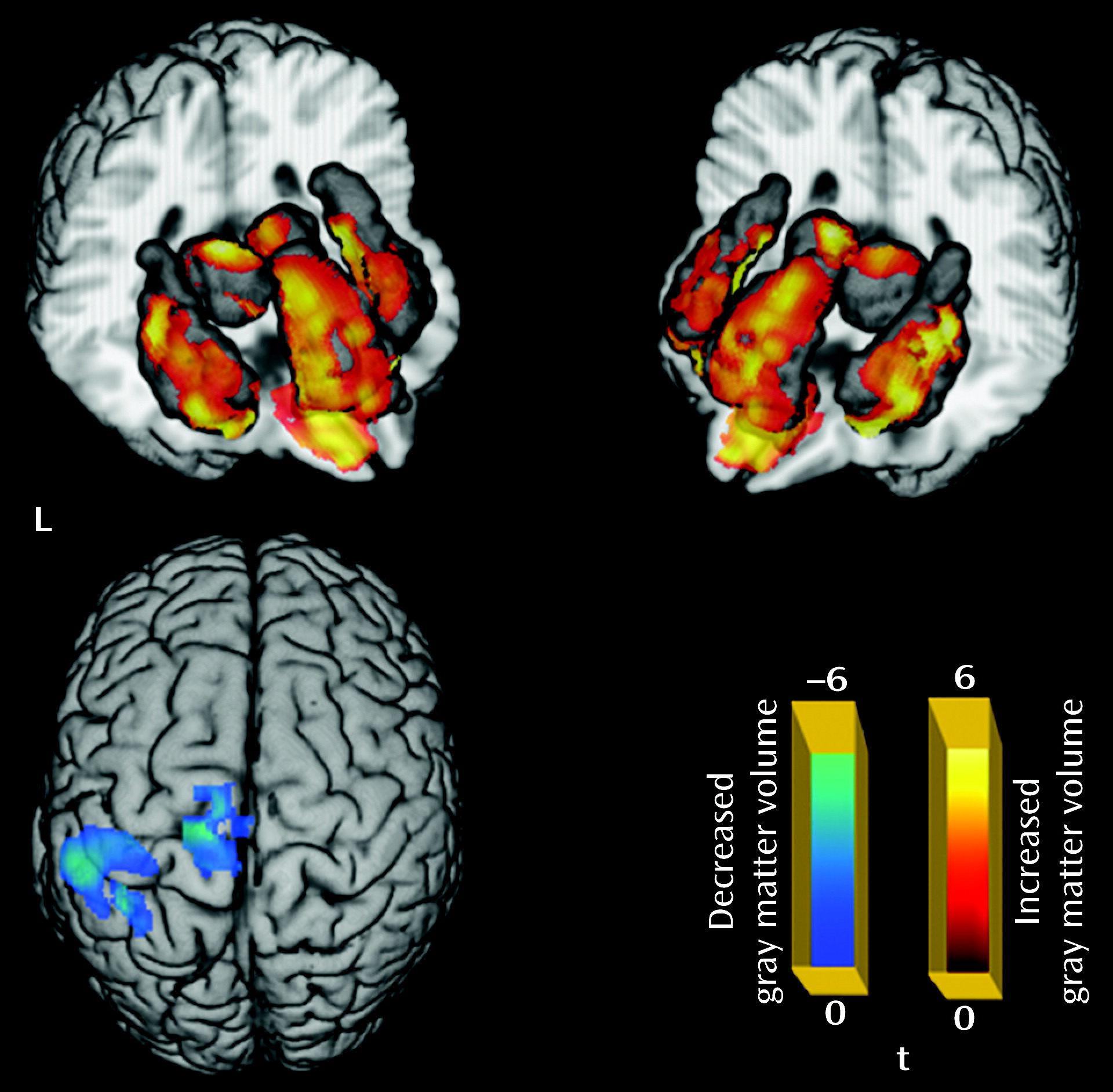
Relative to the comparison group, the patient group showed significantly decreased gray matter volume in the left paracentral and left inferior parietal lobules, and increased gray matter volume in the left and right thalamus, anterior cingulate cortex, insula, and orbital frontal gyrus (p<0.05 corrected with false discovery rate) (
Table 1;
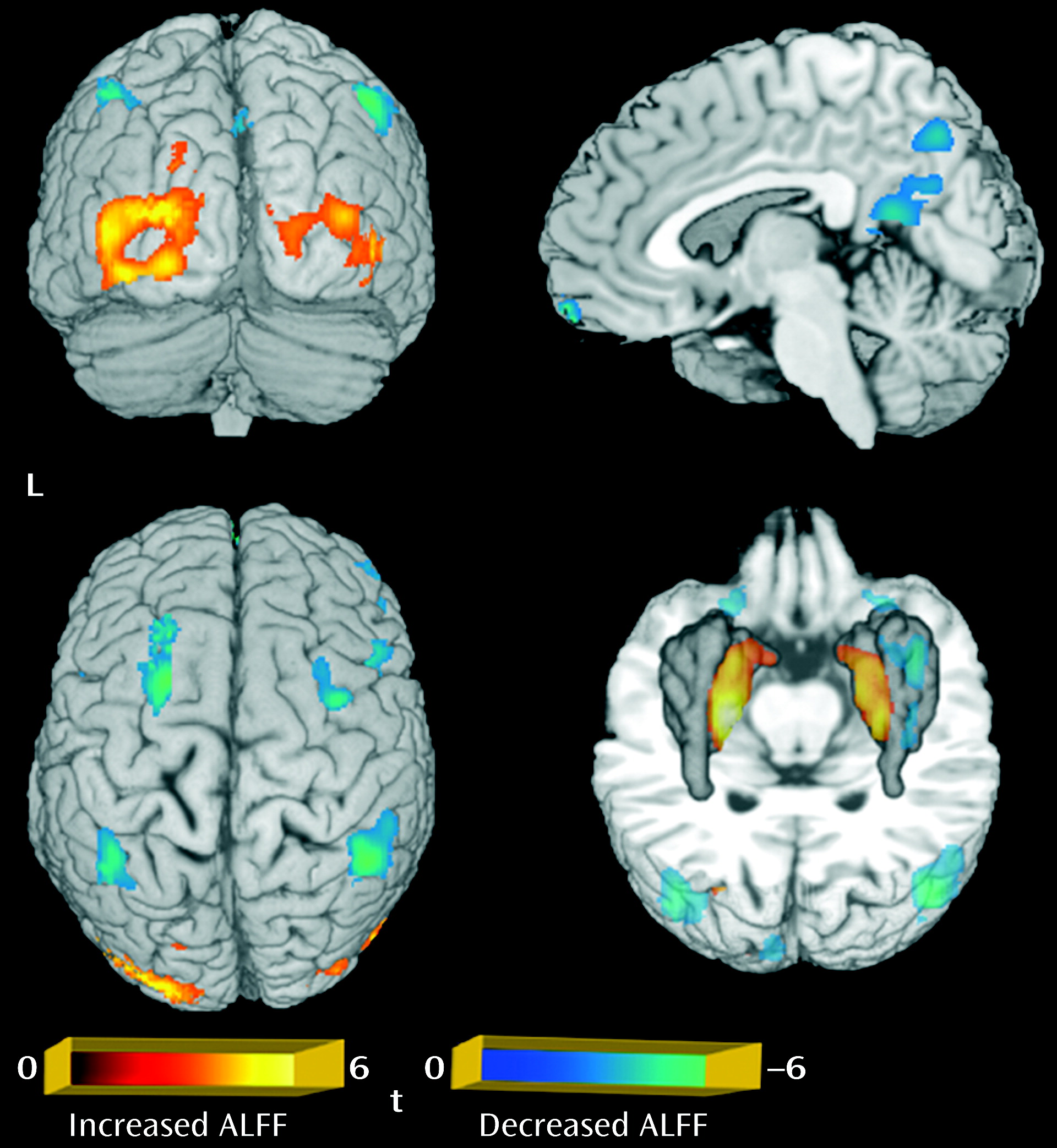
Figure 1). For the amplitude of low-frequency fluctuation analysis, a decreased amplitude of low-frequency fluctuation was observed mainly in the right inferior and left superior frontal gyrus, the medial frontal gyrus bilaterally, the inferior parietal lobule bilaterally, and the precuneus, while an increased amplitude of low-frequency fluctuation was observed bilaterally in the putamen and occipital regions (p<0.05 corrected with AlphaSim) (
Table 1;
Figure 2).
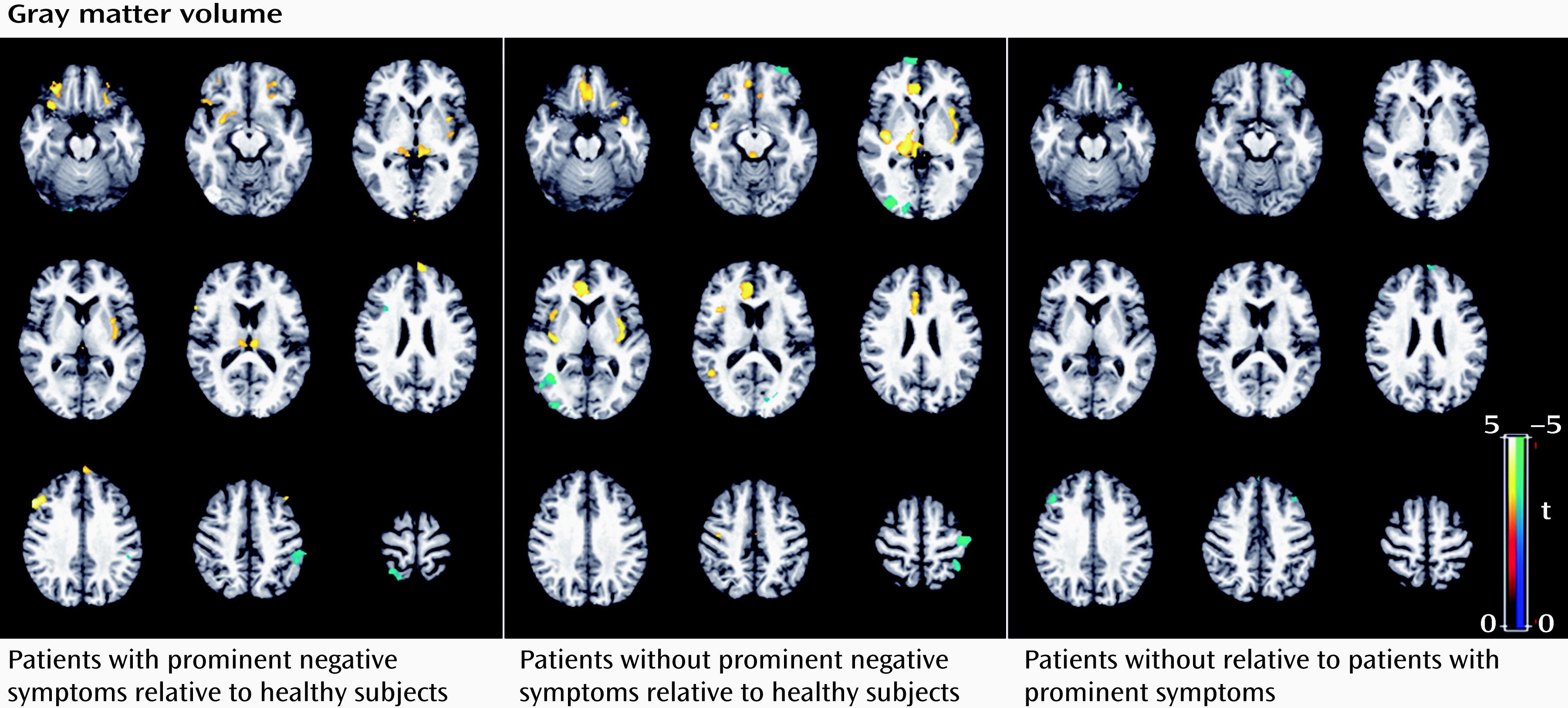
Subgroup analysis revealed that the patients with prominent negative symptoms showed significantly decreased gray matter volume in the right postcentral gyrus and the left inferior parietal lobule, and increased gray matter volume in the left and right thalamus, insula, and dorsolateral prefrontal cortex relative to comparison subjects (p<0.05 corrected with false discovery rate) (
Figure 3; see also Table S2 in the online
data supplement). Relative to comparison subjects, patients without prominent negative symptoms showed significantly decreased gray matter volume in regions including the right middle temporal gyrus, the left middle frontal gyrus, the left postcentral gyrus, and the right middle occipital gyrus, and increased gray matter volume in the left and right thalamus, insula, anterior cingulate cortex, and left inferior frontal gyrus (p<0.05 corrected with false discovery rate) (
Figure 3; see also Table S2 in the
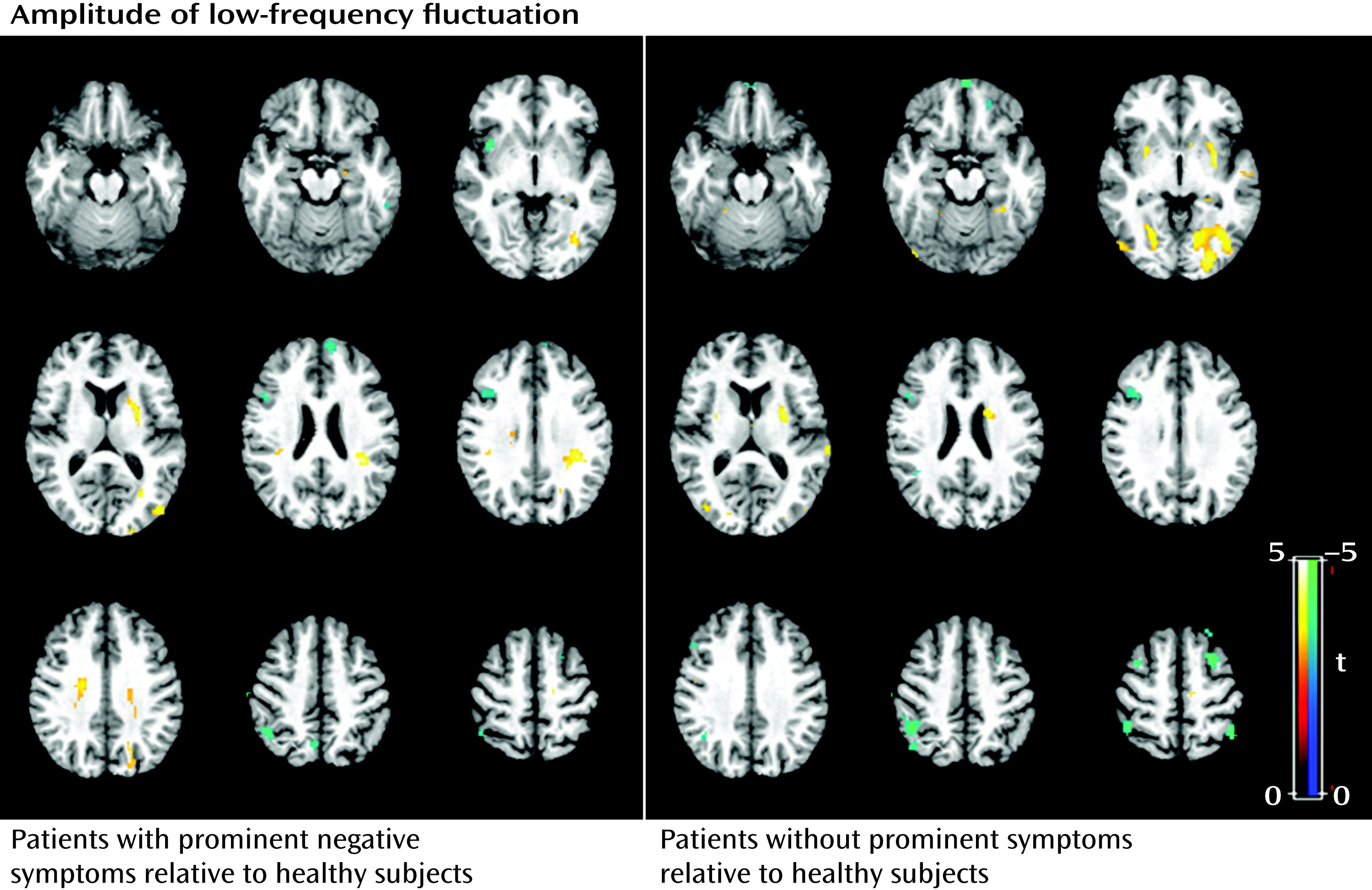
data supplement). The direct comparison between patients without and with prominent negative symptoms revealed smaller gray matter volume in the middle frontal gyrus bilaterally and the left superior frontal gyrus in patients without prominent negative symptoms. For the amplitude of low-frequency fluctuation analysis, patients with and without prominent negative symptoms showed similar patterns of decreased amplitude of low-frequency fluctuation mainly in the left and right medial and middle prefrontal gyrus and the right inferior parietal gyrus, and increased amplitude of low-frequency fluctuation in the putamen and occipital regions bilaterally (p<0.05 corrected with AlphaSim) (
Figure 4; see also Table S2 in the
data supplement). Although the patients without prominent negative symptoms showed more regions with decreased function in the medial prefrontal cortex and the left parietal regions relative to comparison subjects, direct comparisons between the patients with and without prominent negative symptoms revealed no significant difference. Patients with longer and shorter duration of illness did not differ significantly in either the anatomical or functional brain data.
To examine the overlap between gray matter volume and amplitude of low-frequency fluctuation findings, the regions with significant abnormalities in gray matter volume and amplitude of low-frequency fluctuation were overlaid on the same template. There was no overlap of areas with alteration in analyses of gray matter volume and amplitude of low-frequency fluctuation (see Figure S4 in the
data supplement). Furthermore, the average gray matter volume values within the regions with abnormal functional measures were not significantly correlated with the amplitude of low-frequency fluctuation values in either the whole patient group or in subgroups defined by negative symptom severity or illness duration.
Significant positive correlations were observed between untreated illness duration and severity of psychotic symptoms, including PANSS positive symptom scores (r=0.59, p<0.05) and ratings of thought disturbance (r=0.48, p<0.05), activation (r=0.45, p<0.05), paranoia (r=0.50, p<0.05), and anergia (r=0.44, p<0.05). However, no significant correlations were observed between illness duration or positive symptom severity and either changes of gray matter volume or amplitude of low-frequency fluctuation in the patient group.
Discussion
Using the largest sample of drug-naive first-episode schizophrenia patients studied to date with MRI, this study revealed both cerebral anatomical and functional deficits early in the course of illness. However, there was no overlap in the regions with anatomical and functional abnormalities. Gray matter volume changes mainly involved thalamo-cortical networks, while functional changes reflected in the amplitude of low-frequency fluctuation were observed within a fronto-parietal network and the default mode network. Not only did different brain regions show abnormalities in anatomy and physiology measures, but average gray matter volume values within regions having anatomical abnormalities were not significantly associated with the amplitude of low-frequency fluctuation values in those regions. Brain changes in anatomy and function did not correlate with the duration of untreated illness or with positive symptom severity, but some anatomical differences, notably including the increased gray matter volume of the left dorsolateral prefrontal cortex, were identified that varied in relation to negative symptom severity.
It is noteworthy that the patient group showed widespread increases in gray matter volume in multiple brain areas, including the left and right thalamus, insula, anterior cingulate cortex, and orbital frontal gyrus. These findings differ from those of some previous studies that reported decreased gray matter volume within thalamo-cortical networks in chronic as well as first-episode schizophrenia patients (
3–
5,
8,
12–
15). In fact, our previous study in a smaller sample (N=68) of drug-naive first-episode schizophrenia patients also revealed decreased gray matter volume in the right temporal and anterior cingulate cortex (
12). Several factors merit consideration in explaining the regionally greater gray matter volume seen in the present study relative to several previous studies. Differences in patient characteristics across studies may be important, as some other studies have also reported increased gray matter volume in schizophrenia, including a report of such effects in the orbital frontal gyrus and anterior cingulate cortex in a study of 169 patients (
30). One patient characteristic that the present data suggest may be important is severity of negative symptoms. This sample had a higher proportion of patients with prominent negative symptoms (36%) than the sample in our previous study (12%) (
12). The results of the present study indicate that patients without prominent negative symptoms have more regions with decreased volume, especially in right temporal regions, than patients with prominent negative symptoms close to the time of illness onset, which may partly explain the discrepancy with our previous findings (
12). Second, as the patients in the present study were early in the course of illness (average illness duration=6.25 months), possible early-course neuronal pathology, such as preapoptotic osmotic changes or hypertrophy, could increase regional volumes (
31). Progressive gray matter volume loss might be expected after antipsychotic treatment (
6–
10) or in relation to secondary factors or course of illness effects (
6,
32), which could contribute to the absence of reported hypertrophy in previous studies of patients who had a longer duration of illness before MRI scans were obtained (
11). Of note, anatomical and functional changes did not correlate with untreated illness duration or with positive symptoms, and the direct comparison between patients with long and short untreated illness durations revealed no significant differences. These findings suggest a relatively static or slowly evolving process of anatomical changes, which is consistent with a recent study of treated schizophrenia patients (
33) in suggesting the absence of progressive loss of gray matter volume during the early illness course before treatment has begun. Thus, the structural and functional brain changes observed in first-episode patients may be associated with neurodevelopmental problems such as neuronal overgrowth or a deficit in normal pruning during neurogenesis, as suggested by previous studies (
34,
35) or, alternatively, with early illness pathophysiology effects, possibilities that need to be examined in future studies following at-risk individuals. Third, this study had a large sample and may have had the statistical power to detect hypertrophic effects not reported frequently in previous studies. Finally, this study used VBM8, which provides a more optimized method of segmentation and normalization than the VBM2 method in the analysis of gray matter volume (
12). Furthermore, with concern for potential influences of skull-stripping artifacts, especially in the orbital frontal gyrus where deskulling is difficult, we contrasted computerized and manual skull stripping in a subsample of 40 participants and found no differences, indicating that artifacts induced by skull stripping procedures are not likely a cause of the group differences between patients and comparison subjects observed in this study. Although the exact mechanism for increased gray matter volume in first-episode schizophrenia before drug treatment is unclear, our findings indicate that regions within thalamo-cortical networks show early anatomical changes in the course of schizophrenia. Further research is needed on potential neuroinflammatory and other mechanisms that might contribute to these changes in brain anatomy and on how these anatomical changes influence clinical presentation and course of illness.
Deficits in the thalamus and frontal cortex have been widely reported in previous neuroimaging studies of schizophrenia (
4,
9,
36–
39). Alterations in the thalamus have been proposed to be associated with heterogeneous symptoms in schizophrenia, such as hallucinations, delusions, disorganized behaviors and speech, and thought disorder (
5,
36). In contrast, prefrontal abnormalities have been related to negative symptoms and some deficits in higher cognitive function (
40). Several other regions with altered gray matter volume were also identified in the present study, including the insula, inferior parietal lobule, and anterior cingulate cortex (
41). All these regions are located in thalamo-cortical networks, where abnormalities are believed to be central to the pathogenesis of schizophrenia (
10,
42,
43).
In contrast, in the analysis of functional data, abnormalities were located within fronto-parietal and default mode networks, which are thought to be important for decision making, working memory, and general monitoring of internal and external environments (
20,
44,
45). In particular, the decreased amplitude of low-frequency fluctuation in medial prefrontal areas and the inferior parietal lobules bilaterally were consistent with previous studies in drug-naive schizophrenia patients (
19). More interestingly, a small cohort study of antipsychotic-naive patients with first-episode schizophrenia showed significantly increased synchronous regional brain function after 6 weeks of antipsychotic treatment, especially in the left and right prefrontal, medial frontal, and parietal cortex, the left superior temporal cortex, and the right caudate nucleus (
46). These regions include areas where pretreatment alterations were observed in the present study, consistent with the possibility that some of these changes may be shorter-term alterations associated with acute psychosis. Functional deficits in the medial frontal gyrus are thought to be part of the core pathogenesis of schizophrenia and may underlie a number of symptoms, including apathy, lack of emotion, and blunted affect (
47). Besides medial prefrontal regions, we also observed an increased amplitude of low-frequency fluctuation in inferior parietal regions bilaterally. Both inferior parietal regions and prefrontal areas bilaterally play a key role in high-level cognitive and executive processing (
48,
49), and functional activation studies have shown that deficits in these regions are greater during than after episodes of psychosis (
50).
It is interesting that the regions with anatomical and functional changes were so strikingly dissociated and that gray matter volumes in affected regions were not significantly associated with the amplitude of low-frequency fluctuations. Previous studies have also reported different anatomical and functional abnormalities in the same patients (
12,
19,
51), although one recent study (
52) showed reduced linkage between regional gray matter volume and functional activation in schizophrenia patients. One possible explanation is that fMRI may reflect physiological alterations related to acute psychosis, while changes in brain anatomy reflect more stable and long-standing alterations. This possibility is supported by previous findings that functional changes in fronto-parietal and default mode networks were normalized after 6 weeks of antipsychotic treatment and clinical remission (
46), while anatomical deficits in thalamo-cortical networks are more stable and show slow and modest progressive change over the course of illness (
7–
9). While the possibility that fMRI changes more directly reflect psychosis-related neurophysiological changes is appealing, the lack of correlation with positive symptoms on the PANSS and with illness duration suggests that BOLD signal alterations may better track the presence than the severity or duration of psychosis.
Although there were many overlaps in anatomical findings in patients with and without prominent negative symptoms, it is noteworthy that patients with prominent negative symptoms showed more pronounced gray matter volume alterations in some regions, notably the left dorsolateral prefrontal cortex (
53). On the other hand, no differences were seen in the pattern of functional alteration between patients with and without prominent negative symptoms, suggesting that the impact of psychosis on brain physiology may be similar regardless of the severity of more persistent negative symptoms.
In summary, this study provides evidence that anatomical and functional deficits are seen in different brain regions in a large sample of antipsychotic-naive first-episode schizophrenia patients. An important caveat is that resting-state studies do not evaluate the functional integrity of all brain regions equally; default mode regions, for example, normally show more robust effects associated with the resting state, and thus sensitivity to illness effects may be greater in these regions. Activation studies in different regions of interest can provide complementary information with resting-state studies of structure-function relationships. Findings from the present study, along with future work clarifying the causes of the functional and structural changes reported and their dissociation, may provide new insight into the underlying neuropathology of the early course of schizophrenia.