Alcohol dependence is a chronic relapsing disorder, characterized by high levels of craving and the continuation of drinking despite the awareness of negative consequences (
1). During the transition from voluntary to impulsive and ultimately habitual drinking, cues associated with alcohol are hypothesized to increase in salience as a result of Pavlovian drug-cue learning (
2,
3). As a consequence, alcohol cues engender motivational responses in alcohol-dependent patients, which are triggered relatively automatically (
4). Motivational reactivity to alcohol cues has been demonstrated repeatedly in physiological and behavioral studies and is thought to be a key underlying mechanism involved in alcohol craving and alcohol relapse, even after years of abstinence (
5).
Incentive-sensitization models of addiction suggest that fronto-limbic dopaminergic neuroadaptations underlie the brain physiology of alcohol cue reactivity. Alcohol intake has been shown to release dopamine in the ventral tegmental area via interactions with opioid and GABA-ergic neurotransmission, which further projects to mesolimbic structures, such as the nucleus accumbens and the basolateral amygdala, as well as frontal areas (
5,
6). Since dopamine signals motivational relevance, it has been hypothesized to be a key neurobiological substrate of drug-cue learning. For example, neuroimaging studies have shown that when alcohol-dependent patients are exposed to alcohol cues, activation in reinforcement-related mesolimbic areas is evoked (
5,
7). Reactivity in these areas has been positively related to craving (
8–
10), to reward processing (
11–
13), and to alcohol consumption after relapse (
7,
14,
15). Although mesolimbic neuroadaptations have been hypothesized to be sustained after years of abstinence (
2,
3), studies suggest that only a few weeks of behavioral and/or pharmacological therapy may decrease cue-evoked activation in the nucleus accumbens (
16,
17) and amygdala (
18) in alcohol-dependent individuals. Thus, training effects on nucleus accumbens and amygdala activity may be of particular importance for the ability of interventions to change neural cue reactivity.
Behaviorally, alcohol-dependent patients show an automatic approach bias for alcohol cues, that is, a tendency to more quickly approach than avoid these cues on an approach-avoidance task (
9,
19,
20). In this task, participants push and pull pictorial cues with a joystick according to an irrelevant feature such as the format of the cue, and patients have been shown to pull faster than they push alcohol cues (
9,
19,
20). The approach bias may reflect an impulsive response toward drug cues and has been positively associated with drug craving (
21). Recently, the approach-avoidance task has been adapted into a cognitive bias modification training in which subjects implicitly learn to push away and hence avoid alcohol cues. In heavy drinkers, cognitive bias modification training has been shown to decrease the approach bias and reduce posttraining alcohol intake (
22). Moreover, in two recent randomized controlled studies, cognitive bias modification training reduced alcohol craving and relapse rates up to 13% in alcohol-dependent patients, compared with a sham training in which patients pushed and pulled alcohol cues at an equal rate (
19) and a non-training group (
19,
23). Although these findings show the clinical potential of bias modification in alcohol dependence, it is as yet unclear how bias modification affects brain function. For instance, bias modification could directly reduce the incentive salience of alcohol cues and neural alcohol cue reactivity (
2,
24). Understanding the mechanisms underlying cognitive bias modification training can help to further enhance its efficacy and thus further improve the treatment of alcohol dependence.
In this study, using a double-blind randomized design with a sham-training control condition, we examined the effects of cognitive bias modification training on neural reactivity evoked by alcohol cues in alcohol-dependent patients. Patients were randomly assigned to receive either bias modification or sham training, and they performed the approach-avoidance task for 3 weeks. The bias modification group pushed away 90% of alcohol cues, whereas this rate was 50% in the sham training group. Before and after training, neural cue reactivity was measured in functional MRI (fMRI) scans. We expected to find, first, that alcohol cue reactivity would be enhanced in the amygdala and nucleus accumbens across all subjects before training; second, that cue reactivity would decrease in the amygdala and nucleus accumbens as a result of cognitive bias modification; and third, that changes in cue reactivity in these regions would covary with changes in craving.
Method
Participants
The Ethical Committee of the Charité–Universitätsmedizin Berlin approved the study, and participants provided written informed consent after receiving a complete description of the study. Thirty-six male alcohol-dependent inpatients were recruited from the Salus Clinic in Lindow, Germany. Exclusion criteria for all patients were a history of neurological dysfunction, DSM-IV axis I psychiatric disorders other than alcohol dependence (assessed with the Mini International Neuropsychiatric Interview [
25]), abstinence from alcohol for >4 months before participation, and intake of psychoactive medication, as tested by urine drug screening. Patients had to be free of psychoactive medication and other drugs for at least 6 months before participation.
Patients were randomly assigned to receive bias modification training or sham training. Two patients did not complete the training (one in each group), and two patients could not be present on the second day of testing for administrative reasons (both in the bias modification group). The final sample consisted of 15 men in the bias modification training group and 17 in the sham training group. Participants completed the Alcohol Dependence Scale to assess severity of dependence (
26), the matrix reasoning subtest of the WAIS as a proxy for general intelligence (
27), and the State-Trait Anxiety Inventory (
28). The groups did not differ significantly in age, years of education, intelligence scores, or clinical variables (
Table 1) or in number of smokers (12 in the bias modification group [80%] and 15 in the sham training group [88%]). Smokers were abstinent from tobacco for at least 1.5 hours before scanning.
Experimental Tasks
Approach-avoidance task.
The approach-avoidance task was used to measure approach bias before and after training (
29). In response to the format of the cue (landscape or portrait), participants pushed and pulled pictures with a joystick, which increased and decreased the size of the cue, respectively; participants had to respond to a cue within 2 seconds. Twenty practice trials were followed by 80 test trials (20 alcohol push, 20 alcohol pull, 20 soft drink push, 20 soft drink pull) that were presented over two blocks. Picture format to response assignment was counterbalanced, and response type assignment did not differ between the two groups. A set of 40 alcohol and 40 soft drink images was used (
9).
fMRI cue reactivity.
For the fMRI paradigm, the same 80 pictures that were used in the approach-avoidance task were presented over eight blocks per stimulus category. Each block consisted of five stimuli, each presented for 4 seconds. To check whether participants were focused on the task, four oddball blocks were added, containing four alcohol or soft drink stimuli and an oddball cue—a picture of an animal; in these cases, participants had to press a button with their right index finger. The duration of the task was approximately 6 minutes.
Picture rating and craving.
After both scanning sessions, pictures were rated for arousal and valence on a 5-point Likert scale, and alcohol craving was assessed with the Desire for Alcohol Questionnaire (
30).
Cognitive Bias Modification Training
The cognitive bias modification training scheme, which was an adapted version of the approach-avoidance task, consisted of six training sessions over 3 weeks, each consisting of 400 trials (200 alcohol and 200 soft drink) (
19,
23). The experimental bias modification group pushed away 90% and pulled 10% of the alcohol cues (and the reverse for soft drink cues: 10% push and 90% pull). These ratios were 50/50 in the sham training group. Twenty cues were used for training (10 alcohol and 10 soft drink) (
19,
23). To test for effects on cue reactivity based on stimulus category (alcohol versus soft drink) rather than on specific pictures, pictures in the training were different but comparable to cues used before and after training in the avoidance task and fMRI cue reactivity.
fMRI acquisition and preprocessing.
Scanning was done in a 3-T whole-body MRI scanner (Magnetom Trio Tim, Siemens, Germany) equipped with a 12-channel head coil. A standard T2-weighted echo planar imaging sequence was used with the following parameters: sequential descending acquisition, repetition time=2 seconds, echo time=25 ms, flip angle=80°, 64×64 pixels in-plane resolution, 34 slices, slice thickness=3 mm, voxel dimensions=3×3×3 mm3, 0.75-mm gap between slides, field of view=192×192 mm2, 140 images per session.
Functional data analysis was performed with SPM8 (
http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Scans were spatially realigned, slice-time corrected, and normalized to the standard echo planar imaging template. Smoothing was performed with an 8-mm full width at half maximum Gaussian kernel. Participants did not move more than 2 mm or 2 degrees.
Statistical Analysis
For the approach-avoidance task, responses that were missed or incorrect and response times longer than three standard deviations above the mean were discarded based on each participant’s performance. Alcohol approach bias scores were calculated by subtracting median reaction times ([alcohol push − pull] − [soft drink push − pull]). Two-by-two mixed analyses of variance on alcohol approach bias, craving, and picture ratings were calculated, with time (before versus after training) as a within-subject factor and group (bias modification versus sham) as a between-subject factor. Post hoc group comparisons were performed with two-sample t tests at an alpha of 0.05.
Three fMRI regressors—alcohol, soft drink, and oddball blocks (20 seconds each)—were built for every subject and were convolved with the hemodynamic response function with default temporal filtering of 128 seconds. On the single-subject level, two contrasts were calculated. Contrast 1 was alcohol cue reactivity before training: ([alcohol > soft drink] before training); and contrast 2 was alcohol cue reactivity before and after training: ([alcohol > soft drink] before training) − ([alcohol > soft drink] after training). On the second level, t tests were used to calculate alcohol cue reactivity before training in both groups and between-group alcohol cue reactivity before and after training. Post hoc t tests were used in our a priori regions of interest to explore directions of the interaction of time by group.
Based on our hypotheses, anatomically defined left and right nucleus accumbens and amygdala were chosen as regions of interest (
5,
9,
14,
31,
32) and were used for small-volume correction of the results, with a significance threshold of 0.05, family-wise error corrected. Exploratory whole-brain analyses are presented in the
data supplement that accompanies the online edition of this article.
Behavioral approach bias scores, craving, and alcohol picture ratings before training were correlated with blood-oxygen-level-dependent (BOLD) contrast 1 (alcohol cue reactivity before training) using our regions of interest. For behavioral variables showing a positive correlation in our regions of interest before training, we computed difference scores for before and after training and correlated these with significant activations in BOLD contrast 2 (alcohol cue reactivity before and after training).
Results
Behavioral Effects of Cognitive Bias Modification Training
Approach-avoidance task.
Alcohol approach bias scores before and after training, as well as difference scores, were distributed normally in both groups (Kolmogorov-Smirnov test, all p values >0.62). Mean error rates were 3.04% (SD=3.22) before training and 2.65% (SD=3.83) after training, collapsed across the groups. There were no main effects of group or time and no interaction effect of group by time.
For the alcohol approach bias scores, there was no significant interaction effect of group by time, and there were no main effects. Exploratory t tests showed that the groups did not differ before and after training and that a decrease in mean reaction time approached significance in the cognitive bias modification training group (before training, mean=11.90, SD=64.01; after training, mean=−25.53, SD=55.80; t=1.18, df=14, p=0.091) but not in the sham training group (before training, mean=−9.35, SD=122.21; after training, mean=21.50, SD=99.89; t=−0.64, df=16, p=0.53).
Subjective alcohol craving and picture ratings.
For craving scores, there was a main effect of time (F=9.32, df=1, 30, p=0.005; η2=0.23). In both groups, craving scores were higher before training (bias modification group, mean=15.20, SD=6.95; sham training, mean=12.29, SD=5.01) than after (bias modification group, mean=12.33, SD=6.20; sham training, mean=10.36, SD=3.62). There was no significant interaction effect of group by time for craving scores. Exploratory paired t tests showed that the groups did not differ before and after training, but craving scores significantly decreased in the bias modification group (t=3.86, df=14, p=0.002) but not in the sham training group.
There was a significant interaction effect of group by time for arousal ratings of alcohol pictures (F=4.19, df=1, 30, p=0.05; η2=0.12), with arousal ratings having a nearly significant decrease in the bias modification training group (before training, mean=1.02, SD=0.40; after training, mean=0.88, SD=0.51; t=2.01, df=14, p=0.064) but not in the sham training group (before training, mean=0.98, SD=0.34; after training, mean=1.04, SD=0.38; t=0.82, df=16, p=0.43). There were no significant effects of group by time for valence ratings. Before and after training, the groups did not differ in arousal and valence.
Cue-Evoked Brain Activation Within and Between Groups
All patients paid attention to the cue reactivity task, as shown by their responses to all four oddball cues, before and after training.
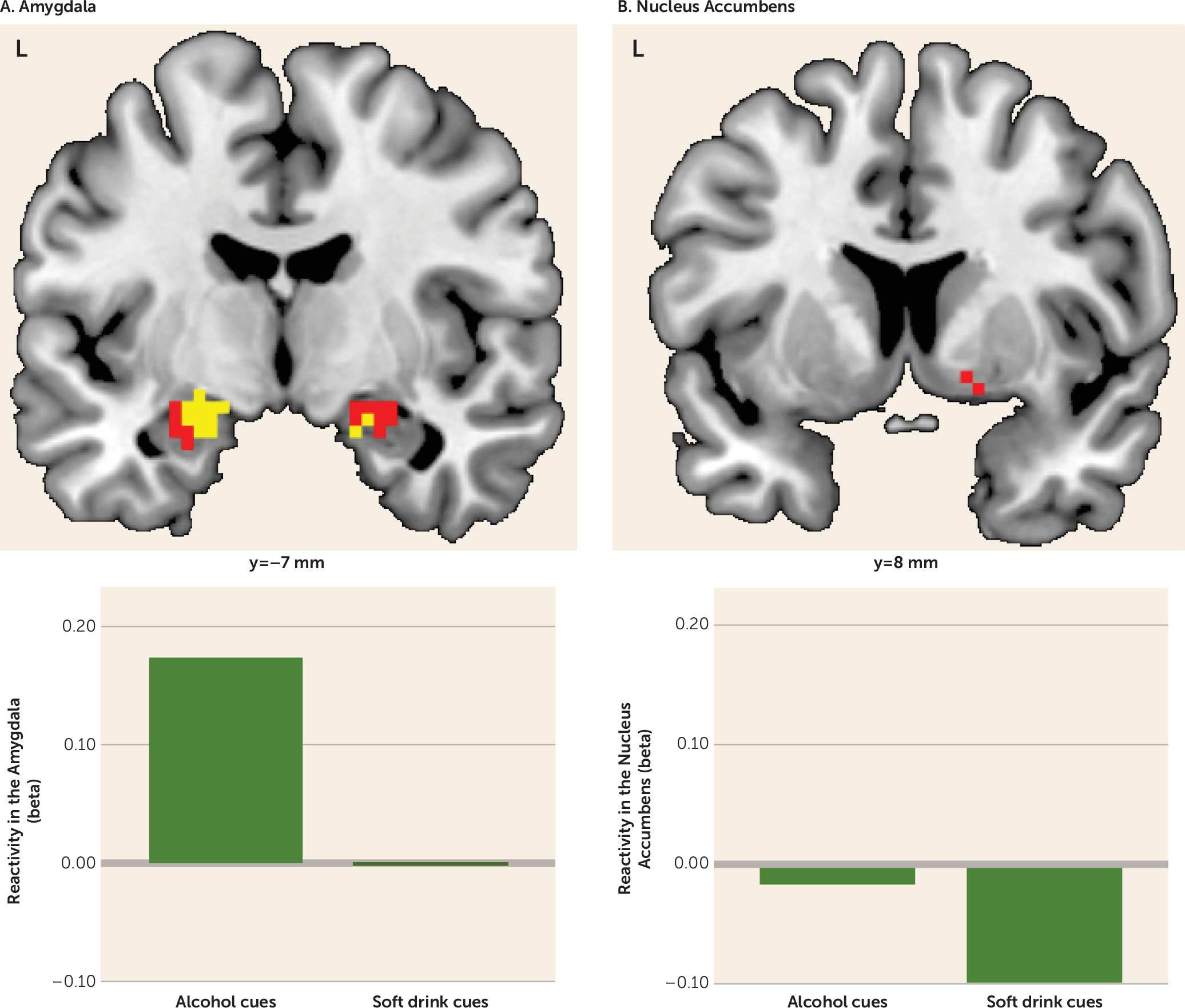
Before training, subjects pooled across both groups showed alcohol cue-evoked activity in the amygdala bilaterally (peak Montreal Neurological Institute coordinates, left side: −21, −7, −14; t=4.98, df=31, p<0.001; right side: 21, −7, −17; t=2.87, df=31, p=0.052) while viewing alcohol cues compared with viewing soft drink cues. In this contrast, the right nucleus accumbens was also activated, although the effect fell short of significance (peak coordinates: 8, 8, −11; t=2.48, df=31, p=0.057).
Figure 1 illustrates the pretraining activations in the amygdala and nucleus accumbens. (See Table S1 in the online
data supplement for whole brain activations showing no relevant between-group differences before training.)
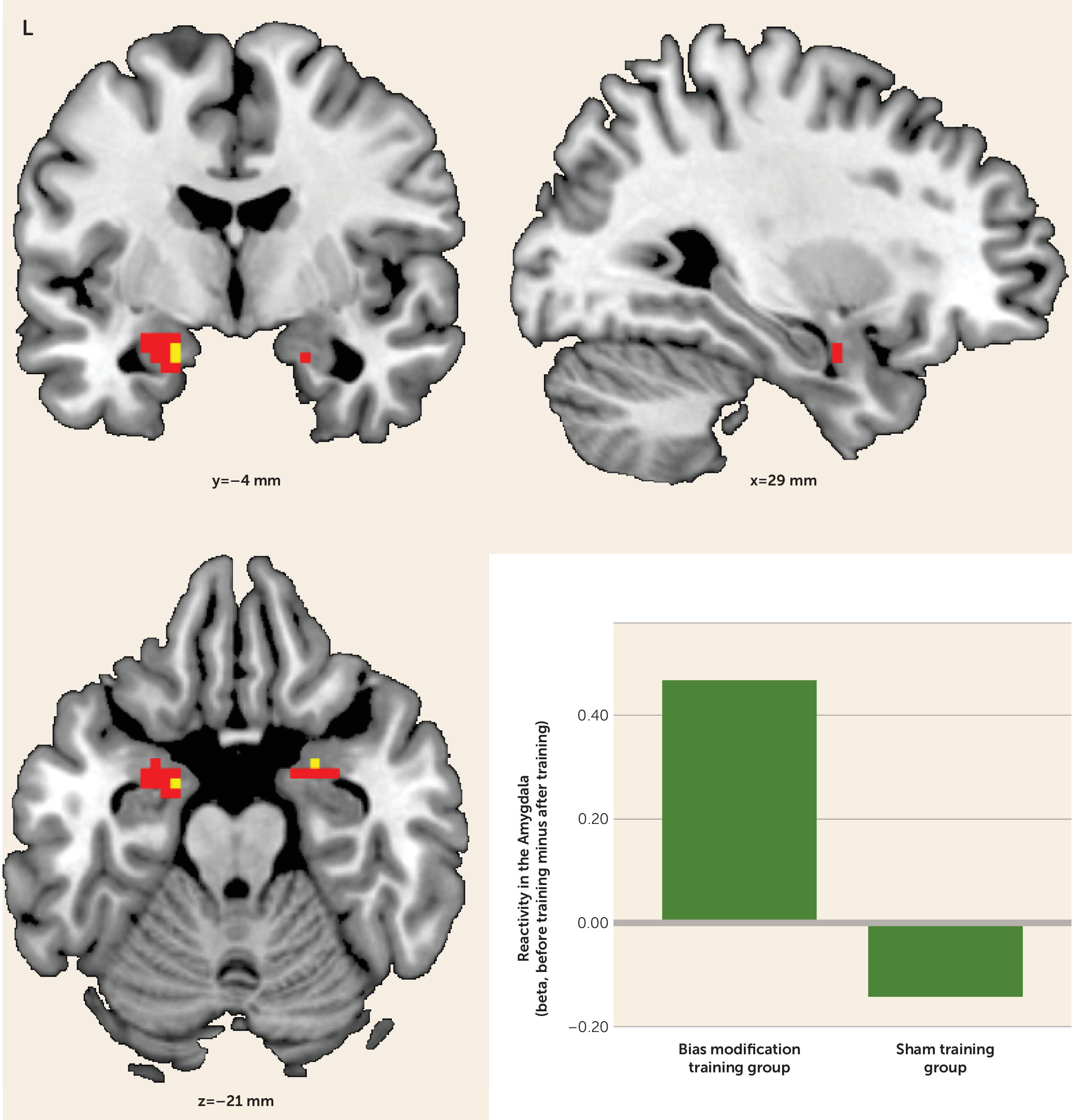
In the assessment of group differences in alcohol cue reactivity before and after training, the bias modification group showed significantly greater reductions in alcohol cue-evoked activation in the amygdala bilaterally (peak coordinates, left side: −15, −1, −23; t=2.97, df=30, p<0.05; right side: 27, 2, −20; t=3.08, df=30, p<0.05) compared with the sham training group (
Figure 2). This effect was not present for the nucleus accumbens, even at a more liberal threshold of p<0.005 uncorrected. After training, the bias modification group had significantly lower activation in the left amygdala than the sham training group (peak coordinates: −15, −1, −26; t=3.86, df=30, p<0.05). (See Table S1 in the online
data supplement for whole brain activations.)
Post hoc t tests on cue reactivity before and after training within groups demonstrated a significant reduction of left and right amygdala activity in the bias modification group (peak coordinates, left side: −27, 2, −17; t=3.58, df=14, p<0.05; right side: 24, 2, −20; t=2.88, df=14, p<0.05). However, this was not the case for the sham training group, even at p<0.005 uncorrected.
Correlations With Behavioral Measures
Before training, both groups’ craving scores significantly correlated with alcohol cue-induced amygdala activity bilaterally (coordinates, left side: −18, −7, −17; t=6.15, df=31, p<0.001); right side: 21, −4, −23; t=3.88, df=31, p<0.01). Craving scores were correlated with activity in the right nucleus accumbens, but the effect fell short of significance (peak coordinates: 15, 11, −8; t=2.15, df=31, p=0.057). Arousal ratings also correlated with cue reactivity in the left and right amygdala (coordinates, left side: −27, −4, −20; t= 3.67, df=31, p=0.01; right side: 21, −1, −14; t=3.46, df=31, p<0.05), and in the right nucleus accumbens, although again falling just short of significance (peak coordinates: 18, 8, −11; t=2.51, df=31, p=0.052). Alcohol approach bias scores and valence ratings did not correlate with alcohol cue-induced activations in our regions of interest.
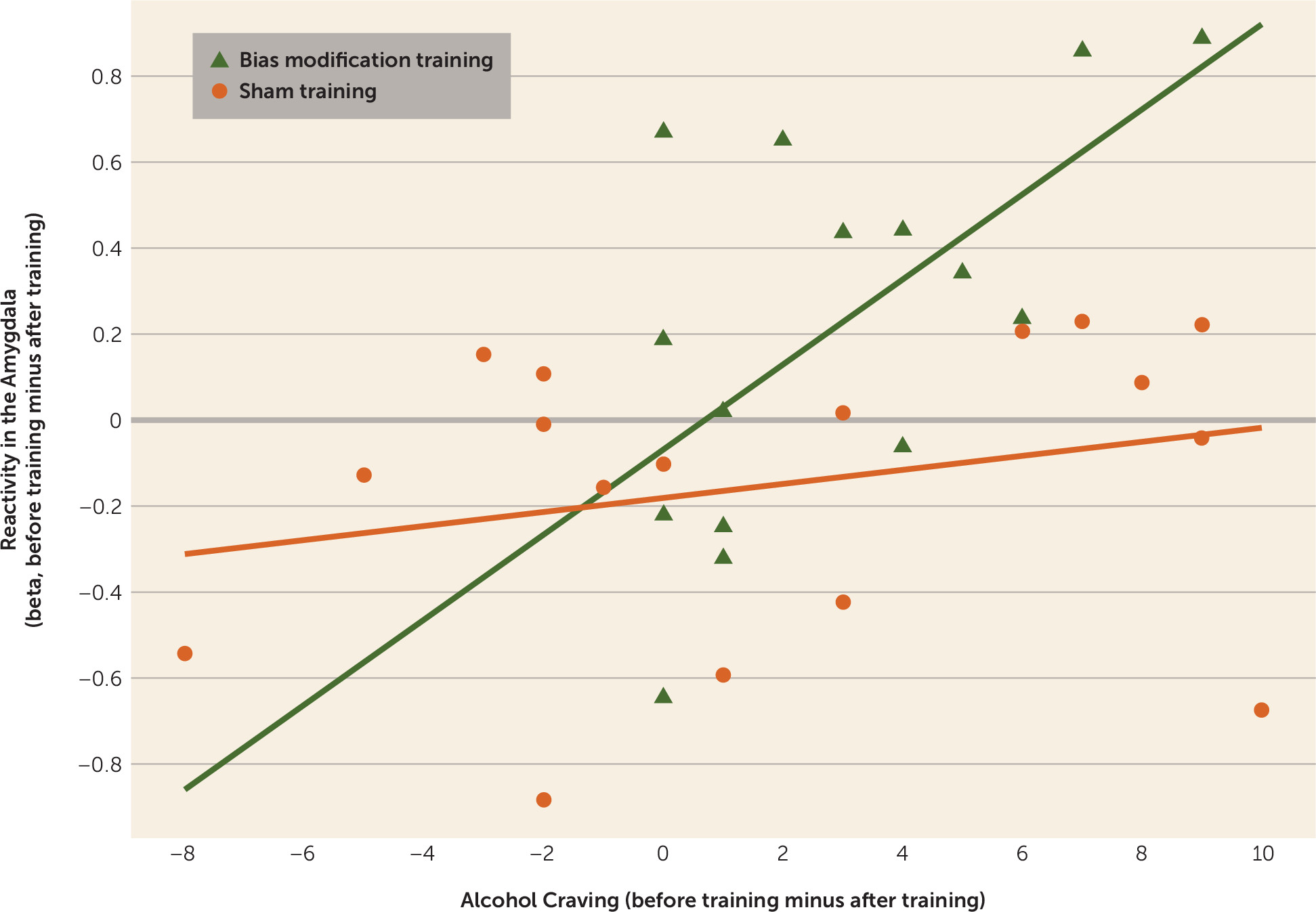
In the bias modification group, the difference in right amygdala activity before and after training correlated positively with the decrease in alcohol craving (peak coordinates: 30, 2, −17; t=3.44, df=14, p<0.05), but not in the sham training group. Moreover, when the correlation slopes of cue reactivity and craving before and after treatment were compared between the two groups, there was an effect in the right amygdala (peak coordinates: 30, 2, −17; t=3.85, df=30, p<0.01), providing stronger evidence for a greater correlation in bias modification (
Figure 3). There were no significant correlations between decreases in arousal ratings and decreases in amygdala activation. See Table S2 in the online
data supplement for exploratory analyses of alcohol cue reactivity and relapse rates 1 year after training.
Discussion
Our aim in this study was to examine the effects of cognitive bias modification training on neural alcohol cue reactivity. The results provide first evidence that cognitive bias modification training can affect cue-induced amygdala activity, an area previously associated with alcohol cue reactivity, alcohol craving, and relapse prediction (
5,
7–
9,
12,
14,
15,
17). Before training, both groups showed alcohol cue reactivity in the amygdala and in the nucleus accumbens (although the latter fell short of significance), which correlated positively with craving scores and arousal ratings of alcohol cues. These findings replicate previous studies of alcohol dependence and suggest that alcohol cue reactivity may be related to clinical severity of dependence (
5,
7–
9,
12,
33). When comparing alcohol cue-evoked brain reactivity before and after training, amygdala activity differed between the two groups: while amygdala activity decreased in the bias modification group, this effect was not observed in the sham training group. Moreover, the decrease in right amygdala activity correlated with a decrease in alcohol craving scores in the bias modification group, but not in the sham training group. Therefore, reduction of alcohol cue-induced amygdala activity may be an important underlying mechanism contributing to the previously reported therapeutic effectiveness of cognitive bias modification training (
19,
23) and may serve as a biomarker for reductions in clinically relevant alcohol craving.
The amygdala has been shown to play a central role in Pavlovian conditioned learning, the modulation of incentive salience to reward cues, and the formation and consolidation of emotional memories (
12,
34). In recent work, a function of the amygdala has been described as the processing of the personal motivational salience of stimuli (
35). The region has been associated with craving while passively viewing drug cues in drug-dependent patients (
12,
36) and in approaching versus avoiding alcohol cues on the approach-avoidance task (
9), and two recent studies found that increased alcohol cue reactivity in the amygdala was predictive of alcohol relapse after abstinence (
14,
15). A study by Schneider et al. (
18) showed that the combination of pharmacological and behavioral therapy reduced amygdala activity when smelling alcohol in alcohol-dependent patients, whereas a healthy comparison group did not show reductions in amygdala activation over the same period. Although the Schneider et al. study could not distinguish whether the effect was due to behavioral or pharmacological interventions, it showed that the amygdala can be flexibly modulated over time with respect to alcohol-induced cue reactivity. Moreover, it has recently been shown (
37) that emotional cue-evoked amygdala activity can be modulated by attentional bias modification in anxious individuals. A possible interpretation of our results, in which bias modification was found to reduce amygdala cue reactivity, is that bias modification reduces the motivational salience of alcohol cues. In line with this interpretation, we found that bias modification reduced arousal ratings of alcohol cues. Moreover, bias modification-induced reductions in right amygdala activation correlated with reductions in alcohol craving.
How might cognitive bias modification cause such a reduction in salience? It may be that this effect is related to findings on inhibition training (
38–
40). These studies have shown that the inhibition of responses to initially positively valenced stimuli results in a devaluation of that stimulus category. Hypothetically, the requirement to consistently perform incongruent actions in approach/avoidance modification (i.e., actively and habitually avoid previously desired alcohol cues) causes a similar effect: patients could solve the avoid-alcohol problem by reducing the overall salience of alcohol cues (
24) and hence reduce behavioral biases associated with them. It therefore may be that reducing overall salience is easier to achieve than changing the automatic response bias without reducing salience. Additional research is needed to provide evidence for or against the hypothesis that the mediating mechanism of cognitive bias modification involves, at least partially, reductions in the salience of alcohol cues.
Despite significant effects of cognitive bias modification on neural cue reactivity, its correlation with craving, and behavioral effects on arousal ratings, we could not replicate the interaction effect of group by time on approach bias scores found by Eberl et al. (
23) and Wiers et al. (
19,
22). Since effects of bias modification on approach bias and alcohol craving are in the hypothesized direction—we observed a reduction in approach bias (falling short of significance) as well as a significant reduction in craving in the bias modification training group but not in the sham training group—it is likely that the lack of effect is due to the relatively small sample size in this study. Although behavioral effects of training have been observed in sample sizes of 200 to 500 (
19,
23), sample sizes of around 15 alcohol-dependent patients have been shown to be sufficient to measure alcohol cue-evoked neural activity (
7,
16) and reductions in cue reactivity over time (
16–
18). Moreover, to allow training effects to generalize to general alcohol stimuli, patients were trained on different cues than those used for behavioral and neural assessments. This was not the case in previous studies, and this conservative approach may have led to less power for the behavioral effect. Nevertheless, our results show that the effects of bias modification generalize to other, nontrained stimuli, at least in terms of neural effects and in arousal ratings of alcohol cues. Furthermore, we scanned patients after 1 month of abstinence on average, which may have reduced the likelihood of detecting effects of training on behavior and brain activation. This may explain the weak initial activation of the nucleus accumbens before training and the fact that we did not observe the hypothesized difference in reductions in the nucleus accumbens between groups. Another limitation is that our study assessed craving as a clinical outcome measure but was not designed to detect between-group differences in relapse. We assessed relapse 1 year after our assessment of abstinence (reported in the online
data supplement), but our study was underpowered to detect cue reactivity effects for relapse. Nevertheless, behavioral craving (
10) and amygdala cue reactivity have been shown to predict relapse in alcohol dependence (
14,
15), providing further evidence that alcohol cue-induced amygdala reactivity is important for clinical success in alcohol dependence. Studies with larger sample sizes are needed to explore whether the neural effects of cognitive bias modification training in the amygdala are not only associated with alcohol craving, but also can predict relapse status.
In conclusion, we show here for the first time that cognitive bias modification training affects alcohol cue reactivity, which was associated with reductions in alcohol craving. These results suggest that bias modification can reduce the motivational salience of drug cues encoded in the amygdala. Such findings can help us better understand the underlying mechanisms of the clinical effects of cognitive bias modification, which can lead to improved training schemes. Furthermore, fMRI measurements may prove useful in predicting whether cognitive bias modification will be effective for individual patients.
Acknowledgments
The authors thank Ulrike Malecki, Silvia Hoffman, Natalie Becht, Jana Paeplow, and Sébastien Lehmann for their assistance in recruitment, screening, and training; Kristin Trapp, Sonja Gröpper, and Jenny Parnack for assistance in data acquisition; Catherine Hindi Attar and Stephanie Spengler for help with data analysis; and the subjects who volunteered to participate in the study.