Heart rate and its variability are determined by a variety of physiological factors, the most prominent of which is the autonomic system. In the resting state, the heart is under tonic inhibitory control by the parasympathetic (vagal) nervous system (
1). Although the parasympathetic and sympathetic nervous systems are typically conceptualized as two opposing components, recent thinking suggests that vagal activity may be withdrawn without a corresponding increase in sympathetic nervous system activity (
2). This metabolically conservative response during challenge may mirror autonomic dysregulation in psychiatric illness during the resting state. It is in this regard that we refer to resting-state heart rate and its variability as surrogate markers of vagally mediated cardiac activity. It is important to acknowledge, however, that measures of heart rate variability and the high-frequency component in particular are more pure indicators of vagal activity than heart rate (
3), which may include sympathetic input. In this study, we examined the impact of depression, anxiety, comorbidity, and antidepressants on resting-state heart rate and its variability, measures we refer to here as “vagal activity” for brevity.
Vagal activity plays an important role in mental and physical well-being (see reference
4 for a review). Increases in vagal activity are associated with positive emotions, social connectedness, and longevity. By contrast, decreases in vagal activity are associated with depression, anxiety, cardiovascular disease, and mortality, findings that may be attributable to the downstream effects of a poorly functioning cholinergic anti-inflammatory reflex. Other mechanisms, including chronically elevated activity in the sympathetic nervous system, are also involved in the pathway from emotion to morbidity. However, we suggest that vagal activity may provide a unique structural link from day-to-day emotion experience to morbidity and mortality from a host of conditions. Within-subject phasic changes in vagal activity reflect a normal response to environmental challenge, while between-subject resting-state tonic differences in vagal activity predict future mortality. In this regard, an early seminal study on the effects of decreased heart rate variability following acute myocardial infarction (
5), as measured by the standard deviation of all normal R-R intervals in 24-hour continuous ECG recordings from Holter monitors, reported a fivefold increase in relative risk of mortality.
We and others have demonstrated that vagal activity is reduced in the mood and anxiety disorders (see reference
4 for a review). In one of the first studies on this topic more than two decades ago (
6), depressed patients with coronary artery disease were found to have a higher heart rate relative to nondepressed patients with coronary artery disease, independent of age, smoking, and beta-blocker medication. Depressed patients also had reduced heart rate variability, although this association fell short of statistical significance. This study was also based on data extracted from 24-hour ambulatory ECG recordings. Reliable and valid measures of heart rate and its variability may also be obtained from shorter recordings under standardized and controlled conditions (
7). Using short-term ECG recordings in another early study (
8), we reported that patients with generalized anxiety disorder displayed lower high-frequency heart rate variability. High-frequency heart rate variability, in particular, is mediated solely by changing levels of vagal activity (
3). We concluded that generalized anxiety disorder and its cardinal feature (worry) are associated with reduced vagal activity. More recently, we demonstrated that patients with comorbid major depressive disorder (which we refer to here as “depression” for brevity) and generalized anxiety disorder (
9; see reference
4 for review) display the most robust reductions in resting-state vagal activity. Our explanation for these findings is that chronic worry and hypervigilance to threat may underpin chronic withdrawal of vagal activity that may subsequently lead to increased morbidity and mortality.
Recent debate has focused on whether mental disorders such as depression and anxiety are associated with reductions in vagal activity (see reference
4 for review) or whether these findings are driven by antidepressant medications (
10,
11). However, studies have seldom used appropriate measures to adequately control for the effects of these variables on vagal activity. Inappropriate control of confounding factors may lead to a phenomenon known as the “reversal paradox,” in which “the association between two variables may be reversed, diminished, or enhanced when another variable is statistically controlled” (
12). Here we control for confounding factors through propensity score weighting using a generalized boosted modeling technique (
13).
ELSA-Brasil is the first large multicenter cohort study of adult health conducted in Brazil. It is an ongoing cohort study designed to investigate the relationship between cardiovascular diseases and diabetes, as well as their social determinants and risk factors. In this regard, vagal activity is an important consideration (
4), and cross-sectional analysis of the ELSA-Brasil cohort at baseline will provide an important foundation for future longitudinal analyses of data from this cohort. Consistent with our previous studies with smaller samples (
9,
16,
17), we hypothesized that individuals diagnosed with mood and anxiety disorders would exhibit reductions in vagal activity, and that use of antidepressants would be associated with further reductions, a finding that would be most apparent in users of tricyclic antidepressants.
Results
Participant Characteristics
Participant characteristics before and after propensity score weighting are presented in the data supplement that accompanies the online edition of this article: Table S1 presents characteristics for the mood and anxiety disorder groupings, and Table S2 does so for the antidepressant groupings.
Impact of Disorder on Vagal Activity
After propensity score weighting, effective sample sizes were as follows: comparison participants with no depressive or anxiety disorders, N=10,346; participants with depression, N=98; participants with phobias, N=121; participants with panic disorder, N=43; participants with generalized anxiety disorder, N=1,183; and participants with comorbid disorders, N=311. Average heart rate was slightly higher in the generalized anxiety disorder group relative to the comparison group (67.30 bpm compared with 66.79 bpm), although the association fell short of statistical significance and was associated with a small effect size (p=0.086, d=0.053). No other groups differed significantly from the comparison group. Sensitivity analysis using propensity score matching revealed similar nonsignificant findings for the generalized anxiety disorder group (N=1,249) relative to the comparison group (N=1,236) (67.23 bpm compared with 67.90 bpm; p=0.065, d=0.074).
Analysis of heart rate variability revealed significant effects for both measures after propensity score weighting; root mean square of successive differences was lower in the generalized anxiety disorder group relative to the comparison group (3.17 compared with 3.21; p=0.015, d=0.075), as was the high-frequency component (5.23 compared with 5.32; p=0.033, d=0.066). No other groups differed significantly from the comparison group. Again, sensitivity analysis using propensity score matching revealed similar findings for the generalized anxiety disorder group relative to the comparison group (root mean square of successive differences: 3.18 compared with 3.25; p=0.004, d=0.116; high-frequency component: 5.29 compared with 5.42; p=0.005, d=0.113).
Impact of Antidepressants on Vagal Activity
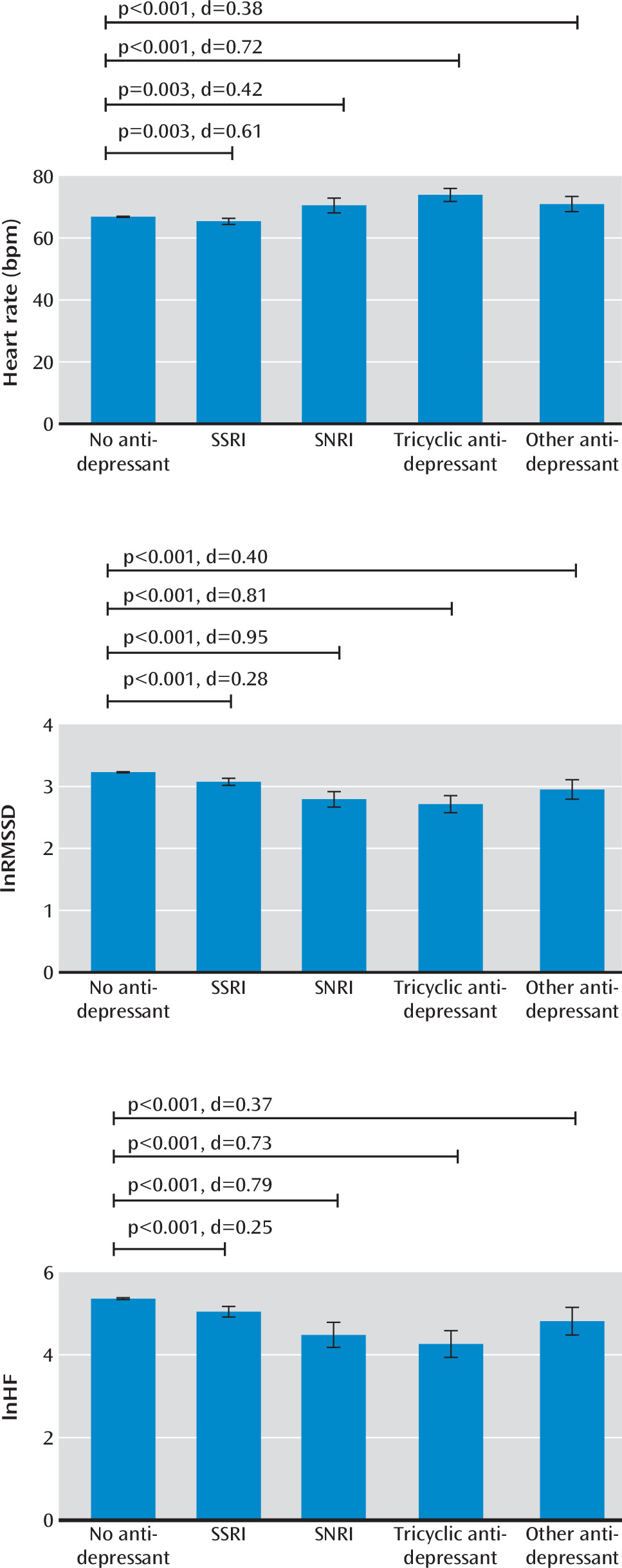
After propensity score weighting, effective sample sizes were as follows: participants not on antidepressants, N=14,069; those on SSRIs, N=356; those on SNRIs, N=52; those on tricyclic antidepressants, N=84; and those on other antidepressants, N=75. As shown in
Figure 1, average heart rate relative to those not on antidepressants (mean=66.87 bpm) was higher in those on tricyclics (mean=73.94 bpm, p<0.001, d=0.721), those on other antidepressants (mean=71.00 bpm, p=0.001, d=0.380), and those on SNRIs (mean=70.55 bpm, p=0.003, d=0.420) and slightly lower among those on SSRIs (mean=65.40 bpm, p=0.003, d=0.161). In addition, all antidepressants were associated with a lower heart rate variability (root mean square of successive differences, tricyclics: mean=2.71, p<0.001, d=0.810; SNRIs: mean=2.79, p<0.001, d=0.952; other antidepressants: mean=2.95, p<0.001, d=0.402; SSRIs: mean=3.08, p<0.001, d=0.280; high-frequency component, tricyclics: mean=4.26, p<0.001, d=0.730; SNRIs: mean=4.48, p<0.001, d=0.789; other antidepressants: mean=4.82, p=0.001, d=0.367; SSRIs: mean=5.05, p<0.001, d=0.254) relative to those not on antidepressants (root mean square of successive differences: mean=3.23; high-frequency component: mean=5.36). These findings were all replicated in sensitivity analysis using propensity score matching (results not shown).
Discussion
The most striking effects from our study relate to the use of antidepressant medications; tricyclic antidepressants, SNRIs, and other antidepressants were associated with moderate to large adverse effects. With respect to the impact of mood and anxiety disorders—an issue that has attracted the most debate—the findings demonstrate that only generalized anxiety disorder is associated with a reduction in vagal activity after controlling for a host of clinical covariates, including biomarkers of metabolic and cardiovascular risk. The effects of generalized anxiety disorder on vagal activity were statistically significant and robust, although effect sizes were small. This is an important finding, as other researchers have recently argued that it is the antidepressant medication, rather than the disorder per se, that has an adverse impact on vagal activity (
10,
11). While it is notable that participants with comorbid depression and anxiety disorders did not display reductions in vagal activity, it is important to bear in mind that many of the factors that adversely affect vagal activity were controlled for in this study.
Mood and anxiety disorders raise metabolic and cardiovascular risk (
21), emphasizing the need for comprehensive cardiovascular risk reduction strategies in all patients with these disorders to minimize subsequent morbidity and mortality. In this study, we showed that only generalized anxiety disorder without comorbidity is associated with reductions in vagal activity. These findings highlight the independent adverse effects of generalized anxiety disorder without comorbidity on vagal activity. It is important to note, however, that generalized anxiety disorder and depression are linked sequentially such that each disorder increases the likelihood of developing the other over time (
22). Longitudinal follow-up of our participants will provide important data to further examine the relationship between depression and generalized anxiety disorder over time and the disorders’ effects on vagal activity. In line with our previous studies on generalized anxiety disorder (
8,
9), we suggest that the cardinal features of this disorder, including chronic worry and hypervigilance to threat, independently contribute to long-term reductions in vagal activity and subsequent increases in morbidity and mortality.
Previous studies of mood disorders and vagal activity have reported contradictory findings, leading to significant debate and discussion. In 2007, Rottenberg (
23) reported findings from a meta-analysis indicating that depression had an adverse impact on vagal activity, a finding associated with a small to medium effect size. He also suggested that low heart rate variability may be a consequence of comorbid anxiety, rather than of depression per se. Licht et al. reported that although heart rate variability is reduced in patients with depression (
24) and those with anxiety disorders (
25), these reductions are driven by antidepressant medications. These findings contrasted with smaller studies that had reported adverse effects of the mood and anxiety disorders, leading to the publication of our meta-analysis in 2010 (
16). We reported that heart rate variability was lower in unmedicated depressed patients relative to healthy comparison subjects, a finding associated with a large effect size when focusing on nonlinear measures of heart rate variability. Recent years have witnessed increasing calls for independent replication of published findings in science (
26,
27). Consistent with this goal, we have examined the impact of mental disorders and antidepressants on vagal activity using a variety of approaches. In addition to meta-analysis, we have replicated reductions in vagal activity in mood and anxiety disorders in multiple independent cohorts of patients (
9,
17,
28). In the largest independent cohort to date, we now report that after controlling for multiple confounding variables, we find that generalized anxiety disorder is robustly associated with reductions in vagal activity, although these reductions are small. It is possible that the lack of findings for depression are related to disorder heterogeneity. For example, specific depression characteristics (e.g., melancholia) may be associated with more robust effects on vagal activity.
We suggest here that the effects we observed for the tricyclics and SNRIs in particular may underpin previously reported increases in risk for cardiovascular morbidity and mortality (
29,
30). There is strong evidence for a continuous increase in risk for cardiovascular and all-cause mortality in men and women with a resting heart rate above 60 bpm, regardless of history of cardiovascular disease (
31). In the present study, we showed that tricyclic antidepressants were associated with an average resting heart rate of ∼74 bpm, while SNRIs and other antidepressants were associated with an average resting heart rate of approximately ∼71 bpm, compared with ∼67 bpm for those not taking antidepressants. In light of increasing concerns about the impact of antidepressant medications (
10,
29,
30), we recently investigated the moderating effects of physical activity on the impact of the SSRI escitalopram on cardiac stress responses (
32). We found that participants who engaged in exercise at least three times a week exhibited a more resilient vagal response to stress relative to irregular exercisers, a finding associated with a moderate effect size. We also observed that acute treatment with escitalopram had a similar impact on irregular exercisers during stress. The moderating effects of regular exercise in long-term users of antidepressants need to be examined in future research. In the present study, the SSRIs were associated with a decrease in heart rate
and its variability. It is particularly interesting that these findings have been reported previously for this class of medication (
10)—findings that were interpreted as both a decrease in sympathetic activity and parallel decreases in net cardiac vagal effects.
While our study has important strengths—particularly the application of propensity score weighting on a large, independent cohort—a number of limitations should also be noted. First, the reliability of short-term measurements of heart rate variability has been questioned (
33). However, we have demonstrated that the protocol on which this study is based shows good reproducibility (
34). Second, we acknowledge that respiration rate may influence estimates of heart rate and its variability. While we did not collect data on respiration rate, the question of whether or not respiration rate should be controlled for remains a divisive one in the field of psychophysiology. Researchers have even cautioned against experimentally controlling for respiration, as this may confound the visceral-medullary feedback system and shift respiratory parameters (
2). Also, the root mean square of successive differences, unlike frequency-based measures, may actually be more robust to changes in breathing patterns (
35). In the present study, key findings were obtained across both measures of heart rate variability, highlighting the robustness of findings. Finally, it should be noted that although we controlled for a variety of important covariates, there are likely other factors that we did not measure or include in our models that may have affected our findings. Thus, there remains the potential for residual confounding factors in our study.
In summary, this study provides much-needed evidence for the impact of mood and anxiety disorders, as well as antidepressants, on resting-state vagal activity after controlling for a large number of confounding factors. Future studies employing a randomized controlled design will provide further important evidence for the impact of specific antidepressants on vagal activity. Further research is also needed to determine whether certain clinical characteristics and their amelioration moderate observed findings. It is possible, for instance, that specific subtypes of depression (e.g., melancholia) or specific symptoms of a depressive episode or an anxiety disorder (e.g., somatic symptoms) have more robust effects on vagal activity. There is also a need for research to determine how and when reduced vagal activity leads to morbidity and mortality from a host of conditions.