Genetic factors have long been hypothesized to confer risk for neuropsychiatric phenotypes, but it has been challenging to identify genetic culprits. The failure to identify risk genes may be in large part a result of the polygenic etiology and phenotypic heterogeneity of neuropsychiatric disorders (
1). These disorders result from complex interactions among multiple genes, tissue-specific epigenetic regulation, and environmental influences, which make identifying the relationships between single genes and distal phenotypes challenging.
An alternative approach is to examine endophenotypes, measurable constructs that confer risk for complex disorders and are thought to lie in greater etiological proximity to genetic factors (
2). One promising endophenotype for late-life neuropsychiatric disorders is small hippocampal volume (
3). Smaller hippocampal volume has been associated with treatment resistance in late-life depression (
4) and predicts progressive cognitive decline in elderly individuals (
5). Therefore, efforts to link hippocampal volume reduction with risk genes may be particularly relevant for late-life neuropsychiatric syndromes.
A novel and biologically plausible gene to investigate in these relationships is
AGTR1, the gene encoding angiotensin-II type 1 (AT
1) receptor in humans. AT
1 receptors are the primary effector of the renin-angiotensin system (RAS) in several organs, including the brain. The RAS is an important regulator of the stress response, and AT
1 receptors are expressed in brain regions that modulate stress and emotion, including the hypothalamus, amygdala, and hippocampus (
6,
7). In animal studies, RAS activation leads to hyperactivity of the stress system and heightened anxious behavior, whereas blockade of AT
1 receptors dampens stress responses and ameliorates anxious and depressive behavior (
6,
8). As the RAS also plays a central role in blood pressure regulation and has been implicated in vascular disease (
9), examining this system may be particularly relevant for older adults with depression, since late-life depression is often characterized by vascular comorbidity (
10).
Despite these theoretical implications, few studies have examined the relationship between genetic variation in
AGTR1 and either neuropsychiatric phenotypes or hippocampal morphology. Studies on the common rs5186
AGTR1 (A-to-C) polymorphism have reported antidepressant response differences between genotypes in elderly depressed individuals (
11–
13). In a broader analysis of single-nucleotide polymorphisms (SNPs) in
AGTR1 (
14), our group reported that allele frequency differences in two
AGTR1 SNPs increased the odds of late-life depression. We also found cross-sectional associations between right hippocampal volume and four
AGTR1 intronic SNPs, namely rs2638363, rs1492103, rs2675511, and rs12721331 (
14). To our knowledge, no studies have examined the effects of
AGTR1 polymorphisms on longitudinal changes in hippocampal morphology in older adults. This is particularly important as reduction in hippocampal volume is associated with subsequent cognitive decline (
5).
To extend our previous findings demonstrating cross-sectional relationships between
AGTR1 and hippocampal morphology (
14), we examined the effects of
AGTR1 genotype on longitudinal change in hippocampal volume. Our a priori hypothesis was that the gene variants we previously associated with smaller cross-sectional hippocampus volume would also be associated with greater hippocampal volume loss over time. We also sought to determine if these gene variants were associated with differences in cognitive function over time, particularly in domains involving the hippocampus, such as episodic memory. To control for the effects of depression that have been shown to lead to smaller hippocampal volumes via chronic hyperactivity of the stress system (
15), we examined two cohorts of elderly depressed and nondepressed individuals. In secondary analyses, we examined whether depression diagnosis or apolipoprotein E (
APOE) genotype had synergistic effects with
AGTR1 genotypes on hippocampal volume change.
Results
Sample Demographics and Baseline Measures
The primary sample included 138 elderly individuals (79 depressed and 59 nondepressed) with genotype data for
AGTR1 SNPs and at least two MRI scans (a baseline and one follow-up assessment). The demographic characteristics and clinical data are summarized in
Table 1. Their ages ranged from 60 to 84 years for the depressed cohort and from 60 to 82 years for the nondepressed cohort. The two groups were similar in genotype frequencies, age, MMSE scores, and baseline left and right hippocampal volumes. However, the percentage of female participants and educational levels were higher in the nondepressed group, and the percentage of patients who reported hypertension was higher in the depressed group. Finally, we found that the number of patients who reported a history of either cardiac complaints or hypertension did not significantly differ between the four
AGTR1 or
APOE genotype groups (data not shown). The maximum number of MRI measures per participant was five, and no significant difference was found for length of study follow-up between diagnostic cohorts (a mean of 1,435.3 days [SD=502.5] for depressed patients and a mean of 1,495.9 days [SD=785.8] for nondepressed patients; Satterthwaite t test=130, df=2, t=0.55, p=0.5855).
Longitudinal Effects of AGTR1 SNPs on Hippocampal Volumes
We created mixed models examining hippocampus volume in the left or right hemisphere as repeatedly measured dependent variables. Independent variables included diagnosis (depressed or nondepressed), age, sex, cerebral volume, baseline hippocampal volume, time, and
AGTR1 SNP genotype. To test the hypothesis that SNPs would differentially affect hippocampal volume over time, we included a SNP-by-time interaction term.
Table 2 summarizes the effects of the four
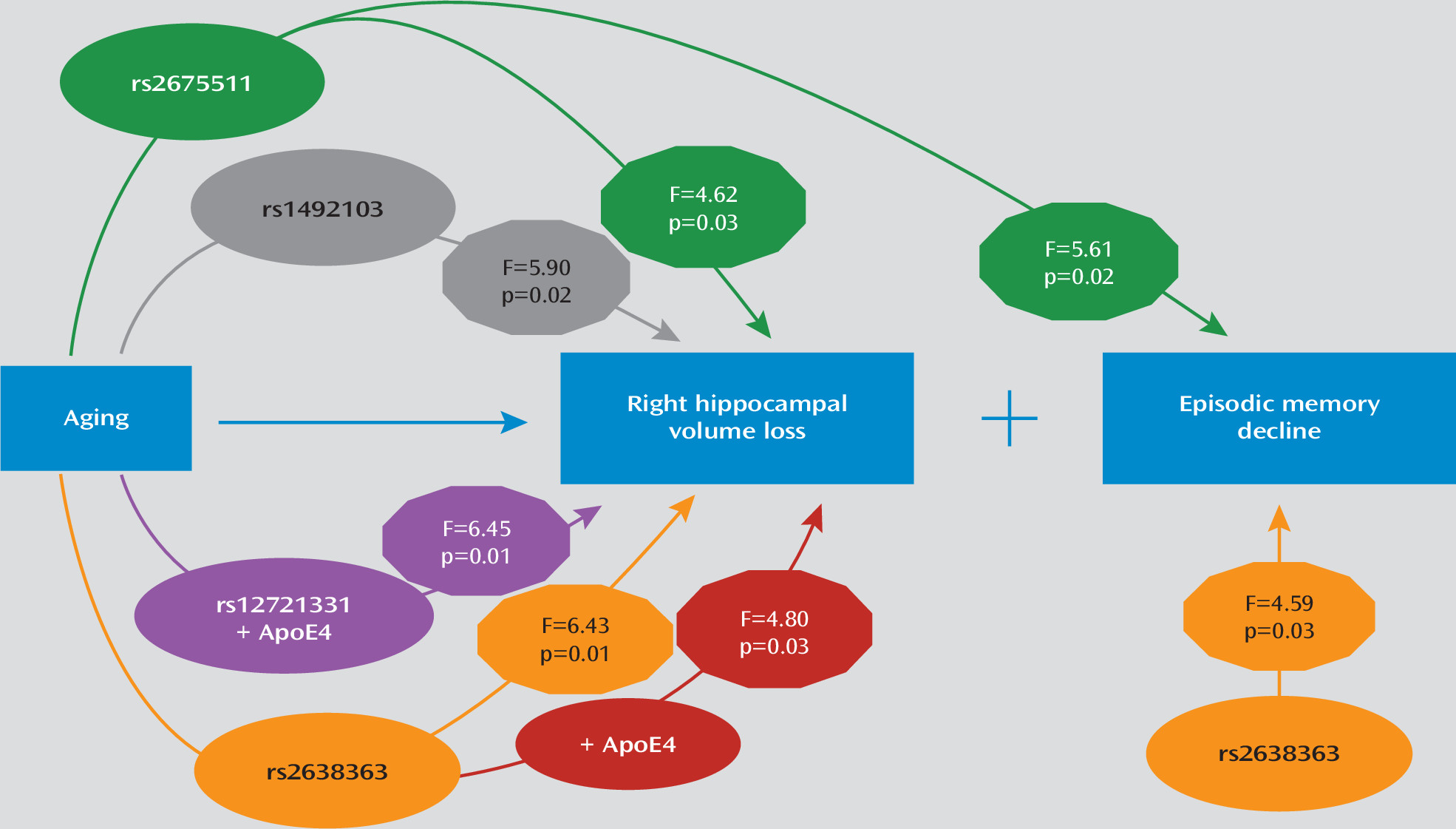
AGTR1 SNPs examined and their interactions with time on left and right hippocampal volumes. When examining models of right hippocampal volume, three of the four SNPs (rs2638363, rs1492103, rs2675511) showed both a statistically significant primary effect and an interactive effect with time (
Table 2). The primary effect of
AGTR1 SNPs replicated our previous findings (
14), despite nearly double the sample size and different MRI field strength. Again, individuals homozygous for the vascular risk alleles (rs2638363 GG, rs1492103 TT, and rs2675511 AA) exhibited smaller hippocampal volumes when compared with individuals who were heterozygous or homozygous for the alternate allele. The SNP-by-time interaction tested for differences between SNP alleles on change in hippocampal volume over time. When examining these interactions, individuals homozygous for the risk alleles exhibited an accelerated decrease in right hippocampal volume over time relative to individuals with alternate genotypes. However, the four SNPs exhibited neither a significant primary effect nor a significant gene-by-time interaction on left hippocampal volume. In other words, the effect of all three SNPs was selective and lateralized to the right hemisphere.
In secondary analyses we tested whether
AGTR1 genetic variation has synergistic effects with depression or
APOE genotype on the rate of hippocampal volume change. The three-way depression-by-
AGTR1-by-time interactions did not reach significance for any of the SNPs for either the left or right hippocampus. However, we did observe significant gene-gene interactions. The
APOE genotype had statistically significant epistatic effects with rs2638363 and rs12721331 for the right hippocampus volume over time (
Table 2). In both cases, the presence of both the risk
AGTR1 allele and the
APOE4 allele was associated with accelerated hippocampal volume loss. We observed a similar trend that did not achieve statistical significance for the interaction between rs1492103,
APOE, and time (
Table 2).
Longitudinal Effects of AGTR1 SNPs on Composite Cognitive Measures
We next tested whether AGTR1 variants confer risk for cognitive decline. A total of 138 elderly participants (63 depressed and 75 nondepressed) had both genotype and neurocognitive assessment data and were included in these analyses. This sample partially overlaps with the sample examining hippocampal volumes, with 81 participants (36 depressed and 45 nondepressed) included in both analyses. As expected, depressed individuals performed significantly worse than nondepressed individuals on univariate comparisons of the four composite cognitive measures of episodic memory (t=5.14, df=106, p<0.0001), executive speed (t=4.36, df=67, p<0.0001), verbal fluency (t=5.18, df=136, p<0.0001), and working memory (t=2.97, df=114, p=0.0037).
We used mixed models to examine the effect of AGTR1 genotypes and their interaction with time on each of the composite cognitive measures. All models controlled for diagnosis (depressed or nondepressed), age, sex, education, time, and baseline cognitive measure score. In these models, only rs2638363 displayed a significant main effect of genotype on composite episodic memory performance (F=4.59, df=1, 126, p=0.0340), but the genotype-by-time interaction did not reach significance. Similarly, rs1492103 exhibited a trend for a direct effect on episodic memory (F=3.85, df=1, 126, p=0.0519), but this did not reach statistical significance. Finally, rs2675511 exhibited a gene-by-time interaction (F=5.61, df=1, 125, p=0.0194) predicting worsening episodic memory performance. In accordance with SNP effects on hippocampal volumes, individuals homozygous for the vascular risk alleles (rs2638363 GG, rs1492103 TT, and rs2675511 AA) exhibited lower episodic memory scores. None of the four genetic variants exhibited either a statistically significant direct effect or interaction with time effect on executive speed, verbal fluency, or working memory.
Discussion
Replicating and extending our previous findings of
AGTR1 effects on right hippocampal morphology (
14), three of the same SNPs predicted longitudinal hippocampal volume change in the right but not the left hemisphere in elderly individuals. The direction of the observed relationships was similar to results from the previous cross-sectional study, that is, each of the previously identified “risk” genotypes (rs2638363 GG, rs1492103 TT, and rs2675511 AA) predicted longitudinally greater shrinkage of the right hippocampus when compared with the alternate genotypes. Intriguingly, these
AGTR1 risk alleles also predicted poorer performance in episodic memory, a neurocognitive measure mediated by the hippocampus, but had no effects on other cognitive measures. Additionally, two risk variants (rs2638363 and rs12721331) showed epistatic effects with the
APOE4 allele, a known risk factor for dementia (
26), on right hippocampal volume loss over time. Importantly, the SNPs examined are in low pairwise linkage disequilibrium (r
2<0.5) and thus represent independent signals. The presence of several signals within the
AGTR1 locus that are independently associated with hippocampal volume changes and memory decline lends further support to the reliability of these associations. Taken together, our findings strongly support that
AGTR1 gene variants may confer vulnerability via both direct and epistatic effects for progressive memory decline by accelerating hippocampal volume loss. The main findings and the supported model are schematically summarized in
Figure 1.
Our study offers novel insights into the role of the renin-angiotensin system (RAS) in hippocampal atrophy and cognitive decline. Hippocampal atrophy has been shown to predict cognitive decline in late life and time-to-conversion to Alzheimer’s disease (
5,
30). Previous animal and human studies support involvement of the RAS in cognitive syndromes (
6,
14,
31). RAS activation modulates cerebral blood flow, increases brain vulnerability to ischemia, and promotes brain inflammation (
6,
32). These effects render this system particularly relevant in late life, where vascular and inflammatory processes are hypothesized to contribute to depressive and cognitive syndromes (
10,
33). On the other hand, AT
1 receptor blockade dampens stress responses, ameliorates anxious and depressive behaviors, and reduces brain inflammation and vulnerability to ischemia (
6,
8,
32). Thus, it is plausible that perturbations in the RAS could heighten the risk for developing dementia by accelerating age-related changes in brain morphology, particularly in brain regions most vulnerable to the effects of aging, such as the hippocampus. Although RAS activity may be modulated by functional variants at the
AGTR1 locus, the molecular effects of the risk
AGTR1 SNPs are currently not clear. Despite their intronic location, these SNPs could have functional effects. For instance, intronic SNPs may be located in enhancer regions that influence the three-dimensional changes in chromatin conformation necessary for transcription regulation (
34). Alternately, these SNPs may be tagging other functional variants in nearby regions of the
AGTR1 locus. Finally, as supported by the epistatic effects with
APOE observed in our study, these SNPs may have functional effects via interaction with other genes that lie in common biological pathways central for the development of brain pathology and dementia. Future studies on the functional effects of these genetic variants are warranted and may shed light on the mechanisms linking
AGTR1 and cognitive syndromes.
Intriguingly, the effects of
AGTR1 variation were lateralized to the right hippocampus, which is in accordance with our previous cross-sectional study (
14). Although the significance of this lateralization is unclear, hippocampal asymmetry may be an important endophenotype that has been underemphasized by previous studies. Hippocampal asymmetry has been observed in patients with severe depression and in nondepressed relatives of depressed individuals (
35,
36). Furthermore, unilateral hippocampal volume changes may be associated with decline in specific cognitive outcomes (
5,
37) and with decreased likelihood of achieving antidepressant remission (
4). Thus, hippocampal asymmetry may be an early step in the pathogenesis of some late-life mood and cognitive syndromes. This asymmetry could be induced by differential hemispheric effects of the RAS on the hippocampus. Notably, activity of angiotensinase, an enzyme that metabolizes angiotensin, has been found to be distributed asymmetrically between the left and right hippocampi of the rat brain (
38,
39). This may also reflect differential activity of angiotensin and potentially asymmetric effects of
AGTR1 genetic variants between the two hemispheres. Whether this mechanism holds true, and the clinical significance of these asymmetries, remains to be determined.
Several limitations should be considered when interpreting our findings. Although we controlled for the effects of depression diagnosis and basic demographic factors on hippocampal morphology, we did not control for antidepressant treatments that may have neurotrophic effects on the hippocampus (
40) and may even reverse hippocampal volume loss in some cases of depression (
37). Depressed participants were treated based on an algorithm rather than a rigid clinical trial. Although this makes our approach comparable to clinical practice, it makes it challenging to elucidate the effects of antidepressants. Data on antidepressant use prior to enrollment, as well as specific treatment modalities, duration, and dosages used over the study period, were not known for each participant. Thus, it is possible that variable treatments between genotype groups may have influenced our results. However, the assignment of treatment modalities occurred randomly and treating clinicians were blind to genotype groups, and thus systematic errors cannot account for our findings. Another limitation was our focus on variation at a single genetic locus and a single brain region. This was based on our sample size and the a priori plausible involvement of
AGTR1 and hippocampal pathology in late-life neuropsychiatric syndromes. Larger longitudinal studies are warranted for a more systematic examination of the multiple brain regions, genes, and biological pathways involved in the pathogenesis of these syndromes. Our study shows that the RAS is one such pathway that should be included in future pathogenetic models.
In summary, this is the first study to our knowledge to explore the longitudinal effects of AGTR1 genetic variation on hippocampal morphology and cognitive decline. In contrast with cross-sectional approaches that cannot establish temporal relationships, the present study reveals that older adults homozygous for AGTR1 risk variants exhibit accelerated hippocampal volume loss and memory decline. Our study exemplifies how examining the longitudinal effects of biologically plausible genotypes on relevant endophenotypes may provide novel insights into the pathogenesis of complex phenotypes. It further suggests that molecules involved in the RAS may serve as early therapeutic targets for late-life neuropsychiatric syndromes.